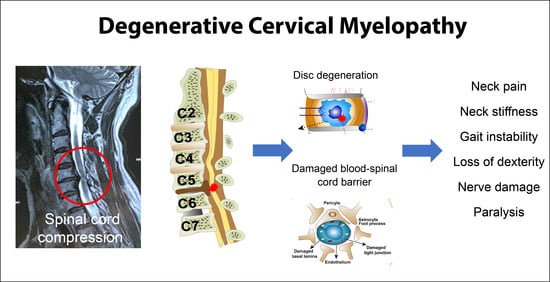
Degenerative Cervical Myelopathy: Insights into Its Pathobiology and Molecular Mechanisms
Abstract
1. Introduction
2. Epidemiology
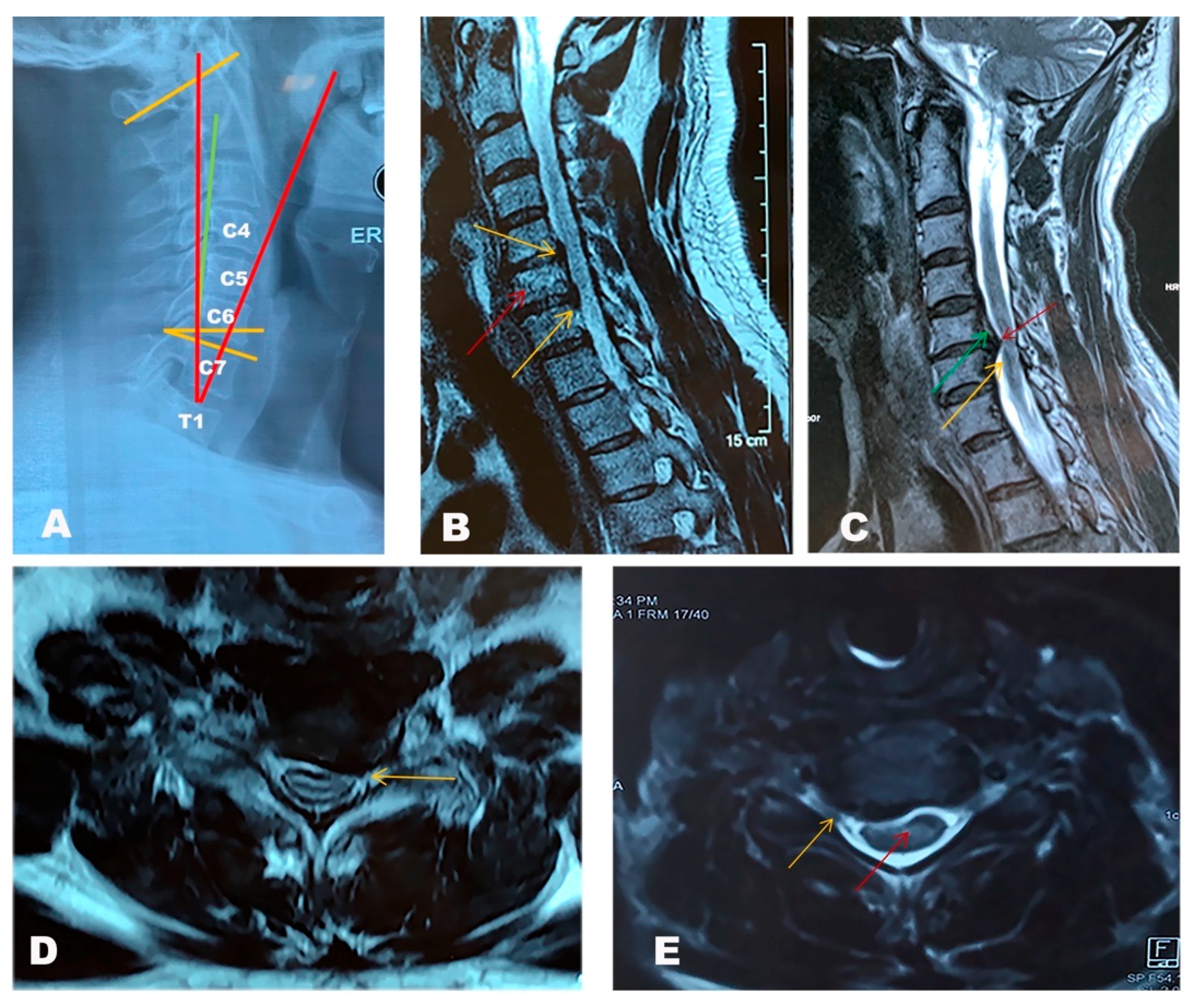
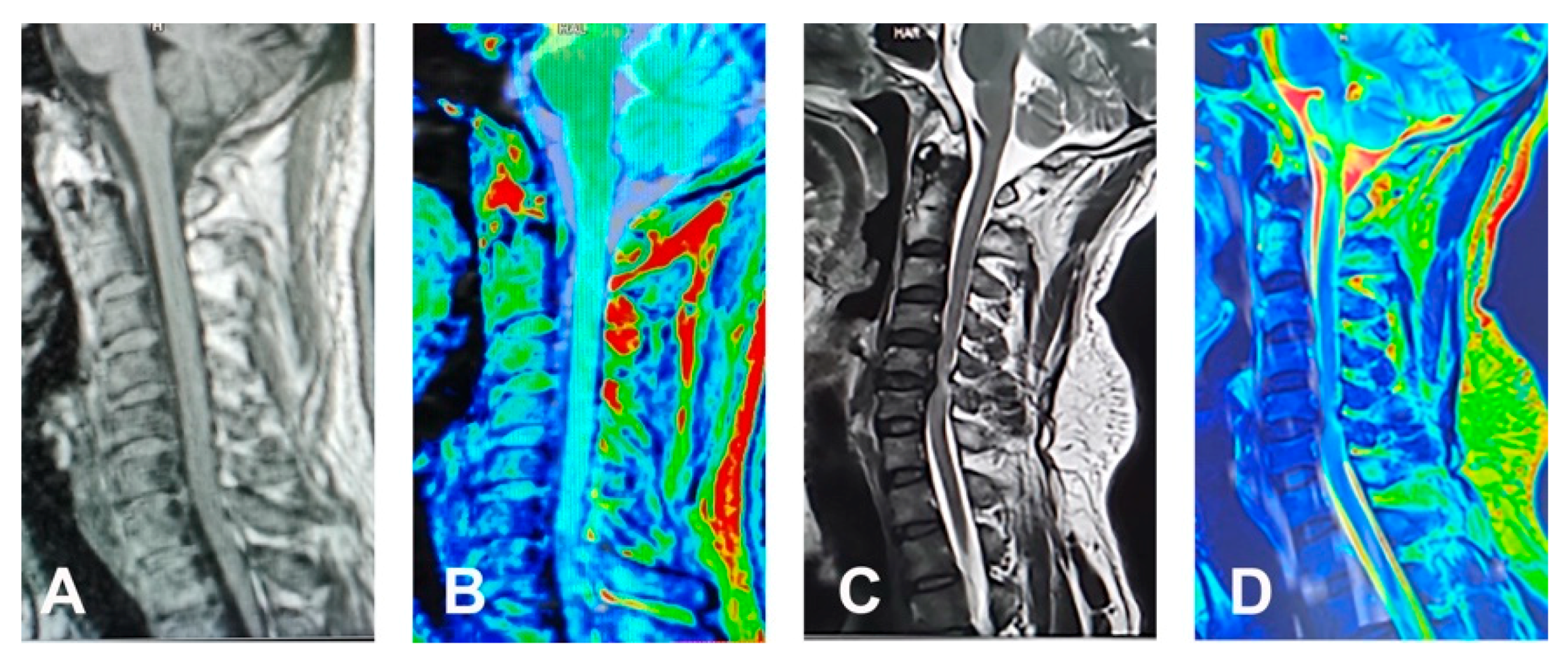
3. Diagnosis
| Sign/Symptom | Description | Explanation | Sensitivity | Specificity |
|---|---|---|---|---|
| Hyperreflexia | Reflex greater than 3 on a 0 to 4 scale. (0: absent, 1: hypoactive, 2: normal, 3: hyperactive without clonus, 4: very hyperactive often with clonus. | Interruption of corticospinal and other descending pathways that influence the two-neuron reflex arc due to a suprasegmental lesion. Normally, the cerebral cortex or a number of brainstem nuclei influence the sensory input of the muscle by inhibiting the motor neuron in the anterior horn of the spinal cord. If a descending tract carrying these inhibitory signals is lost, the reflex is augmented. | 72% | 43% |
| Hyperreflexia Biceps | Percussion or tapping of the biceps tendon, close to its insertion in the ulna. Greater than 3 on a 0–4 scale. | Mainly C5. Small C6 component. | 62% | 49% |
| Hyperreflexia Brachioradialis (BR) | Percussion of the BR tendon distally. Greater than 3 on a 0–4 scale. | Evaluates neurologic integrity of C6. | 21% | 89% |
| Hyperreflexia Triceps | Percussion on the distal tendon of the triceps muscle. | Evaluates C7 neurologic integrity. | 36% | 78% |
| Hyperreflexia Patella | Percussion on the patellar tendon, with quadriceps relaxed. | Evaluates L4 neurologic integrity. | 33% | 76% |
| Hyperreflexia Achilles | Percussion in the Achilles tendon, with a relaxed gastro-soleus muscle. | Evaluates S1 neurologic integrity. | 26% | 81% |
| Hoffman | Hand in neutral position, flicking of the distal phalanx of the middle finger causes flexion of the distal phalanx of the thumb and second and third phalanx of the second finger. | Thought to represent a lesion in the corticospinal tracts [18]. | 59% | 84% |
| Inverted Brachioradialis reflex (IBR) | When eliciting a BR reflex, there is contraction of the finger flexors with diminished BR reflex. | Thought to be caused by a lesion at C5-C6 (damage to the alpha motoneurons) and hyper-active response levels below (C8) [19]. | 51% | 81% |
| Clonus | Forcefully dorsiflexing the ankle and maintaining pressure on the sole of the foot while observing for rhythmic beats of ankle flexion and extension. More than 3 beats required. | Hyper-active stretch reflexes in clonus are believed to be caused by self- excitation, which is not inhibited by the corticospinal tract (if there is an injury in the spinal cord) [20]. | 13% | 100% |
| Babinski | Firmly run a pointy instrument, on the lateral part of the sole of the foot, from the heel to the base of the toes. Positive if extension of the Hallux occurs. | The normal response to plantar stimuli is abolished by an upper motor neuron lesion. It is replaced by Babinski’s reflex, where the upward going toe (although anatomically it looks like extension) is part of a flexor reflex, disinhibited by loss of upper motor neurone control, and its receptive field may extend in some instances to the leg or thigh [21]. | 13% | 100% |
| Radiologic Measures | Normal Values | Explanation |
|---|---|---|
| Cobb C1–7/C2–7 angle | 18 degrees +/− 12 degrees | The angle between the line parallel to the inferior endplate of C1/C2 to parallel to the inferior endplate of C7. |
| C7 slope | Normal values vary according to the individual cervical lordosis | Angle between a horizontal line and the superior endplate of C7 |
| T1 slope | Normal values vary according to the individual cervical lordosis | Angle between horizontal plane at T1 endplate |
| Cervical sagittal vertical alignment (SVA) | 15 mm +/− 11 mm | The distance from the posterior, superior corner of C7 to the plumbline from the centroid of C2 |
| Cervical tilt | 43 degrees +/− 6 degrees | The angle between two lines, both originating from the centre of the T1 upper end plate; one is vertical to the T1 upper end plate and the other passes through the tip of the dens |
| Modic Type | T1 Findings | T2 Findings | Clinical Correlation |
|---|---|---|---|
| 1 | Hypointense | Hyperintense | Represent bone marrow oedema and inflammation |
| 2 | Hyperintense | Isointense | Conversion of normal hemopoietic bone marrow into fatty marrow as a result of ischemia |
| 3 | Hypointense | Hypointense | Represent subchondral bone sclerosis |
| Name | Scoring Method | Radiologic Findings | Correlation to Symptoms | Limitations | Advantages |
|---|---|---|---|---|---|
| Nurick | 0–5. The higher the grade, the more severe the deficit. | No | Affected by gait function (++), lower limbs paresis and paraesthesia and vegetative symptoms (+). | Less accurate post-op scoring; Does not pick up upper extremity disfunction | Evaluates economic situation in connection to gait function. |
| mJOA | 0–17. The lower the score, the more severe the deficits. Normal: 16–17, grade 1: 12–15, grade 2: 8–11, grade 3: 0–7. Upper extremity 23.5%; lower extremity 23.5%; sensory 35.4%; bladder and bowel 17.6% | No | Affected by paraesthesia of lower limbs and paresis of upper limbs (++) and dysdiadochokinesia and vegetative symptoms (+). | Does not take economic factors into consideration | Good for assessing outcomes (post-intervention). |
| CMS | Upper and lower extremity are analysed separately. 0–5 each. The higher the grade, the more severe the deficit. | Weak correlation between low severity in the lower limb score and C-Spine mal-alignment | Affected by dysdiadochokinesia, gait function and paresis of upper extremity (++) and vegetative symptoms (+) | Does not take economic factors into consideration | Good for assessing function/symptoms of upper/lower extremities/as it evaluates them individually. Good at assessing clinical state and grade of severity of CSM. |
| EMS | 5–18. The lower the score the more severe the deficits. Normal function: 17+, grade 1: 13–16, grade 2: 9–12, grade 3: 5–8. Upper extremity 27.8%, lower extremity 22.2%, coordination 16.7%, paraesthesia/pain 16.0%, bladder and bowel function 16.7% | No | Affected by dysdiadochokinesia (++) and paresis of the upper extremity and vegetative symptoms (+) | Good at assessing clinical state and grade of severity of CSM. Better sensitivity to reveal functional deficit (by assessing proprioception/coordination). | |
| Prolo scale | 2–10. The lower the score the more severe the deficits. Normal function: 9+, grade 1: 7 + 8, grade 2: 5 + 6, grade 3: 2–4. Economic status 50%; functional status 50%. | No | Mildly affected by vegetative symptoms (+) | Does not reflect clinical symptoms significantly -Not good for pre-op assessing the grade of severity | Good correlation between high pre-op scores and better outcomes. Good for assessing normalisation¨ and rehabilitation (regained ability for work or for leisure time). |
4. Natural History
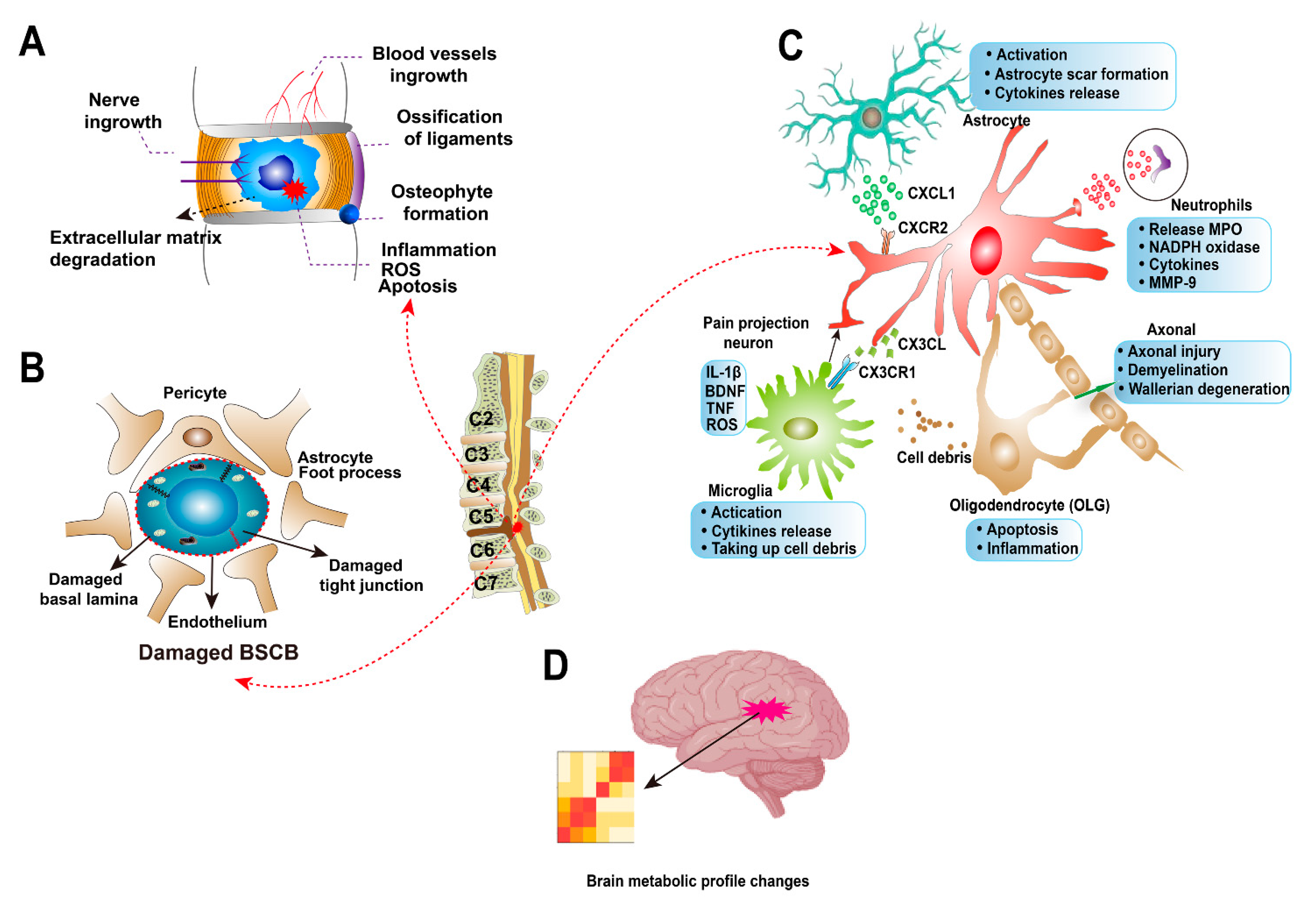
5. Pathophysiology
5.1. Spinal Cord Compression and Ischemic Injury
5.2. Spine Deformity and Instability
5.3. Ossification of the Ligaments
5.4. Biomechanical Changes
6. Risk Factors
6.1. Aging
6.2. Genetic Polymorphism
| Gene | DCM Features | Reference |
|---|---|---|
| Brain-derived neurotrophic factor (BDNF) | Worse mJOA and Nurick scores | [81] |
| Osteoprotegerin (OPG) | Worse mJOA score | [82] |
| Osteopontin (OPN) | Worse mJOA score | [83] |
| Hypoxia inducible factor-1α (HIF-1α) | Worse mJOA score | [84] |
| Apolipoprotein E (APOE) | Worse mJOA score | [85,86] |
| BMPs (BMP4, BMP9, BMPR1A) | Radiographic severity of DCM | [87,88] |
| RUNX2 | Responsible for OPLL | [89] |
| BMP2 | Responsible for OPLL | [90] |
| Vitamin D receptor (VDR) | Radiologic changes and mJOA scores | [89,91] |
| Vitamin D binding protein (VDBP) | Radiologic changes and mJOA scores | [91] |
| Collagen IX | Radiologic changes and mJOA scores | [92] |
| Collagen α2(XI) | Radiographic severity of DCM | [93] |
6.3. Microbes
7. Molecular Features
7.1. Cervical Intervertebral Disc Degeneration
7.2. Blood-Spinal Cord Barrier Dysfunction
7.3. Axonal Injury
7.4. Astrocytes
7.5. Microglia and Neutrophils
7.6. Oligodendrocytes
7.7. Brain Reorganization
8. Treatment
8.1. Non-Surgical Treatment
8.2. Surgical Treatment
9. Future Directions
10. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tracy, J.A.; Bartleson, J.D. Cervical Spondylotic Myelopathy. Neurologist 2010, 16, 176–187. [Google Scholar] [CrossRef]
- Emery, S.E.; Bohlman, H.H.; Bolesta, M.J.; Jones, P.K. Anterior Cervical Decompression and Arthrodesis for the Treatment of Cervical Spondylotic Myelopathy. Two to Seventeen-Year Follow-up. J. Bone Jt. Surg. Am. 1998, 80, 941–951. [Google Scholar] [CrossRef]
- Badhiwala, J.H.; Ahuja, C.S.; Akbar, M.A.; Witiw, C.D.; Nassiri, F.; Furlan, J.C.; Curt, A.; Wilson, J.R.; Fehlings, M.G. Degenerative cervical myelopathy—Update and future directions. Nat. Rev. Neurol. 2020, 16, 108–124. [Google Scholar] [CrossRef]
- Boden, S.D.; McCowin, P.R.; Davis, D.; Dina, T.S.; Mark, A.S.; Wiesel, S. Abnormal magnetic-resonance scans of the cervical spine in asymptomatic subjects. A prospective investigation. J. Bone Jt. Surg. Am. 1990, 72, 1178–1184. [Google Scholar] [CrossRef]
- Nakashima, H.; Yukawa, Y.; Suda, K.; Yamagata, M.; Ueta, T.; Kato, F. Abnormal Findings on Magnetic Resonance Images of the Cervical Spines in 1211 Asymptomatic Subjects. Spine 2015, 40, 392–398. [Google Scholar] [CrossRef]
- Fontes, R.B.D.V.; Baptista, J.S.; Rabbani, S.R.; Traynelis, V.C.; Liberti, E.A. Structural and Ultrastructural Analysis of the Cervical Discs of Young and Elderly Humans. PLoS ONE 2015, 10, e0139283. [Google Scholar] [CrossRef] [PubMed]
- Kato, F.; Yukawa, Y.; Suda, K.; Yamagata, M.; Ueta, T. Normal morphology, age-related changes and abnormal findings of the cervical spine. Part II: Magnetic resonance imaging of over 1200 asymptomatic subjects. Eur. Spine J. 2012, 21, 1499–1507. [Google Scholar] [CrossRef] [PubMed]
- Gibson, J.; Nouri, A.; Krueger, B.; Lakomkin, N.; Nasser, R.; Gimbel, D.; Cheng, J. Degenerative Cervical Myelopathy: A Clinical Review. Yale J. Biol. Med. 2018, 91, 43–48. [Google Scholar] [PubMed]
- Boogaarts, H.D.; Bartels, R.H.M.A. Prevalence of cervical spondylotic myelopathy. Eur. Spine J. 2015, 24 (Suppl. 2), 139–141. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, J.; Xu, B.; Yang, H.; Zhu, Q. A model of acute central cervical spinal cord injury syndrome combined with chronic injury in goats. Eur. Spine J. 2017, 26, 56–63. [Google Scholar] [CrossRef]
- Tan, L.A.; Riew, K.D.; Traynelis, V.C. Cervical Spine Deformity—Part 2: Management Algorithm and Anterior Techniques. Neurosurgery 2017, 81, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Rhee, J.; Hamasaki, T.; Heflin, J.; Freedman, B. Prevalence of Physical Signs in Cervical Myelopathy: A Prospective Controlled Study. Spine 2009, 34, 890–895. [Google Scholar] [CrossRef] [PubMed]
- Harrop, J.S.; Naroji, S.; Maltenfort, M.; Anderson, D.G.; Albert, T.; Ratliff, J.K.; Ponnappan, R.K.; Rihn, J.A.; Smith, H.E.; Hilibrand, A.; et al. Cervical myelopathy: A clinical and radiographic evaluation and correlation to cervical spondylotic myelopathy. Spine 2010, 35, 620–624. [Google Scholar] [CrossRef]
- Tan, L.A.; Riew, K.D.; Traynelis, V.C. Cervical Spine Deformity—Part 1: Biomechanics, Radiographic Parameters, and Classification. Neurosurgery 2017, 81, 197–203. [Google Scholar] [CrossRef]
- Lebl, D.R.; Hughes, A.; Cammisa, F.P., Jr.; O’Leary, P.F. Cervical Spondylotic Myelopathy: Pathophysiology, Clinical Presentation, and Treatment. HSS J. 2011, 7, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Acharya, S.; Srivastava, A.; Virmani, S.; Tandon, R. Resolution of Physical Signs and Recovery in Severe Cervical Spondylotic Myelopathy After Cervical Laminoplasty. Spine 2010, 35, E1083–E1087. [Google Scholar] [CrossRef] [PubMed]
- Kalsi-Ryan, S.; Karadimas, S.K.; Fehlings, M.G. Cervical spondylotic myelopathy: The clinical phenomenon and the current pathobiology of an increasingly prevalent and devastating disorder. Neuroscientist 2013, 19, 409–421. [Google Scholar] [CrossRef]
- Glaser, J.A.; Curé, J.K.; Bailey, K.L.; Morrow, D.L. Cervical spinal cord compression and the Hoffmann sign. Iowa Orthop. J. 2001, 21, 49–52. [Google Scholar]
- Estanol, B.V.; Marin, O.S. Mechanism of the inverted supinator reflex. A clinical and neurophysiological study. J. Neurol. Neurosurg. Psychiatry 1976, 39, 905–908. [Google Scholar] [CrossRef]
- Boyraz, I.; Uysal, H.; Koc, B.; Sarman, H. Clonus: Definition, mechanism, treatment. Med. Glas. Zenica 2015, 12, 19–26. [Google Scholar]
- Lance, J.W. The Babinski sign. J. Neurol. Neurosurg. Psychiatry 2002, 73, 360–362. [Google Scholar] [CrossRef]
- Bydon, A.; Ritzl, E.K.; Sciubba, D.M.; Witham, T.F.; Xu, R.; Sait, M.; Wolinsky, J.-P.; Gokaslan, Z.L. A role for motor and somatosensory evoked potentials during anterior cervical discectomy and fusion for patients without myelopathy: Analysis of 57 consecutive cases. Surg. Neurol. Int. 2011, 2, 133. [Google Scholar] [CrossRef] [PubMed]
- Morishita, Y.; Hida, S.; Naito, M.; Matsushima, U. Evaluation of cervical spondylotic myelopathy using somatosensory-evoked potentials. Int. Orthop. 2005, 29, 343–346. [Google Scholar] [CrossRef][Green Version]
- Lyu, R.K.; Tang, L.M.; Chen, C.; Chang, H.S.; Wu, Y.R. The use of evoked potentials for clinical correlation and surgical outcome in cervical spondylotic myelopathy with intramedullary high signal intensity on MRI. J. Neurol. Neurosurg. Psychiatry 2004, 75, 256–261. [Google Scholar] [PubMed]
- Lo, Y.L. How has electrophysiology changed the management of cervical spondylotic myelopathy? Eur. J. Neurol. 2008, 15, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Xing, R.; Zhou, G.; Chen, Q.; Liang, Y.; Dong, J. MRI to measure cervical sagittal parameters: A comparison with plain radiographs. Arch. Orthop. Trauma Surg. 2017, 137, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Scheer, J.K.; Tang, J.A.; Smith, J.S.; Acosta, F.L., Jr.; Protopsaltis, T.S.; Blondel, B.; Bess, S.; Shaffrey, C.I.; Deviren, V.; Lafage, V.; et al. Cervical spine alignment, sagittal deformity, and clinical implications: A review. J. Neurosurg. Spine 2013, 19, 141–159. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Cho, C.B.; Song, J.H.; Kim, S.W.; Ha, Y.; Oh, J.K. T1 Slope and Cervical Sagittal Alignment on Cervical CT Radiographs of Asymptomatic Persons. J. Korean Neurosurg. Soc. 2013, 53, 356–359. [Google Scholar] [CrossRef] [PubMed]
- Blease Graham, C.; Wippold, F.J.; Bae, K.T.; Pilgram, T.K.; Shaibani, A.; Kido, D.K. Comparison of CT myelography performed in the prone and supine positions in the detection of cervical spinal stenosis. Clin. Radiol. 2001, 56, 35–39. [Google Scholar] [CrossRef]
- Abiola, R.; Rubery, P.; Mesfin, A. Ossification of the Posterior Longitudinal Ligament: Etiology, Diagnosis, and Outcomes of Nonoperative and Operative Management. Glob. Spine J. 2016, 6, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Modic, M.T.; Steinberg, P.M.; Ross, J.S.; Masaryk, T.J.; Carter, J.R. Degenerative disk disease: Assessment of changes in vertebral body marrow with MR imaging. Radiology 1988, 166 (Pt 1), 193–199. [Google Scholar] [CrossRef]
- Gao, X.; Li, J.; Shi, Y.; Li, S.; Shen, Y. Asymmetrical degenerative marrow (Modic) changes in cervical spine: Prevalence, correlative factors, and surgical outcomes. J. Orthop. Surg. Res. 2018, 13, 85. [Google Scholar] [CrossRef] [PubMed]
- Mann, E.; Peterson, C.K.; Hodler, J.; Pfirrmann, C.W.A. The evolution of degenerative marrow (Modic) changes in the cervical spine in neck pain patients. Eur. Spine J. 2014, 23, 584–589. [Google Scholar] [CrossRef] [PubMed]
- Yoshimatsu, H.; Nagata, K.; Goto, H.; Sonoda, K.; Ando, N.; Imoto, H.; Mashima, T.; Takamiya, Y. Conservative treatment for cervical spondylotic myelopathy. prediction of treatment effects by multivariate analysis. Spine J. 2001, 1, 269–273. [Google Scholar] [CrossRef]
- Fehlings, M.G.; Rao, S.C.; Tator, C.H.; Skaf, G.; Arnold, P.; Benzel, E.; Dickman, C.; Cuddy, B.; Green, B.; Hitchon, P.; et al. The optimal radiologic method for assessing spinal canal compromise and cord compression in patients with cervical spinal cord injury. Part II: Results of a multicenter study. Spine 1999, 24, 605–613. [Google Scholar] [CrossRef]
- Furlan, J.C.; Kailaya-Vasan, A.; Aarabi, B.; Fehlings, M.G. A Novel Approach to Quantitatively Assess Posttraumatic Cervical Spinal Canal Compromise and Spinal Cord Compression: A Multicenter Responsiveness Study. Spine 2011, 36, 784–793. [Google Scholar] [CrossRef] [PubMed]
- Sritharan, K.; Chamoli, U.; Kuan, J.; Diwan, A.D. Assessment of degenerative cervical stenosis on T2-weighted MR imaging: Sensitivity to change and reliability of mid-sagittal and axial plane metrics. Spinal Cord 2020, 58, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Nouri, A.; Tetreault, L.; Côté, P.; Zamorano, J.J.; Dalzell, K.; Fehlings, M.G. Does Magnetic Resonance Imaging Improve the Predictive Performance of a Validated Clinical Prediction Rule Developed to Evaluate Surgical Outcome in Patients With Degenerative Cervical Myelopathy? Spine 2015, 40, 1092–1100. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zeitoun, D.; Rangel, A.; Lazennec, J.Y.; Catonné, Y.; Pascal-Moussellard, H. Preoperative evaluation of the cervical spondylotic myelopathy with flexion-extension magnetic resonance imaging: About a prospective study of fifty patients. Spine 2011, 36, E1134–E1139. [Google Scholar] [CrossRef]
- Dalbayrak, S.; Yaman, O.; Firidin, M.N.; Yilmaz, T.; Yilmaz, M. The contribution of cervical dynamic magnetic resonance imaging to the surgical treatment of cervical spondylotic myelopathy. Turk. Neurosurg. 2015, 25, 36–42. [Google Scholar]
- Rutman, A.M.; Peterson, D.J.; Cohen, W.A.; Mossa-Basha, M. Diffusion Tensor Imaging of the Spinal Cord: Clinical Value, Investigational Applications, and Technical Limitations. Curr. Probl. Diagn. Radiol. 2018, 47, 257–269. [Google Scholar] [CrossRef] [PubMed]
- D’Avanzo, S.; Ciavarro, M.; Pavone, L.; Pasqua, G.; Ricciardi, F.; Bartolo, M.; Solari, D.; Somma, T.; De Divitiis, O.; Cappabianca, P.; et al. The Functional Relevance of Diffusion Tensor Imaging in Patients with Degenerative Cervical Myelopathy. J. Clin. Med. 2020, 9, 1828. [Google Scholar] [CrossRef]
- Wang, K.; Zhu, S.; Mueller, B.; Lim, K.; Liu, Z.; He, B. A New Method to Derive White Matter Conductivity From Diffusion Tensor MRI. IEEE Trans. Biomed. Eng. 2008, 55, 2481–2486. [Google Scholar] [CrossRef]
- Wang, K.; Chen, Z.; Zhang, F.; Song, Q.; Hou, C.; Tang, Y.; Wang, J.; Chen, S.; Bian, Y.; Hao, Q.; et al. Evaluation of DTI Parameter Ratios and Diffusion Tensor Tractography Grading in the Diagnosis and Prognosis Prediction of Cervical Spondylotic Myelopathy. Spine 2017, 42, E202–E210. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Guan, L.; Hai, Y.; Liu, Y.; Ding, H.; Chen, X. Multi-shot echo-planar diffusion tensor imaging in cervical spondylotic myelopathy. Bone Jt. J. 2020, 102-B, 1210–1218. [Google Scholar] [CrossRef] [PubMed]
- Dalitz, K.; Vitzthum, H.-E. Evaluation of five scoring systems for cervical spondylogenic myelopathy. Spine J. 2019, 19, e41–e46. [Google Scholar] [CrossRef]
- Herdmann, J.; Linzbach, M.; Krzan, M. The European Myelopathy Score. In Advances in Neurosurgery; Baucher, B.L., Brock, M., Klinger, M., Eds.; Springer: Berlin, Germany, 1994. [Google Scholar]
- Vitzthum, H.-E.; Dalitz, K. Analysis of five specific scores for cervical spondylogenic myelopathy. Eur. Spine J. 2007, 16, 2096–2103. [Google Scholar] [CrossRef]
- Revanappa, K.K.; Rajshekhar, V. Comparison of Nurick grading system and modified Japanese Orthopaedic Association scoring system in evaluation of patients with cervical spondylotic myelopathy. Eur. Spine J. 2011, 20, 1545–1551. [Google Scholar] [CrossRef]
- Clarke, E.; Robinson, P.K. Cervical myelopathy: A complication of cervical spondylosis. Brain 1956, 79, 483–510. [Google Scholar] [CrossRef]
- Lees, F.; Turner, J.W. Natural history and prognosis of cervical spondylosis. Br. Med. J. 1963, 2, 1607–1610. [Google Scholar] [CrossRef]
- Bednarik, J.; Sládková, D.; Kadaňka, Z.; Dušek, L.; Keřkovský, M.; Voháňka, S.; Novotný, O.; Urbánek, I.; Němec, M. Are subjects with spondylotic cervical cord encroachment at increased risk of cervical spinal cord injury after minor trauma? J. Neurol. Neurosurg. Psychiatry 2011, 82, 779–781. [Google Scholar] [CrossRef]
- Katoh, S.; Ikata, T.; Hirai, N.; Okada, Y.; Nakauchi, K. Influence of minor trauma to the neck on the neurological outcome in patients with ossification of the posterior longitudinal ligament (OPLL) of the cervical spine. Spinal Cord 1995, 33, 330–333. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ichihara, K.; Taguchi, T.; Sakuramoto, I.; Kawano, S.; Kawai, S. Mechanism of the spinal cord injury and the cervical spondylotic myelopathy: New approach based on the mechanical features of the spinal cord white and gray matter. J. Neurosurg. 2003, 99 (Suppl. 3), 278–285. [Google Scholar] [CrossRef]
- Tanabe, F.; Yone, K.; Kawabata, N.; Sakakima, H.; Matsuda, F.; Ishidou, Y.; Maeda, S.; Abematsu, M.; Komiya, S.; Setoguchi, T. Accumulation of p62 in degenerated spinal cord under chronic mechanical compression: Functional analysis of p62 and autophagy in hypoxic neuronal cells. Autophagy 2011, 7, 1462–1471. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.R.; Liu, T.; Kiehl, T.-R.; Fehlings, M.G. Human neuropathological and animal model evidence supporting a role for Fas-mediated apoptosis and inflammation in cervical spondylotic myelopathy. Brain 2011, 134 (Pt 5), 1277–1292. [Google Scholar] [CrossRef]
- Morishita, Y.; Naito, M.; Hymanson, H.; Miyazaki, M.; Wu, G.; Wang, J.C. The relationship between the cervical spinal canal diameter and the pathological changes in the cervical spine. Eur. Spine J. 2009, 18, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Levine, D.N. Pathogenesis of cervical spondylotic myelopathy. J. Neurol. Neurosurg. Psychiatry 1997, 62, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Brain, W.R.; Knight, G.C.; Bull, J.W.D. Discussion on rupture of the intervertebral disc in the cervical region. Proc. R. Soc. Med. 1948, 41, 509–516. [Google Scholar] [PubMed]
- Mair, W.G.P.; Druckman, R. The pathology of spinal cord lesions and their relation to the clinical features in protrusion of cervical intervertebral discs: A Report of Four Cases. Brain 1953, 76, 70–91. [Google Scholar] [CrossRef] [PubMed]
- Gooding, M.R.; Wilson, C.B.; Hoff, J.T. Experimental cervical myelopathy. Effects of ischemia and compression of the canine cervical spinal cord. J. Neurosurg. 1975, 43, 9–17. [Google Scholar] [CrossRef]
- Gooding, M.R.; Wilson, C.B.; Hoff, J.T. Experimental cervical myelopathy: Autoradiographic studies of spinal cord blood flow patterns. Surg. Neurol. 1976, 5, 233–239. [Google Scholar] [PubMed]
- Kurokawa, R.; Murata, H.; Ogino, M.; Ueki, K.; Kim, P. Altered Blood Flow Distribution in the Rat Spinal Cord under Chronic Compression. Spine 2011, 36, 1006–1009. [Google Scholar] [CrossRef]
- Smith, J.S.; Lafage, V.; Ryan, D.J.; Shaffrey, C.I.; Schwab, F.J.; Patel, A.A.; Brodke, D.S.; Arnold, P.M.; Riew, K.D.; Traynelis, V.C.; et al. Association of myelopathy scores with cervical sagittal balance and normalized spinal cord volume: Analysis of 56 preoperative cases from the AOSpine North America Myelopathy study. Spine 2013, 38 (Suppl. 1), S161–S70. [Google Scholar] [CrossRef]
- Ames, C.P.; Blondel, B.; Scheer, J.K.; Schwab, F.J.; Le Huec, J.C.; Massicotte, E.M.; Patel, A.A.; Traynelis, V.C.; Kim, H.J.; Shaffrey, C.I.; et al. Cervical radiographical alignment: Comprehensive assessment techniques and potential importance in cervical myelopathy. Spine 2013, 38 (Suppl. 1), S149–S60. [Google Scholar] [CrossRef] [PubMed]
- Goel, A. Role of Subaxial Spinal and Atlantoaxial Instability in Multisegmental Cervical Spondylotic Myelopathy. Acta Neurochir. Suppl. 2019, 125, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Goel, A.; Dhar, A.; Shah, A.; Jadhav, D.; Bakale, N.; Vaja, T.; Jadhav, N. Central or Axial Atlantoaxial Dislocation as a Cause of Cervical Myelopathy: A Report of Outcome of 5 Cases Treated by Atlantoaxial Stabilization. World Neurosurg. 2019, 121, e908–e916. [Google Scholar] [CrossRef]
- Ohara, Y. Ossification of the Ligaments in the Cervical Spine, Including Ossification of the Anterior Longitudinal Ligament, Ossification of the Posterior Longitudinal Ligament, and Ossification of the Ligamentum Flavum. Neurosurg. Clin. N. Am. 2018, 29, 63–68. [Google Scholar] [CrossRef]
- Matsunaga, S.; Sakou, T. Ossification of the posterior longitudinal ligament of the cervical spine: Etiology and natural history. Spine 2012, 37, E309–E314. [Google Scholar] [CrossRef]
- McAfee, P.C.; Regan, J.J.; Bohlman, H.H. Cervical cord compression from ossification of the posterior longitudinal ligament in non-orientals. J. Bone Jt. Surg. Br. 1987, 69, 569–575. [Google Scholar] [CrossRef]
- Li, H.; Jiang, L.S.; Dai, L.Y. Hormones and growth factors in the pathogenesis of spinal ligament ossification. Eur. Spine J. 2007, 16, 1075–1084. [Google Scholar] [CrossRef]
- Kato, Y.; Kanchiku, T.; Imajo, Y.; Kimura, K.; Ichihara, K.; Kawano, S.; Hamanaka, D.; Yaji, K.; Taguchi, T. Biomechanical study of the effect of degree of static compression of the spinal cord in ossification of the posterior longitudinal ligament. J. Neurosurg. Spine 2010, 12, 301–305. [Google Scholar] [CrossRef]
- Takahashi, K.; Ozawa, H.; Sakamoto, N.; Minegishi, Y.; Sato, M.; Itoi, E. Influence of intramedullary stress on cervical spondylotic myelopathy. Spinal Cord 2013, 51, 761–764. [Google Scholar] [CrossRef][Green Version]
- Kato, Y.; Kataoka, H.; Ichihara, K.; Imajo, Y.; Kojima, T.; Kawano, S.; Hamanaka, D.; Yaji, K.; Taguchi, T. Biomechanical study of cervical flexion myelopathy using a three-dimensional finite element method. J. Neurosurg. Spine 2008, 8, 436–441. [Google Scholar] [CrossRef]
- Nagata, K.; Yoshimura, N.; Muraki, S.; Hashizume, H.; Ishimoto, Y.; Yamada, H.; Takiguchi, N.; Nakagawa, Y.; Oka, H.; Kawaguchi, H.; et al. Prevalence of cervical cord compression and its association with physical performance in a population-based cohort in Japan: The Wakayama Spine Study. Spine 2012, 37, 1892–1898. [Google Scholar] [CrossRef]
- Davies, B.M.; Mowforth, O.D.; Smith, E.K.; Kotter, M.R. Degenerative cervical myelopathy. BMJ 2018, 360, k186. [Google Scholar] [CrossRef]
- Okada, E.; Matsumoto, M.; Ichihara, D.; Chiba, K.; Toyama, Y.; Fujiwara, H.; Momoshima, S.; Nishiwaki, Y.; Hashimoto, T.; Ogawa, J.; et al. Aging of the cervical spine in healthy volunteers: A 10-year longitudinal magnetic resonance imaging study. Spine 2009, 34, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; Ye, I.B.; Cheung, Z.B.; Kim, J.S.; Cho, S.K. Age-related Changes in Cervical Sagittal Alignment: A Radiographic Analysis. Spine 2019, 44, E1144–E1150. [Google Scholar] [CrossRef] [PubMed]
- Bull, J.; El Gammal, T.; Popham, M. A possible genetic factor in cervical spondylosis. Br. J. Radiol. 1969, 42, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.A.; Spiker, W.R.; Daubs, M.; Brodke, D.S.; Cannon-Albright, L.A. Evidence of an Inherited Predisposition for Cervical Spondylotic Myelopathy. Spine 2012, 37, 26–29. [Google Scholar] [CrossRef] [PubMed]
- Abode-Iyamah, K.O.; Stoner, K.E.; Grossbach, A.J.; Viljoen, S.V.; McHenry, C.L.; Petrie, M.A.; Dahdaleh, N.S.; Grosland, N.M.; Shields, R.K.; Howard, M.A.; et al. Effects of brain derived neurotrophic factor Val66Met polymorphism in patients with cervical spondylotic myelopathy. J. Clin. Neurosci. 2016, 24, 117–121. [Google Scholar] [CrossRef]
- Yu, H.-M.; Chen, X.-L.; Wei, W.; Yao, X.-D.; Sun, J.-Q.; Su, X.-T.; Lin, S.-F. Effect of osteoprotegerin gene polymorphisms on the risk of cervical spondylotic myelopathy in a Chinese population. Clin. Neurol. Neurosurg. 2018, 175, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wu, N.; Guo, K.; Yuan, F.; Ran, B. OPN Polymorphism is Associated with the Susceptibility to Cervical Spondylotic Myelopathy and its Outcome After Anterior Cervical Corpectomy and Fusion. Cell. Physiol. Biochem. 2014, 34, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-C.; Hou, X.-W.; Shao, J.; Ji, Y.-J.; Li, L.; Zhou, Q.; Yu, S.-M.; Mao, Y.-L.; Zhang, H.-J.; Zhang, P.-C.; et al. HIF-1α Polymorphism in the Susceptibility of Cervical Spondylotic Myelopathy and Its Outcome after Anterior Cervical Corpectomy and Fusion Treatment. PLoS ONE 2014, 9, e110862. [Google Scholar] [CrossRef]
- Setzer, M.; Hermann, E.; Seifert, V.; Marquardt, G. Apolipoprotein E Gene Polymorphism and the Risk of Cervical Myelopathy in Patients With Chronic Spinal Cord Compression. Spine 2008, 33, 497–502. [Google Scholar] [CrossRef]
- Setzer, M.; Vrionis, F.D.; Hermann, E.J.; Seifert, V.; Marquardt, G. Effect of apolipoprotein E genotype on the outcome after anterior cervical decompression and fusion in patients with cervical spondylotic myelopathy. J. Neurosurg. Spine 2009, 11, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Feng, J.; Liu, Z.-Z.; Wan, H.; Li, J.-H.; Lin, X. A new haplotype in BMP4 implicated in ossification of the posterior longitudinal ligament (OPLL) in a Chinese population. J. Orthop. Res. 2012, 30, 748–756. [Google Scholar] [CrossRef]
- Ren, Y.; Liu, Z.-Z.; Feng, J.; Wan, H.; Li, J.-H.; Wang, H.; Lin, X. Association of a BMP9 Haplotype with Ossification of the Posterior Longitudinal Ligament (OPLL) in a Chinese Population. PLoS ONE 2012, 7, e40587. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Y.; Chen, Y.; Shi, G.; Yuan, W. RUNX2 Polymorphisms Associated with OPLL and OLF in the Han Population. Clin. Orthop. Relat. Res. 2010, 468, 3333–3341. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, D.; Yang, Z.; Tian, B.; Li, J.; Meng, X.; Wang, Z.; Yang, H.; Lin, X. Association of bone morphogenetic protein-2 gene polymorphisms with susceptibility to ossification of the posterior longitudinal ligament of the spine and its severity in Chinese patients. Eur. Spine J. 2008, 17, 956–964. [Google Scholar] [CrossRef] [PubMed]
- Song, D.W.; Wu, Y.D.; Tian, D.D. Association of VDR-FokI and VDBP-Thr420Lys polymorphisms with cervical spondylotic myelopathy: A case-control study in the population of China. J. Clin. Lab. Anal. 2019, 33, e22669. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Shi, J.; Chen, X.; Xu, G.; Li, L.; Jia, L. The role of smoking status and collagen IX polymorphisms in the susceptibility to cervical spondylotic myelopathy. Genet. Mol. Res. 2012, 11, 1238–1244. [Google Scholar] [CrossRef] [PubMed]
- Maeda, S.; Ishidou, Y.; Koga, H.; Taketomi, E.; Ikari, K.; Komiya, S.; Takeda, J.; Sakou, T.; Inoue, I. Functional Impact of Human Collagen α2(XI) Gene Polymorphism in Pathogenesis of Ossification of the Posterior Longitudinal Ligament of the Spine. J. Bone Miner. Res. 2001, 16, 948–957. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X.; Zhang, X.; Ren, H.; Huang, B.; Chen, J.; Liu, J.; Shan, Z.; Zhu, Z.; Zhao, F. Low virulence bacterial infections in cervical intervertebral discs: A prospective case series. Eur. Spine J. 2018, 27, 2496–2505. [Google Scholar] [CrossRef] [PubMed]
- Bivona, L.J.; Camacho, J.E.; Usmani, F.; Nash, A.; Bruckner, J.J.; Hughes, M.; Bhandutia, A.K.; Koh, E.Y.; Banagan, K.E.; Gelb, D.E.; et al. The Prevalence of Bacterial Infection in Patients Undergoing Elective ACDF for Degenerative Cervical Spine Conditions: A Prospective Cohort Study With Contaminant Control. Glob. Spine J. 2021, 11, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Rajasekaran, S.; Soundararajan, D.C.R.; Tangavel, C.; Muthurajan, R.; Anand, K.S.S.V.; Matchado, M.S.; Nayagam, S.M.; Shetty, A.P.; Kanna, R.M.; Dharmalingam, K. Human intervertebral discs harbour a unique microbiome and dysbiosis determines health and disease. Eur. Spine J. 2020, 29, 1621–1640. [Google Scholar] [CrossRef]
- Nakashima, H.; Yukawa, Y.; Suda, K.; Yamagata, M.; Ueta, T.; Kato, F. Cervical Disc Protrusion Correlates With the Severity of Cervical Disc Degeneration: A Cross-Sectional Study of 1211 Relatively Healthy Volunteers. Spine 2015, 40, E774-9. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.E.; Bosworth, T.R.; Cribb, A.M.; Taylor, J.R. The chemical morphology of age-related changes in human intervertebral disc glycosaminoglycans from cervical, thoracic and lumbar nucleus pulposus and annulus fibrosus. J. Anat. 1994, 184 (Pt 1), 73–82. [Google Scholar]
- Zhu, W.; Sha, S.; Liu, Z.; Li, Y.; Xu, L.; Zhang, W.; Qiu, Y.; Zhu, Z. Influence of the Occipital Orientation on Cervical Sagittal Alignment: A Prospective Radiographic Study on 335 Normal Subjects. Sci. Rep. 2018, 8, 15336. [Google Scholar] [CrossRef]
- Kumaresan, S.; Yoganandan, N.; Pintar, F.A.; Maiman, D.J.; Goel, V.K. Contribution of disc degeneration to osteophyte formation in the cervical spine: A biomechanical investigation. J. Orthop. Res. 2001, 19, 977–984. [Google Scholar] [CrossRef]
- Friedenberg, Z.B.; Miller, W.T. Degenerative disc disease of the cervical spine. J. Bone Jt. Surg. Am. 1963, 45, 1171–1178. [Google Scholar] [CrossRef]
- Kartha, S.; Zeeman, M.E.; Baig, H.A.; Guarino, B.B.; Winkelstein, B.A. Upregulation of BDNF and NGF in Cervical Intervertebral Discs Exposed to Painful Whole-Body Vibration. Spine 2014, 39, 1542–1548. [Google Scholar] [CrossRef] [PubMed]
- Sitte, I.; Klosterhuber, M.; Lindtner, R.A.; Freund, M.C.; Neururer, S.B.; Pfaller, K.; Kathrein, A. Morphological changes in the human cervical intervertebral disc post trauma: Response to fracture-type and degeneration grade over time. Eur. Spine J. 2016, 25, 80–95. [Google Scholar] [CrossRef]
- Kepler, C.K.; Ponnappan, R.K.; Tannoury, C.A.; Risbud, M.V.; Anderson, D.G. The molecular basis of intervertebral disc degeneration. Spine J. 2013, 13, 318–330. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Li, S.; Geng, W.; Luo, R.; Liu, W.; Tu, J.; Wang, K.; Kang, L.; Yin, H.; Wu, X.; et al. Sirtuin 3-dependent mitochondrial redox homeostasis protects against AGEs-induced intervertebral disc degeneration. Redox Biol. 2018, 19, 339–353. [Google Scholar] [CrossRef] [PubMed]
- Molinos, M.; Almeida, C.R.; Caldeira, J.; Cunha, C.; Gonçalves, R.M.; Barbosa, M.A. Inflammation in intervertebral disc degeneration and regeneration. J. R. Soc. Interface 2015, 12, 20141191. [Google Scholar] [CrossRef]
- Bartanusz, V.; Jezova, D.; Alajajian, B.; Digicaylioglu, M. The blood-spinal cord barrier: Morphology and Clinical Implications. Ann. Neurol. 2011, 70, 194–206. [Google Scholar] [CrossRef]
- Lev, N.; Gilgun-Sherki, Y.; Offen, D.; Melamed, E. Chapter 13—The Role of Oxidative Stress in the Pathogenesis of Multiple Sclerosis: Current State. In Oxidative Stress and Neurodegenerative Disorders; Qureshi, G.A., Parvez, S.H., Eds.; Elsevier Science B.V.: Amsterdam, The Netherlands, 2007; pp. 283–295. [Google Scholar]
- Maikos, J.T.; Shreiber, D.I. Immediate Damage to The Blood-Spinal Cord Barrier Due to Mechanical Trauma. J. Neurotrauma 2007, 24, 492–507. [Google Scholar] [CrossRef]
- Cohen, D.M.; Patel, C.B.; Ahobila-Vajjula, P.; Sundberg, L.M.; Chacko, T.; Liu, S.-J.; Narayana, P.A. Blood-spinal cord barrier permeability in experimental spinal cord injury: Dynamic contrast-enhanced MRI. NMR Biomed. 2009, 22, 332–341. [Google Scholar] [CrossRef]
- Tian, D.-S.; Liu, J.-L.; Xie, M.-J.; Zhan, Y.; Qu, W.-S.; Yu, Z.-Y.; Tang, Z.-P.; Pan, D.-J.; Wang, W. Tamoxifen attenuates inflammatory-mediated damage and improves functional outcome after spinal cord injury in rats. J. Neurochem. 2009, 109, 1658–1667. [Google Scholar] [CrossRef] [PubMed]
- Blume, C.; Geiger, M.F.; Brandenburg, L.O.; Müller, M.; Mainz, V.; Kalder, J.; Albanna, W.; Clusmann, H.; Mueller, C.A. Patients with degenerative cervical myelopathy have signs of blood spinal cord barrier disruption, and its magnitude correlates with myelopathy severity: A prospective comparative cohort study. Eur. Spine J. 2020, 29, 986–993. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, N.; Oichi, T.; Kato, S.; Sato, Y.; Hasebe, H.; Hirai, S.; Taniguchi, Y.; Matsubayashi, Y.; Mori, H.; Tanaka, S.; et al. Spinal cord swelling in patients with cervical compression myelopathy. BMC Musculoskelet. Disord. 2019, 20, 284. [Google Scholar] [CrossRef]
- Oklinski, M.K.; Lim, J.-S.; Choi, H.-J.; Oklinska, P.; Skowronski, M.T.; Kwon, T.-H. Immunolocalization of Water Channel Proteins AQP1 and AQP4 in Rat Spinal Cord. J. Histochem. Cytochem. 2014, 62, 598–611. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, R.S.; Parker, J.; Syed, Y.A.; Edgley, S.A.; Young, A.; Fawcett, J.W.; Jeffery, N.D.; Franklin, R.J.M.; Kotter, M.R. Axonal plasticity underpins the functional recovery following surgical decompression in a rat model of cervical spondylotic myelopathy. Acta Neuropathol. Commun. 2016, 4, 89. [Google Scholar] [CrossRef] [PubMed]
- Salvadores, N.; Sanhueza, M.; Manque, P.; Court, F.A. Axonal Degeneration during Aging and Its Functional Role in Neurodegenerative Disorders. Front. Neurosci. 2017, 11, 451. [Google Scholar] [CrossRef]
- Adalbert, R.; Coleman, M.P. Review: Axon pathology in age-related neurodegenerative disorders. Neuropathol. Appl. Neurobiol. 2013, 39, 90–108. [Google Scholar] [CrossRef]
- Deckwerth, T.L.; Johnson, E.M. Neurites Can Remain Viable after Destruction of the Neuronal Soma by Programmed Cell Death (Apoptosis). Dev. Biol. 1994, 165, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, D.; Sargsyan, S.; Monk, P.N.; Shaw, P.J. Astrocyte function and role in motor neuron disease: A future therapeutic target? Glia 2009, 57, 1251–1264. [Google Scholar] [CrossRef]
- Anderson, M.A.; Burda, J.E.; Ren, Y.; Ao, Y.; O’Shea, T.M.; Kawaguchi, R.; Coppola, R.K.G.; Khakh, B.S.; Deming, T.J. Astrocyte scar formation aids central nervous system axon regeneration. Nature 2016, 532, 195–200. [Google Scholar] [CrossRef]
- Chung, W.-S.; Clarke, L.E.; Wang, G.X.; Stafford, B.K.; Sher, A.; Chakraborty, C.; Joung, J.; Foo, L.C.; Thompson, A.; Chen, C.; et al. Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature 2013, 504, 394–400. [Google Scholar] [CrossRef]
- Song, I.; Dityatev, A. Crosstalk between glia, extracellular matrix and neurons. Brain Res. Bull. 2018, 136, 101–108. [Google Scholar] [CrossRef]
- Yovich, J.V.; Gould, D.H.; LeCouteur, R. Chronic cervical compressive myelopathy in horses: Patterns of astrocytosis in the spinal cord. Aust. Vet. J. 1991, 68, 334. [Google Scholar] [CrossRef]
- Moon, E.S.; Karadimas, S.K.; Yu, W.-R.; Austin, J.W.; Fehlings, M.G. Riluzole attenuates neuropathic pain and enhances functional recovery in a rodent model of cervical spondylotic myelopathy. Neurobiol. Dis. 2014, 62, 394–406. [Google Scholar] [CrossRef]
- Ozawa, H.; Wu, Z.J.; Tanaka, Y.; Kokubun, S. Morphologic Change and Astrocyte Response to Unilateral Spinal Cord Compression in Rabbits. J. Neurotrauma 2004, 21, 944–955. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Barres, B.A. Reactive Astrocytes: Production, Function, and Therapeutic Potential. Immunity 2017, 46, 957–967. [Google Scholar] [CrossRef] [PubMed]
- Vidal, P.M.; Karadimas, S.K.; Ulndreaj, A.; Laliberte, A.M.; Tetreault, L.; Forner, S.; Wang, J.; Foltz, W.D.; Fehlings, M.G. Delayed decompression exacerbates ischemia-reperfusion injury in cervical compressive myelopathy. JCI Insight 2017, 2, 11. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Han, Q.; Chen, G.; Huang, Y.; Zhao, L.-X.; Berta, T.; Gao, Y.-J.; Qingjian, H. Toll-like receptor 4 contributes to chronic itch, alloknesis, and spinal astrocyte activation in male mice. Pain 2016, 157, 806–817. [Google Scholar] [CrossRef]
- Putatunda, R.; Hala, T.J.; Chin, J.; Lepore, A.C. Chronic at-level thermal hyperalgesia following rat cervical contusion spinal cord injury is accompanied by neuronal and astrocyte activation and loss of the astrocyte glutamate transporter, GLT1, in superficial dorsal horn. Brain Res. 2014, 1581, 64–79. [Google Scholar] [CrossRef] [PubMed]
- David, S.; Kroner, A. Repertoire of microglial and macrophage responses after spinal cord injury. Nat. Rev. Neurosci. 2011, 12, 388–399. [Google Scholar] [CrossRef]
- Fleming, J.C.; Norenberg, M.D.; Ramsay, D.A.; Dekaban, G.A.; Marcillo, A.E.; Saenz, A.D.; Pasquale-Styles, M.; Dietrich, W.D.; Weaver, L.C. The cellular inflammatory response in human spinal cords after injury. Brain 2006, 129, 3249–3269. [Google Scholar] [CrossRef]
- Harrison, J.K.; Jiang, Y.; Chen, S.; Xia, Y.; Maciejewski, D.; McNamara, R.K.; Streit, W.J.; Salafranca, M.N.; Adhikari, S.; Thompson, D.A.; et al. Role for neuronally derived fractalkine in mediating interactions between neurons and CX3CR1-expressing microglia. Proc. Natl. Acad. Sci. USA 1998, 95, 10896–10901. [Google Scholar] [CrossRef]
- Yamaura, I.; Yone, K.; Nakahara, S.; Nagamine, T.; Baba, H.; Uchida, K.; Komiya, S. Mechanism of Destructive Pathologic Changes in the Spinal Cord Under Chronic Mechanical Compression. Spine 2002, 27, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Fumagalli, S.; Perego, C.; Ortolano, F.; De Simoni, M.-G. CX3CR1 deficiency induces an early protective inflammatory environment in ischemic mice. Glia 2013, 61, 827–842. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Gan, Y.; Liu, Q.; Yin, J.-X.; Liu, Q.; Shi, J.; Shi, F.-D. CX3CR1 deficiency suppresses activation and neurotoxicity of microglia/macrophage in experimental ischemic stroke. J. Neuroinflamm. 2014, 11, 26. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, M. Microglia in the spinal cord and neuropathic pain. J. Diabetes Investig. 2016, 7, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Gwak, Y.S.; Hulsebosch, C.E. Remote astrocytic and microglial activation modulates neuronal hyperexcitability and below-level neuropathic pain after spinal injury in rat. Neuroscience 2009, 161, 895–903. [Google Scholar] [CrossRef]
- Juurlink, B.H.J.; Thorburne, S.K.; Hertz, L. Peroxide-scavenging deficit underlies oligodendrocyte susceptibility to oxidative stress. Glia 1998, 22, 371–378. [Google Scholar] [CrossRef]
- Bai, L.; Lennon, D.P.; Caplan, A.I.; DeChant, A.; Hecker, J.; Kranso, J.; Zaremba, A.; Miller, R.H. Hepatocyte growth factor mediates mesenchymal stem cell–induced recovery in multiple sclerosis models. Nat. Neurosci. 2012, 15, 862–870. [Google Scholar] [CrossRef]
- Kim, B.S.; Jin, Y.-H.; Meng, L.; Hou, W.; Kang, H.S.; Park, H.S.; Koh, C.-S. IL-1 signal affects both protection and pathogenesis of virus-induced chronic CNS demyelinating disease. J. Neuroinflamm. 2012, 9, 217. [Google Scholar] [CrossRef] [PubMed]
- Giacoppo, S.; Galuppo, M.; Iori, R.; De Nicola, G.R.; Cassata, G.; Bramanti, P.; Mazzon, E. Protective Role of (RS)-glucoraphanin Bioactivated with Myrosinase in an Experimental Model of Multiple Sclerosis. CNS Neurosci. Ther. 2013, 19, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-Q.; Tang, C.-L.; Sun, S.-Q.; Yang, C.; Xu, J.; Wang, K.-J.; Lu, W.-T.; Huang, J.; Zhuo, F.; Qiu, G.-P.; et al. Demyelination Initiated by Oligodendrocyte Apoptosis through Enhancing Endoplasmic Reticulum–Mitochondria Interactions and Id2 Expression after Compressed Spinal Cord Injury in Rats. CNS Neurosci. Ther. 2014, 20, 20–31. [Google Scholar] [CrossRef]
- McGavern, D.B.; Murray, P.D.; Rodriguez, M. Quantitation of spinal cord demyelination, remyelination, atrophy, and axonal loss in a model of progressive neurologic injury. J. Neurosci. Res. 1999, 58, 492–504. [Google Scholar] [CrossRef]
- Lassmann, H.; Bradl, M. Multiple sclerosis: Experimental models and reality. Acta Neuropathol. 2017, 133, 223–244. [Google Scholar] [CrossRef] [PubMed]
- Ackery, A.; Robins, S.; Fehlings, M.G. Inhibition of Fas-Mediated Apoptosis through Administration of Soluble Fas Receptor Improves Functional Outcome and Reduces Posttraumatic Axonal Degeneration after Acute Spinal Cord Injury. J. Neurotrauma 2006, 23, 604–616. [Google Scholar] [CrossRef] [PubMed]
- Emery, E.; Aldana, P.; Bunge, M.B.; Puckett, W.; Srinivasan, A.; Keane, R.W.; Bethea, J.; Levi, A.D.O. Apoptosis after traumatic human spinal cord injury. J. Neurosurg. 1998, 89, 911–920. [Google Scholar] [CrossRef]
- Liu, H.; MacMillian, E.L.; Jutzeler, C.R.; Ljungberg, E.; MacKay, A.L.; Kolind, S.H.; Mädler, B.; Li, D.K.B.; Dvorak, M.F.; Curt, A.; et al. Assessing structure and function of myelin in cervical spondylotic myelopathy: Evidence of demyelination. Neurology 2017, 89, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Yu, H.-J.; Gan, S.-W.; Gong, R.; Mou, K.-J.; Xue, J.; Sun, S.-Q. p53-Mediated oligodendrocyte apoptosis initiates demyelination after compressed spinal cord injury by enhancing ER-mitochondria interaction and E2F1 expression. Neurosci. Lett. 2017, 644, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Iwasawa, R.; Mahul-Mellier, A.-L.; Datler, C.; Pazarentzos, E.; Grimm, S. Fis1 and Bap31 bridge the mitochondria-ER interface to establish a platform for apoptosis induction. EMBO J. 2011, 30, 556–568. [Google Scholar] [CrossRef]
- Palam, L.R.; Baird, T.D.; Wek, R.C. Phosphorylation of eIF2 Facilitates Ribosomal Bypass of an Inhibitory Upstream ORF to Enhance CHOP Translation. J. Biol. Chem. 2011, 286, 10939–10949. [Google Scholar] [CrossRef]
- Lebeaupin, C.; Proics, E.; De Bieville, C.H.D.; Rousseau, D.; Bonnafous, S.; Patouraux, S.; Adam, G.; Lavallard, V.J.; Rovere, C.; Le Thuc, O.; et al. ER stress induces NLRP3 inflammasome activation and hepatocyte death. Cell Death Dis. 2015, 6, e1879. [Google Scholar] [CrossRef]
- Lee, Y.B.; Yune, T.Y.; Baik, S.Y.; Shin, Y.H.; Dua, S.; Rhimb, H.; Lee, E.B.; Kim, Y.C.; Shin, M.L.; Markelonis, G.J.; et al. Role of Tumor Necrosis Factor-α in Neuronal and Glial Apoptosis after Spinal Cord Injury. Exp. Neurol. 2000, 166, 190–195. [Google Scholar] [CrossRef]
- Takenouchi, T.; Setoguchi, T.; Yone, K.; Komiya, S. Expression of apoptosis signal-regulating kinase 1 in mouse spinal cord under chronic mechanical compression: Possible involvement of the stress-activated mitogen-activated protein kinase pathways in spinal cord cell apoptosis. Spine 2008, 33, 1943–1950. [Google Scholar] [CrossRef]
- Ye, P.; Kollias, G.; D’Ercole, A.J. Insulin-like growth factor-I ameliorates demyelination induced by tumor necrosis factor-α in transgenic mice. J. Neurosci. Res. 2007, 85, 712–722. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.G.; Muruve, D.A.; Power, C. Inflammasomes in the CNS. Nat. Rev. Neurosci. 2014, 15, 84–97. [Google Scholar] [CrossRef]
- Abais, J.M.; Xia, M.; Zhang, Y.; Boini, K.M.; Li, P.-L. Redox Regulation of NLRP3 Inflammasomes: ROS as Trigger or Effector? Antioxid. Redox Signal. 2015, 22, 1111–1129. [Google Scholar] [CrossRef]
- Bononi, A.; Pinton, P. Study of PTEN subcellular localization. Methods 2015, 77–78, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Y.-B.; Giffard, R.G. ER-Mitochondria Crosstalk during Cerebral Ischemia: Molecular Chaperones and ER-Mitochondrial Calcium Transfer. Int. J. Cell Biol. 2012, 2012, 493934. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, M.B.; Malmqvist, T.; Clarke, E.; Hubens, C.J.; Grist, J.; Hobbs, C.; Trigo, D.; Risling, M.; Angeria, M.; Damberg, P.; et al. Neuronal RARβ Signaling Modulates PTEN Activity Directly in Neurons and via Exosome Transfer in Astrocytes to Prevent Glial Scar Formation and Induce Spinal Cord Regeneration. J. Neurosci. 2015, 35, 15731–15745. [Google Scholar] [CrossRef]
- Harrington, E.P.; Zhao, C.; Fancy, S.P.; Kaing, S.; Franklin, R.J.; Rowitch, D.H. Oligodendrocyte PTEN is required for myelin and axonal integrity, not remyelination. Ann. Neurol. 2010, 68, 703–716. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Holly, L.T.; Albistegui-DuBois, R.; Yan, X.; Marehbian, J.; Newton, J.M.; Dobkin, B.H. Compensatory cerebral adaptations before and evolving changes after surgical decompression in cervical spondylotic myelopathy. J. Neurosurg. Spine 2008, 9, 538–551. [Google Scholar] [CrossRef]
- Tam, S.; Barry, R.L.; Bartha, R.; Duggal, N. Changes in functional magnetic resonance imaging cortical activation after decompression of cervical spondylosis: Case report. Neurosurgery 2010, 67, E863-4, Discussion E864. [Google Scholar] [CrossRef]
- Holly, L.T.; Dong, Y.; Albistegui-DuBois, R.; Marehbian, J.; Dobkin, B. Cortical reorganization in patients with cervical spondylotic myelopathy. J. Neurosurg. Spine 2007, 6, 544–551. [Google Scholar] [CrossRef]
- Duggal, N.; Rabin, D.; Bartha, R.; Barry, R.L.; Gati, J.S.; Kowalczyk, I.; Fink, M. Brain reorganization in patients with spinal cord compression evaluated using fMRI. Neurology 2010, 74, 1048–1054. [Google Scholar] [CrossRef] [PubMed]
- Craciunas, S.C.; Gorgan, M.R.; Ianosi, B.; Lee, P.; Burris, J.; Cirstea, C.M. Remote motor system metabolic profile and surgery outcome in cervical spondylotic myelopathy. J. Neurosurg. Spine 2017, 26, 668–678. [Google Scholar] [CrossRef]
- Kadaňka, Z.; Bednarik, J.; Novotný, O.; Urbánek, I.; Dušek, L. Cervical spondylotic myelopathy: Conservative versus surgical treatment after 10 years. Eur. Spine J. 2011, 20, 1533–1538. [Google Scholar] [CrossRef] [PubMed]
- Tetreault, L.A.; Rhee, J.; Prather, H.; Kwon, B.K.; Wilson, J.R.; Martin, A.R.; Andersson, I.B.; Dembek, A.H.; Pagarigan, K.T.; Dettori, J.R.; et al. Change in Function, Pain, and Quality of Life Following Structured Nonoperative Treatment in Patients With Degenerative Cervical Myelopathy: A Systematic Review. Glob. Spine J. 2017, 7 (Suppl. 3), 42S–52S. [Google Scholar] [CrossRef]
- Zhang, Y.; Bhavnani, B.R. Glutamate-induced apoptosis in neuronal cells is mediated via caspase-dependent and independent mechanisms involving calpain and caspase-3 proteases as well as apoptosis inducing factor (AIF) and this process is inhibited by equine estrogens. BMC Neurosci. 2006, 7, 49. [Google Scholar] [CrossRef] [PubMed]
- Perez, E.; Wang, X.; Simpkins, J.W. Chapter 22—Role of Antioxidant Activity of Estrogens in their Potent Neuroprotection. In Oxidative Stress and Neurodegenerative Disorders; Qureshi, G.A., Parvez, S.H., Eds.; Elsevier Science B.V.: Amsterdam, The Netherlands, 2007; pp. 503–524. [Google Scholar]
- Miranda, J.D.; Colón, J.M. Tamoxifen: An FDA approved drug with neuroprotective effects for spinal cord injury recovery. Neural Regen. Res. 2016, 11, 1208–1211. [Google Scholar] [CrossRef] [PubMed]
- Ha, K.-Y.; Kim, Y.-H.; Rhyu, K.-W.; Kwon, S.-E. Pregabalin as a neuroprotector after spinal cord injury in rats. Eur. Spine J. 2008, 17, 864–872. [Google Scholar] [CrossRef] [PubMed]
- Onakpoya, I.J.; Thomas, E.T.; Lee, J.J.; Goldacre, B.; Heneghan, C.J. Benefits and harms of pregabalin in the management of neuropathic pain: A rapid review and meta-analysis of randomised clinical trials. BMJ Open 2019, 9, e023600. [Google Scholar] [CrossRef] [PubMed]
- Abduljabbar, F.H.; Teles, A.R.; Bokhari, R.; Weber, M.; Santaguida, C. Laminectomy with or Without Fusion to Manage Degenerative Cervical Myelopathy. Neurosurg. Clin. N. Am. 2018, 29, 91–105. [Google Scholar] [CrossRef] [PubMed]
- Pal, G.P.; Sherk, H.H. The Vertical Stability of the Cervical Spine. Spine 1988, 13, 447–449. [Google Scholar] [CrossRef] [PubMed]
- Manzano, G.R.; Casella, G.; Wang, M.Y.; Vanni, S.; Levi, A.D. A Prospective, Randomized Trial Comparing Expansile Cervical Laminoplasty and Cervical Laminectomy and Fusion for Multilevel Cervical Myelopathy. Neurosurgery 2012, 70, 264–277. [Google Scholar] [CrossRef] [PubMed]
- Bartels, R.H.; Groenewoud, H.; Peul, W.C.; Arts, M.P. Lamifuse: Results of a randomized controlled trial comparing laminectomy without and with fusion for cervical spondylotic myelopathy. J. Neurosurg. Sci. 2017, 61, 134–139. [Google Scholar] [PubMed]
- Chiaki, H.; Seisuke, T. Bilateral multilevel laminectomy with or without posterolateral fusion for cervical spondylotic myelopathy: Relationship to type of onset and time until operation. J. Neurosurg. 1996, 85, 447–451. [Google Scholar]
- Denaro, V.; Di Martino, A. Cervical Spine Surgery: An Historical Perspective. Clin. Orthop. Relat. Res. 2011, 469, 639–648. [Google Scholar] [CrossRef]
- Wilson, J.R.; Tetreault, L.A.; Kim, J.; Shamji, M.F.; Harrop, J.S.; Mroz, T.; Cho, S.; Fehlings, M.G. State of the Art in Degenerative Cervical Myelopathy: An Update on Current Clinical Evidence. Neurosurgery 2017, 80, S33–S45. [Google Scholar] [CrossRef]
- Hirano, Y.; Ohara, Y.; Mizuno, J.; Itoh, Y. History and Evolution of Laminoplasty. Neurosurg. Clin. N. Am. 2018, 29, 107–113. [Google Scholar] [CrossRef]
- Fehlings, M.G.; Santaguida, C.; Tetreault, L.; Arnold, P.; Barbagallo, G.; Defino, H.; Kale, S.; Zhou, Q.; Yoon, T.S.; Kopjar, B. Laminectomy and fusion versus laminoplasty for the treatment of degenerative cervical myelopathy: Results from the AOSpine North America and International prospective multicenter studies. Spine J. 2017, 17, 102–108. [Google Scholar] [CrossRef]
- Cheung, W.Y.; Arvinte, D.; Wong, Y.W.; Luk, K.D.K.; Cheung, K.M.C. Neurological recovery after surgical decompression in patients with cervical spondylotic myelopathy—A prospective study. Int. Orthop. 2008, 32, 273–278. [Google Scholar] [CrossRef]
- Karadimas, S.K.; Laliberte, A.M.; Tetreault, L.; Chung, Y.S.; Arnold, P.; Foltz, W.D.; Fehlings, M.G. Riluzole blocks perioperative ischemia-reperfusion injury and enhances postdecompression outcomes in cervical spondylotic myelopathy. Sci. Transl. Med. 2015, 7, 316ra194. [Google Scholar] [CrossRef]
- Smith, P.D.; Puskas, F.; Meng, X.; Lee, J.H.; Cleveland, J.C.; Weyant, M.J.; Fullerton, D.; Reece, T.B. The Evolution of Chemokine Release Supports a Bimodal Mechanism of Spinal Cord Ischemia and Reperfusion Injury. Circulation 2012, 126 (Suppl. 1), S110–S117. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Hallenbeck, J.M.; Ruetzler, C.; Bol, D.; Thomas, K.; Berman, N.E.J.; Vogel, S.N. Overexpression of Monocyte Chemoattractant Protein 1 in the Brain Exacerbates Ischemic Brain Injury and is Associated with Recruitment of Inflammatory Cells. J. Cereb. Blood Flow Metab. 2003, 23, 748–755. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Dong, Q.; Lyu, B.; Wang, J.; Quan, Y.; Gong, S. The expression of bradykinin and its receptors in spinal cord ischemia-reperfusion injury rat model. Life Sci. 2019, 218, 340–345. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tu, J.; Vargas Castillo, J.; Das, A.; Diwan, A.D. Degenerative Cervical Myelopathy: Insights into Its Pathobiology and Molecular Mechanisms. J. Clin. Med. 2021, 10, 1214. https://doi.org/10.3390/jcm10061214
Tu J, Vargas Castillo J, Das A, Diwan AD. Degenerative Cervical Myelopathy: Insights into Its Pathobiology and Molecular Mechanisms. Journal of Clinical Medicine. 2021; 10(6):1214. https://doi.org/10.3390/jcm10061214
Chicago/Turabian StyleTu, Ji, Jose Vargas Castillo, Abhirup Das, and Ashish D. Diwan. 2021. "Degenerative Cervical Myelopathy: Insights into Its Pathobiology and Molecular Mechanisms" Journal of Clinical Medicine 10, no. 6: 1214. https://doi.org/10.3390/jcm10061214
APA StyleTu, J., Vargas Castillo, J., Das, A., & Diwan, A. D. (2021). Degenerative Cervical Myelopathy: Insights into Its Pathobiology and Molecular Mechanisms. Journal of Clinical Medicine, 10(6), 1214. https://doi.org/10.3390/jcm10061214