Vertigo in Patients with Degenerative Cervical Myelopathy
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
2.2. Participants
- Patients with previous surgery on the cervical spine (possibly limiting the rotation of the spine);
- Patients with other than degenerative cervical cord compressions or other non-compressive myelopathies.
2.3. Clinical Evaluation
- Gait disturbance;
- Numb and/or clumsy hands;
- Lhermitte’s phenomenon;
- Bilateral arm paresthesias;
- Weakness of lower or upper extremities;
- Urinary urgency, or incontinence.
- Corticospinal tract signs;
- Hyperreflexia/clonus;
- Spasticity;
- Pyramidal signs (Babinski reflex or Hoffman’s sign);
- Spastic paresis of any of the extremities (most frequently, lower spastic paraparesis);
- Flaccid paresis of one or two upper extremities;
- Atrophy of the hand muscles;
- Sensory involvement in various distributions in upper or lower extremities;
- Gait ataxia.
- Age;
- Sex.
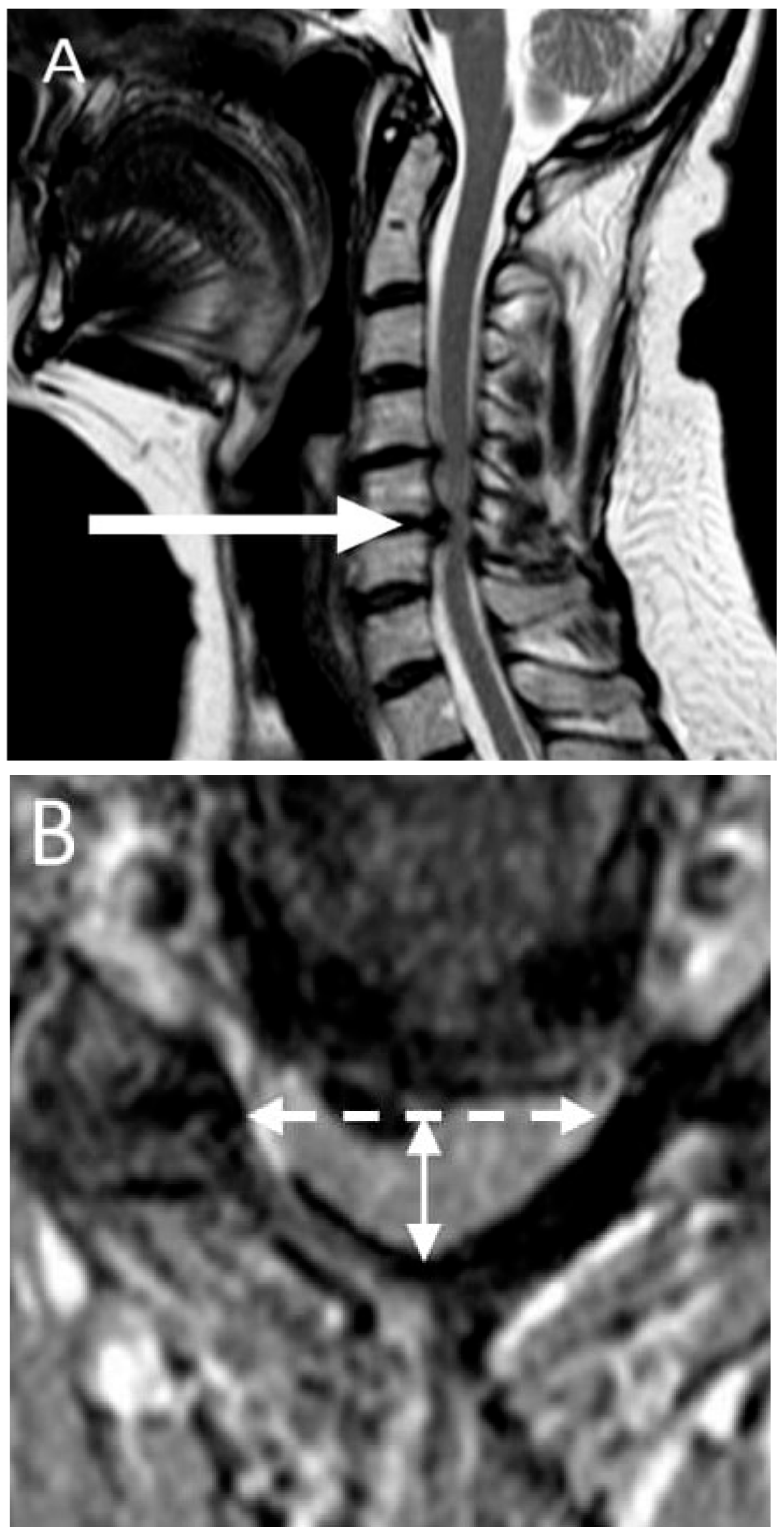
2.4. Imaging
2.5. Vertigo Questionnaire
2.6. Uncontrolled Blood Pressure
2.7. Orthostatic Hypotension
2.8. Benign Paroxysmal Positional Vertigo
2.9. Ultrasound of Carotid and Vertebral Arteries
2.10. Cervical Torsion Test
3. Results
3.1. Study Cohort
3.2. Dizziness/Vertigo
4. Discussion
Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kovacs, E.; Wang, X.; Grill, E. Economic burden of vertigo: A systematic review. Health Econ. Rev. 2019, 9, 37. [Google Scholar] [CrossRef] [PubMed]
- Thompson-Harvey, A.; Hain, T.C. Symptoms in cervical vertigo. Laryngoscope 2019, 4, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Reiley, A.S.; Vickory, F.M.; Funderburg, S.E.; Cesario, R.A.; Clendaniel, R.A. How to diagnose cervicogenic dizziness. Arch. Physiother. 2017, 7, 12. [Google Scholar] [CrossRef]
- Colledge, N.R.; Barr-Hamilton, R.M.; Lewis, S.J.; Sellar, R.J.; Wilson, J.A. Evaluation of investigations to diagnose the cause of dizziness in elderly people: A community based controlled study. BMJ 1996, 313, 788–792. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S. Importance of cervicogenic general dizziness. J. Rural Med. 2018, 13, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Strupp, M.; Dlugaiczyk, J.; Ertl-Wagner, B.B.; Rujescu, D.; Westhofen, M.; Dieterich, M. Vestibular Disorders. Dtsch. Aerzteblatt Online 2020, 117, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Milligan, J.; Ryan, K.; Fehlings, M.; Bauman, C. Degenerative Cervical Myelopathy. Can. Fam. Physician 2019, 65, 619–624. [Google Scholar]
- Kalsi-Ryan, S.; Karadimas, S.K.; Fehlings, M.G. Cervical Spondylotic Myelopathy. Neuroscientist 2012, 19, 409–421. [Google Scholar] [CrossRef]
- Martin, A.R.; De Leener, B.; Cohen-Adad, J.; Cadotte, D.W.; Nouri, A.; Wilson, J.R.; Tetreault, L.; Crawley, A.P.; Mikulis, D.J.; Ginsberg, H.; et al. Can microstructural MRI detect subclinical tissue injury in subjects with asymptomatic cervical spinal cord compression? A prospective cohort study. BMJ Open 2018, 8, e019809. [Google Scholar] [CrossRef]
- Tetreault, L.; Kopjar, B.; Nouri, A.; Arnold, P.; Barbagallo, G.; Bartels, R.; Qiang, Z.; Singh, A.; Zileli, M.; Vaccaro, A.; et al. The modified Japanese Orthopaedic Association scale: Establishing criteria for mild, moderate and severe impairment in patients with degenerative cervical myelopathy. Eur. Spine J. 2016, 26, 78–84. [Google Scholar] [CrossRef]
- Kadanka, Z.; Adamova, B.; Keřkovský, M.; Dusek, L.; Jurová, B.; Vlckova, E.; Bednarik, J. Predictors of symptomatic myelopathy in degenerative cervical spinal cord compression. Brain Behav. 2017, 7, e00797. [Google Scholar] [CrossRef]
- Strupp, M.; Dieterich, M.; Brandt, T. The Treatment and Natural Course of Peripheral and Central Vertigo. Dtsch. Aerzteblatt Online 2013, 110, 505–516. [Google Scholar] [CrossRef]
- Filippopulos, F.M.; Albers, L.; Straube, A.; Gerstl, L.; Blum, B.; Langhagen, T.; Jahn, K.; Heinen, F.; Von Kries, R.; Landgraf, M.N. Vertigo and dizziness in adolescents: Risk factors and their population attributable risk. PLoS ONE 2017, 12, e0187819. [Google Scholar] [CrossRef]
- Freeman, R.; Wieling, W.; Axelrod, F.B.; Benditt, D.G.; Benarroch, E.E.; Biaggioni, I.; Cheshire, W.; Chelimsky, T.C.; Cortelli, P.; Gibbons, C.H.; et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin. Auton. Res. 2011, 21, 69–72. [Google Scholar] [CrossRef]
- Bhattacharyya, N.; Gubbels, S.P.; Schwartz, S.R.; Edlow, J.A.; El-Kashlan, H.; Fife, T.; Holmberg, J.M.; Mahoney, K.; Hollingsworth, D.B.; Roberts, R.; et al. Clinical Practice Guideline: Benign Paroxysmal Positional Vertigo (Update). Otolaryngol. Neck Surg. 2017, 156, S1–S47. [Google Scholar] [CrossRef]
- Warlow, C. MRC European Carotid Surgery Trial: Interim results for symptomatic patients with severe (70–99%) or with mild (0–29%) carotid stenosis. Lancet 1991, 337, 1235–1243. [Google Scholar] [CrossRef]
- Cloud, G.; Markus, H. Diagnosis and management of vertebral artery stenosis. Qjm Int. J. Med. 2003, 96, 27–54. [Google Scholar] [CrossRef]
- Koch, S.; Bustillo, A.J.; Campo, B.; Campo, N.; Campo-Bustillo, I.; McClendon, M.S.; Katsnelson, M.; Romano, J.G. Prevalence of vertebral artery origin stenosis and occlusion in outpatient extracranial ultrasonography. J. Vasc. Interv. Neurol. 2014, 7, 29–33. [Google Scholar]
- L’Heureux-Lebeau, B.; Godbout, A.; Berbiche, D.; Saliba, I. Evaluation of Paraclinical Tests in the Diagnosis of Cervicogenic Dizziness. Otol. Neurotol. 2014, 35, 1858–1865. [Google Scholar] [CrossRef]
- Reddy, R.S.; Tedla, J.S.; Dixit, S.; Abohashrh, M. Cervical proprioception and its relationship with neck pain intensity in subjects with cervical spondylosis. BMC Musculoskelet. Disord. 2019, 20, 447. [Google Scholar] [CrossRef]
- Dieterich, M.; Brandt, T. Perception of Verticality and Vestibular Disorders of Balance and Falls. Front. Neurol. 2019, 10, 172. [Google Scholar] [CrossRef] [PubMed]
- Radtke, A.; Lempert, T.; Von Brevern, M.; Feldmann, M.; Lezius, F.; Neuhauser, H. Prevalence and complications of orthostatic dizziness in the general population. Clin. Auton. Res. 2011, 21, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Moreira, M.D.; Trelha, C.S.; Marchiori, L.L.D.M.; Lopes, A.R. Association between complaints of dizziness and hypertension in non-institutionalized elders. Int. Arch. Otorhinolaryngol. 2014, 17, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Deng, Y.; Zhang, T.; Wang, M. Clinical characteristics and treatment outcomes for benign paroxysmal positional vertigo comorbid with hypertension. Acta Oto-Laryngol. 2016, 137, 482–484. [Google Scholar] [CrossRef]
- Xue, H.; Chong, Y.; Jiang, Z.D.; Liu, Z.L.; Ding, L.; Yang, S.L.; Wang, L.; Xiang, W.P. Etiological analysis on patients with vertigo or dizziness. Chin. Med. J. 2018, 98, 1227–1230. [Google Scholar]
- Kim, H.A.; Bisdorff, A.; Bronstein, A.M.; Lempert, T.; Rossi-Izquierdo, M.; Staab, J.P.; Strupp, M.; Kim, J.-S. Hemodynamic orthostatic dizziness/vertigo: Diagnostic criteria. J. Vestib. Res. 2019, 29, 45–56. [Google Scholar] [CrossRef]
- Britt, C.J.; Ward, B.K.; Owusu, Y.; Friedland, D.; Russell, J.O.; Weinreich, H.M. Assessment of a Statistical Algorithm for the Prediction of Benign Paroxysmal Positional Vertigo. JAMA Otolaryngol. Neck Surg. 2018, 144, 883. [Google Scholar] [CrossRef]
- Dieterich, M.; Staab, J. Functional dizziness: From phobic postural vertigo and chronic subjective dizziness to persistent postural-perceptual dizziness. Curr. Opin. Neurol. 2017, 30, 107–113. [Google Scholar] [CrossRef]
- Bécares-Martínez, C.; López-Llames, A.; Martín-Pagán, A.; Cores-Prieto, A.E.; Arroyo-Domingo, M.; Marco-Algarra, J.; Morales-Suárez-Varela, M. Cervical spine radiographs in patients with vertigo and dizziness. Radiol. Med. 2019, 125, 272–279. [Google Scholar] [CrossRef]
- Judy, B.F.; Theodore, N. Bow Hunter’s Syndrome. World Neurosurg. 2021, 148, 127–128. [Google Scholar] [CrossRef]
- Chu, E.C.P.; Chin, W.L.; Bhaumik, A. Cervicogenic dizziness. Oxf. Med Case Rep. 2019, 2019, 476–478. [Google Scholar] [CrossRef]
- De Jong, P.T.V.M.; De Jong, J.M.B.V.; Cohen, B.; Jongkees, L.B.W. Ataxia and nystagmus induced by injection of local anesthetics in the neck. Ann. Neurol. 1977, 1, 240–246. [Google Scholar] [CrossRef]
- Liu, X.-M.; Pan, F.-M.; Yong, Z.-Y.; Ba, Z.-Y.; Wang, S.-J.; Liu, Z.; Zhao, W.-D.; Wu, D.-S. Does the longus colli have an effect on cervical vertigo? Med. 2017, 96, e6365. [Google Scholar] [CrossRef]
- Rendon, R.; Mannoia, K.; Reiman, S.; Hitchman, L.; Shutze, W. Rotational vertebral artery occlusion secondary to completely extraosseous vertebral artery. J. Vasc. Surg. Cases Innov. Tech. 2019, 5, 14–17. [Google Scholar] [CrossRef]
- Simon, H.; Niederkorn, K.; Horner, S.; Duft, M.; Schröckenfuchs, M. Effect of head rotation on the vertebrobasilar system. A transcranial Doppler ultrasound contribution to the physiology. HNO 1994, 42, 614–618. [Google Scholar]
- Wang, Z.; Wang, X.; Yuan, W.; Jiang, D. Degenerative pathological irritations to cervical PLL may play a role in presenting sympathetic symptoms. Med. Hypotheses 2011, 77, 921–923. [Google Scholar] [CrossRef]
- Bayrak, I.K.; Durmus, D.; Bayrak, A.O.; Diren, B.; Canturk, F.; Diren, H.B. Effect of cervical spondylosis on vertebral arterial flow and its association with vertigo. Clin. Rheumatol. 2008, 28, 59–64. [Google Scholar] [CrossRef]
- Chandratheva, A.; Werring, D.; Kaski, D. Vertebrobasilar insufficiency: An insufficient term that should be retired. Pract. Neurol. 2020, 2–3. [Google Scholar] [CrossRef]

| Patients No | Gender | Age | mJOA Score | Maximum Cervical Cord Compression Level | Signs of Myelopathy on MRI | CR | ICA Stenosis | VA Stenosis |
|---|---|---|---|---|---|---|---|---|
| 1 | F | 46 | 17 | C5/6 | no | 0.31 | no | none |
| 2 | M | 44 | 17 | C4/5 | no | 0.3 | no | none |
| 3 | F | 60 | 17 | C6/7 | yes | 0.32 | no | none |
| 4 | F | 60 | 16 | C6/7 | no | 0.37 | no | none |
| 5 | F | 51 | 17 | C4/5 | no | 0.44 | no | none |
| 6 | M | 43 | 16 | C4/5 | no | 0.45 | no | none |
| 7 | M | 71 | 15 | C5/6 | yes | 0.35 | yes | none |
| 8 | M | 60 | 17 | C4/5 | no | 0.3 | no | none |
| 9 | M | 65 | 16 | C3/4 | yes | 0.4 | no | none |
| 10 | M | 51 | 16 | C5/6 | yes | 0.43 | no | none |
| 11 | M | 65 | 17 | C5/6 | no | 0.36 | no | none |
| 12 | F | 50 | 17 | C5/6 | no | 0.36 | no | none |
| 13 | F | 63 | 11 | C5/6 | yes | 0.36 | yes | none |
| 14 | F | 71 | 16 | C5/6 | no | 0.41 | no | none |
| 15 | M | 58 | 16 | C4/5 | no | 0.43 | no | none |
| 16 | F | 69 | 12 | C4/5 | yes | 0.39 | no | none |
| 17 | M | 60 | 15 | C6/7 | yes | 0.42 | no | none |
| 18 | F | 59 | 16 | C6/7 | no | 0.40 | no | none |
| 19 | M | 63 | 17 | C5/6 | yes | 0.42 | no | none |
| 20 | M | 52 | 16 | C5/6 | no | 0.28 | no | none |
| 21 | M | 69 | 15 | C5/6 | no | 0.3 | no | none |
| 22 | F | 57 | 16 | C5/6 | yes | 0.38 | no | none |
| 23 | M | 82 | 17 | C6/7 | no | 0.36 | no | none |
| 24 | F | 59 | 15 | C5/6 | yes | 0.36 | no | none |
| 25 | M | 67 | 13 | C5/6 | yes | 0.49 | yes | none |
| 26 | M | 64 | 15 | C5/6 | no | 0.41 | no | none |
| 27 | M | 45 | 17 | C3/4 | no | 0.37 | no | none |
| 28 | M | 77 | 9 | C4/5 | yes | 0.41 | no | none |
| 29 | F | 40 | 17 | C5/6 | no | 0.43 | no | none |
| 30 | M | 59 | 17 | C5/6 | yes | 0.44 | no | none |
| 31 | F | 51 | 17 | C5/6 | no | 0.39 | no | none |
| 32 | M | 48 | 15 | C5/6 | yes | 0.21 | no | none |
| 33 | F | 48 | 17 | C5/6 | no | 0.23 | no | none |
| 34 | F | 59 | 17 | C5/6 | no | 0.33 | no | none |
| 35 | F | 48 | 17 | C4/5 | yes | 0.23 | no | none |
| 36 | F | 52 | 17 | C4/5 | no | 0.44 | no | none |
| 37 | M | 58 | 16 | C5/6 | yes | 0.38 | no | none |
| 38 | M | 68 | 17 | C5/6 | no | 0.39 | no | none |
| Patients No | Type of Vertigo | Vertigo According to Body Movement | Cervical Torsion Test | Hallpike Test | Drop in BP ≥ 20/10 mmHg after at Least 3 min of Standing | Upright Tilt Table Test | Uncontrolled AH Detection | Final Aetiology of Dizziness |
|---|---|---|---|---|---|---|---|---|
| 1 | none | none | negative | negative | No | NA | NA | |
| 2 | none | none | negative | negative | No | NA | NA | |
| 3 | none | none | negative | negative | No | NA | NA | |
| 4 | none | none | negative | negative | No | NA | NA | |
| 5 | unspecified dizziness | also present when sitting or lying down | negative | negative | No | NA | 24 h monitoring | uncontrolled AH |
| 6 | none | none | negative | negative | No | NA | NA | |
| 7 | spinning | also present when sitting or lying down | negative | negative | No | NA | self-measurement | uncontrolled AH |
| 8 | none | none | negative | negative | No | NA | NA | |
| 9 | none | none | negative | negative | No | NA | NA | |
| 10 | blackout when standing | triggered by a change of position | negative | negative | Yes | NA | orthostatic vertigo | |
| 11 | none | none | negative | negative | No | NA | NA | |
| 12 | blackout when standing | triggered by a change of position | negative | negative | No | positive | orthostatic vertigo | |
| 13 | swaying | only present when standing or walking | negative | negative | No | NA | 24 h monitoring | uncontrolled AH |
| 14 | blackout when standing | triggered by a change of position | negative | negative | Yes | NA | orthostatic vertigo | |
| 15 | none | none | negative | negative | No | NA | NA | |
| 16 | swaying | also present when sitting or lying down | negative | negative | No | NA | psychogenic vertigo | |
| 17 | none | none | negative | negative | No | NA | NA | |
| 18 | swaying | only present when standing or walking | negative | negative | No | NA | self-measurement | uncontrolled AH |
| 19 | none | none | negative | negative | No | NA | NA | |
| 20 | none | none | negative | negative | No | NA | NA | |
| 21 | blackout when standing | triggered by a change of position | negative | negative | Yes | NA | orthostatic vertigo | |
| 22 | none | none | negative | positive | No | NA | NA | |
| 23 | blackout when standing | triggered by a change of position | negative | negative | Yes | NA | orthostatic vertigo | |
| 24 | spinning | triggered by head movement | negative | positive | No | NA | BPPV | |
| 25 | blackout when standing | triggered by a change of position | negative | negative | Yes | NA | orthostatic vertigo | |
| 26 | blackout when standing | triggered by a change of position | negative | negative | Yes | NA | orthostatic vertigo | |
| 27 | none | none | negative | negative | No | NA | NA | |
| 28 | spinning | trigered by head movement | negative | positive | No | NA | BPPV | |
| 29 | none | none | negative | negative | No | NA | NA | |
| 30 | none | none | negative | negative | No | NA | NA | |
| 31 | spinning | triggered by head movement | negative | positive | No | NA | BPPV | |
| 32 | blackout when standing | trigered by a change of position | negative | negative | No | positive | orthostatic vertigo | |
| 33 | spinning | triggered by head movement | negative | positive | No | NA | BPPV | |
| 34 | swaying | only present when standing or walking | negative | negative | No | NA | self-measurement | uncompensated AH |
| 35 | none | none | negative | negative | No | NA | NA | |
| 36 | none | none | negative | negative | No | NA | NA | |
| 37 | none | none | negative | negative | No | NA | NA | |
| 38 | none | none | negative | negative | No | NA | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kadanka, Z., Jr.; Kadanka, Z., Sr.; Jura, R.; Bednarik, J. Vertigo in Patients with Degenerative Cervical Myelopathy. J. Clin. Med. 2021, 10, 2496. https://doi.org/10.3390/jcm10112496
Kadanka Z Jr., Kadanka Z Sr., Jura R, Bednarik J. Vertigo in Patients with Degenerative Cervical Myelopathy. Journal of Clinical Medicine. 2021; 10(11):2496. https://doi.org/10.3390/jcm10112496
Chicago/Turabian StyleKadanka, Zdenek, Jr., Zdenek Kadanka, Sr., Rene Jura, and Josef Bednarik. 2021. "Vertigo in Patients with Degenerative Cervical Myelopathy" Journal of Clinical Medicine 10, no. 11: 2496. https://doi.org/10.3390/jcm10112496
APA StyleKadanka, Z., Jr., Kadanka, Z., Sr., Jura, R., & Bednarik, J. (2021). Vertigo in Patients with Degenerative Cervical Myelopathy. Journal of Clinical Medicine, 10(11), 2496. https://doi.org/10.3390/jcm10112496






