The Effect of a Comprehensive, Interdisciplinary Medication Review on Quality of Life and Medication Use in Community Dwelling Older People with Polypharmacy
Abstract
1. Introduction
2. Methods
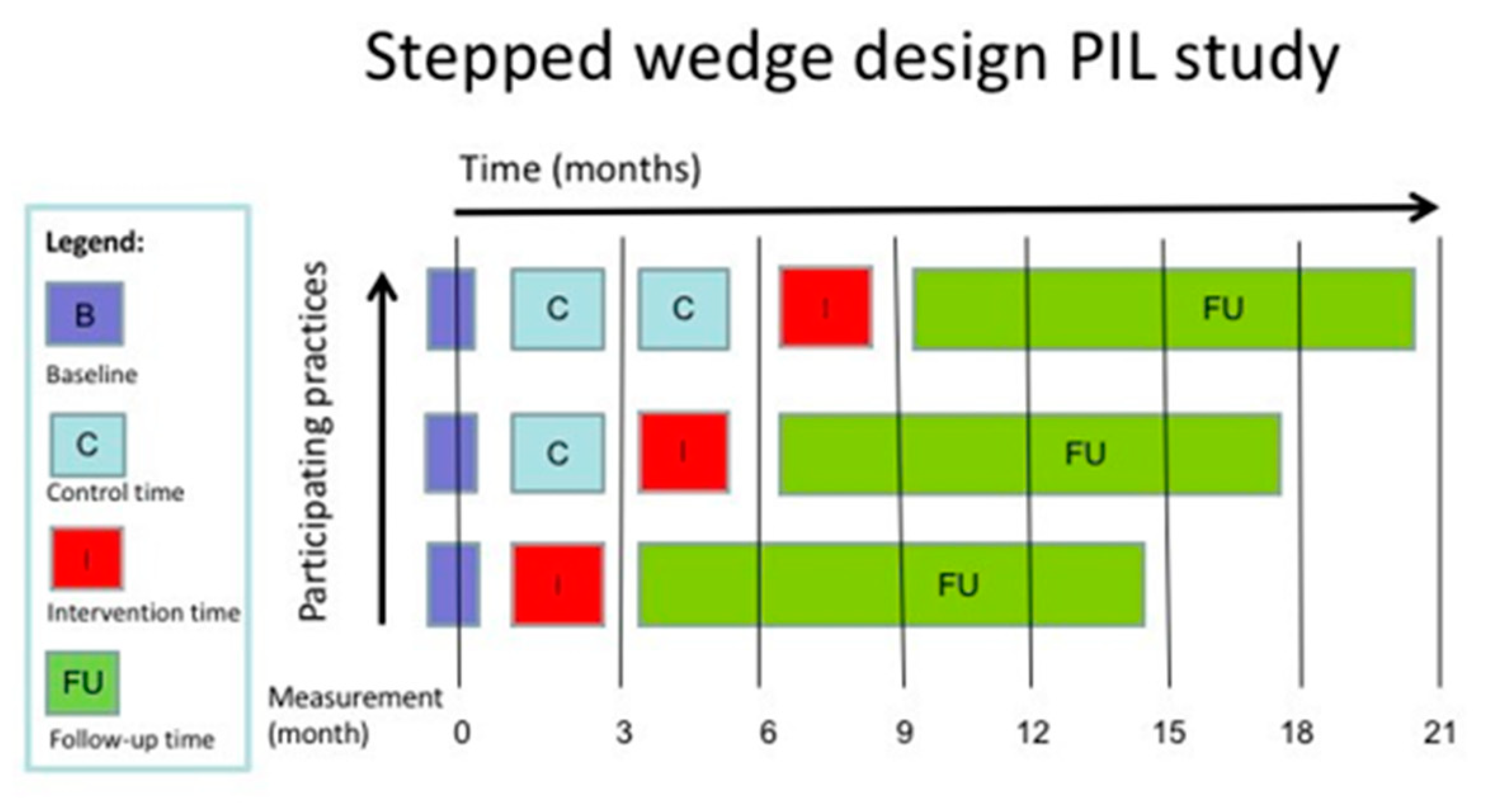
2.1. Design
2.2. Sample and Recruitment
2.3. Randomisation and Masking
2.4. Control Period
2.5. Intervention
2.6. Follow-Up Period
2.7. Data Collection
2.8. Secondary Outcomes
2.9. Analysis
2.10. Baseline Characteristics
2.11. Data are Number of Participants (%), Unless Otherwise Stated 2.10. Primary Outcome
2.12. Secondary Outcomes
3. Main Results
3.1. Strengths and Limitations
3.2. Comparison to Previous Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Trial Registration
References
- Nielen, M.M.J.; Poos, M.J.J.C.; Gommer, A.M. De Gezondheidsmonitor: Chronische Ziekten en Multimorbiditeit. Available online: https://www.volksgezondheidenzorg.info/onderwerp/chronische-ziekten-en-multimorbiditeit/cijfers-context/huidige-situatie (accessed on 23 December 2020).
- Masnoon, N.; Shakib, S.; Kalisch-Ellett, L.; Caughey, G.E. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017, 17, 230. [Google Scholar] [CrossRef]
- Waller, J.L.; Maclean, J.R. Updating the Beers criteria for potentially inappropriate medication use in older adults. Arch. Intern. Med. 2003, 163, 2716–2724. [Google Scholar]
- Garfinkel, D.; Bilek, A. Inappropriate medication use and polypharmacy in older people. BMJ 2020, 369, m2023. [Google Scholar] [CrossRef] [PubMed]
- Davies, L.E.; Spiers, G.; Kingston, A.; Todd, A.; Adamson, J.; Hanratty, B. Adverse Outcomes of Polypharmacy in Older People: Systematic Review of Reviews. J. Am. Med. Dir. Assoc. 2020, 21, 181–187. [Google Scholar] [CrossRef]
- Eriksen, C.U.; Kyriakidis, S.; Christensen, L.D.; Jacobsen, R.; Laursen, J.; Christensen, M.B.; Frølich, A. Medication-related experiences of patients with polypharmacy: A systematic review of qualitative studies. BMJ Open 2020, 10, e036158. [Google Scholar] [CrossRef] [PubMed]
- Vass, M.; Hendriksen, C. Polypharmacy and older people—. Z. Gerontol. Geriatr. 2005, 38, i14–i17. [Google Scholar] [CrossRef] [PubMed]
- Salisbury, C.; Johnson, L.; Purdy, S.; Valderas, J.M.; Montgomery, A. Epidemiology and impact of multimorbidity in primary care: A retrospective cohort study. Br. J. Gen. Pract. 2011, 61, e12–e21. [Google Scholar] [CrossRef]
- Holmes, H.M.; Todd, A. The role of patient preferences in deprescribing. Clin. Geriatr. Med. 2017, 33, 165–175. [Google Scholar] [CrossRef]
- Todd, A.; Jansen, J.; Colvin, J.; McLachlan, A.J. The deprescribing rainbow: A conceptual framework highlighting the importance of patient context when stopping medication in older people. BMC Geriatr. 2018, 18, 1–8. [Google Scholar] [CrossRef]
- Hussey, M.A.; Hughes, J.P. Design and analysis of stepped wedge cluster randomized trials. Contemp. Clin. Trials 2007, 28, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.A.; Lilford, R.J. The stepped wedge trial design: A systematic review. BMC Med. Res. Methodol. 2006, 6, 54. [Google Scholar] [CrossRef]
- Ware, J.E., Jr. SF-36 health survey update. Spine (Phila Pa 1976) 2000, 25, 3130–3139. [Google Scholar] [CrossRef]
- Herdman, M.; Gudex, C.; Lloyd, A.; Janssen, M.; Kind, P.; Parkin, D.E.; Bonsel, G.J.; Badia, X. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual. Life Res. 2011, 20, 1727–1736. [Google Scholar] [CrossRef]
- Katz, S. Assessing self-maintenance: Activities of daily living, mobility, and instrumental activities of daily living. J. Am. Geriatr. Soc. 1983, 31, 721–727. [Google Scholar] [CrossRef] [PubMed]
- ATC/DDD Index. Available online: https://www.whocc.no/atc_ddd_index/ (accessed on 23 December 2020).
- Rankin, A.; Cadogan, C.A.; Patterson, S.M.; Kerse, N.; Cardwell, C.R.; Bradley, M.C.; Ryan, C.; Hughes, C. Interventions to improve the appropriate use of polypharmacy for older people. Cochrane Database Syst. Rev. 2018, 9, CD008165. [Google Scholar] [CrossRef] [PubMed]
- Walters, S.J.; Munro, J.F.; Brazier, J.E. Using the SF-36 with older adults: A cross-sectional community-based survey. Age Ageing 2018, 30, 337–343. [Google Scholar] [CrossRef]
- Suijker, J.J.; van Rijn, M.; ter Riet, G.; van Charante, E.M.; de Rooij, S.E.; Buurman, B.M. Minimal important change and minimal detectable change in activities of daily living in community-living older people. J. Nutr. Health Aging, 2017; 21, 165–172. [Google Scholar] [CrossRef]
- Muth, C.; Uhlmann, L.; Haefeli, W.E.; Rochon, J.; Akker, M.V.D.; Perera, R.; Güthlin, C.; Beyer, M.; Oswald, F.; Valderas, J.M.; et al. Effectiveness of a complex intervention on Prioritising Multimedication in Multimorbidity (PRIMUM) in primary care: Results of a pragmatic cluster randomised controlled trial. BMJ Open 2018, 8, e017740. [Google Scholar] [CrossRef] [PubMed]
- Nishtala, P.S.; Salahudeen, M.S. Temporal trends in polypharmacy and hyperpolypharmacy in older New Zealanders over a 9-year period: 2005–2013. Gerontology 2015, 61, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Jansen, J.; Naganathan, V.; Carter, S.M.; McLachlan, A.J.; Nickel, B.; Irwig, L.; Bonner, C.; Doust, J.; Colvin, J.; Heaney, A.; et al. Too much medicine in older people? Deprescribing through shared decision making. BMJ 2016, 353, i2893. [Google Scholar] [CrossRef]
- Beuscart, J.-B.; Knol, W.; Cullinan, S.; Schneider, C.; Dalleur, O.; Boland, B.; Thevelin, S.; Jansen, P.A.F.; O’Mahony, D.; Rodondi, N.; et al. International core outcome set for clinical trials of medication review in multi-morbid older patients with polypharmacy. BMC Med. 2018, 16, 21. [Google Scholar] [CrossRef]
- Huiskes, V.J.B.; Burger, D.M.; van den Ende, C.H.M.; van den Bemt, B.J.F. Effectiveness of medication review: A systematic review and meta-analysis of randomized controlled trials. BMC Fam. Pract. 2017, 18, 5. [Google Scholar] [CrossRef]
- Verdoorn, S.; Kwint, H.; Blom, J.W.; Gussekloo, J.; Bouvy, M.L. Effects of a clinical medication review focused on personal goals, quality of life and complaints in older persons with polypharmacy: A randomized clinical trial ( DREAMeR-study). PLoS Med. 2019, 16, e1002798. [Google Scholar] [CrossRef] [PubMed]
- Gellad, W.F.; Grenard, J.L.; Marcum, Z.A. A systematic review of barriers to medication adherence in the elderly: Looking beyond cost and regimen complexity. Am. J. Geriatr. Pharmacother. 2011, 9, 11–23. [Google Scholar] [CrossRef] [PubMed]

| Baseline | Intervention | Follow-Up | |||
|---|---|---|---|---|---|
| Start | End | Intermediate | End of Study | ||
| Data provided by GPs (GP’s EPR *) | |||||
| Current medication list | X | X | X | X | |
| Laboratory results 1 | X | ||||
| Diagnoses (ICPC-1) | X | X | |||
| Correspondence with specialist(s) involved | X | ||||
| Data provided by pharmacists (pharmacy’s EPR) | |||||
| Current medication list | X | X | X | ||
| Medication delivery details and adherence | X | X | X | ||
| Data provided by patients | |||||
| Home visits | |||||
| Current medication use reported by patient | X | X | |||
| Use of over-the-counter medication | X | X | |||
| Adverse drug effects | X | X | |||
| Knowledge of medication use | X | X | |||
| Appropriate use of prescribed medication | X | X | |||
| Correct storage and use of medication | X | X | |||
| Use of medication-related devices/assistance | X | X | |||
| Use of food/drinks that can influence health status | X | X | |||
| Mobility | X | X | |||
| Stool and micturition habits | X | X | |||
| Height | X | X | |||
| Weight | X | X | |||
| Patient questionnaires (by mail) | |||||
| Quality of life (SF-36, EQ-5D) | X | X | X | X | X |
| Activities of daily living (ADL-I-ADL) | X | X | X | X | X |
| Date and country of birth, gender, education | X | ||||
| Postal code, living situation | X | X | X | X | X |
| Age | |
| 60–69 | 304 (39.6) |
| 70–79 | 300 (39.0) |
| ≥80 | 166 (21.6) |
| Sex | |
| Female | 359 |
| Male | 409 |
| Missing | 2 |
| Country of birth | |
| The Netherlands | 722 (94.6) |
| Other European country | 27 (3.5) |
| Indonesia | 11 (1.4) |
| Other | 3 (0.3) |
| Missing | 7 |
| Level of education | |
| None | 185 (24.3) |
| Low | 358 (47.1) |
| Middle | 129 (17.0) |
| High | 88 (11.6) |
| Missing | 10 |
| Living arrangements | |
| Independent, with someone | 509 (67.1) |
| Independent, alone | 213 (28.1) |
| Nursing home | 37 (4.9) |
| Missing | 11 |
| Average number of medications/patient | |
| Reported by the patient | 8.0 sd 2.6 |
| From the GP records | 7.4 sd 2.5 |
| From the pharmacist records | 7.5 sd 2.4 |
| Outcome | Baseline (N = 768) ‡ | Intervention | Follow-Up ¥ | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Start (N = 746) | Intermediate (N = 624) | End of Study (N = 508) | ||||||||||
| Quality of life: SF-36 * | Mean | SD | Median | Mean | SD | Median | Mean | SD | Median | Mean | SD | Median |
| Physical functioning | 57.9 | 28.5 | 60 | 58.3 | 29.0 | 60 | 58.1 | 29.3 | 60 | 58.4 | 28.1 | 60 |
| Social functioning | 77.0 | 25.1 | 87.5 | 76.8 | 24.9 | 87.5 | 74.7 | 25.8 | 75 | 75.7 | 24.1 | 75 |
| Role physical | 54.4 | 44.4 | 66.7 | 54.4 | 44.8 | 75 | 53.7 | 44.9 | 75 | 50.5 | 44.4 | 50 |
| Role emotional | 77.0 | 37.7 | 100 | 75.8 | 38.0 | 100 | 73.6 | 38.9 | 100 | 74.0 | 39.7 | 100 |
| Mental Health | 76.7 | 17.7 | 80 | 76.4 | 18.1 | 80 | 75.6 | 17.8 | 80 | 74.8 | 18.1 | 80 |
| Vitality | 61.8 | 19.8 | 65 | 62.0 | 20.0 | 65 | 61.2 | 19.5 | 65 | 60.0 | 19.1 | 60 |
| Pain | 66.0 | 25.7 | 67.3 | 66.2 | 26.1 | 67.3 | 64.6 | 26.2 | 67.3 | 64.7 | 24.4 | 67.3 |
| General health | 53.4 | 18.3 | 55 | 54.1 | 18.5 | 55 | 52.9 | 18.1 | 50 | 52.7 | 17.9 | 50 |
| Quality of life(EQ-5D)$ | 7.6 | 2.1 | 8.1 | 7.7 | 2.1 | 8.1 | 7.6 | 2.2 | 8.1 | 7.6 | 2.0 | 8.1 |
| Activities of daily living (ADL/iADL)# | ||||||||||||
| ADL | 0.49 | 0.91 | 0 | 0.50 | 0.90 | 0 | 0.54 | 0.97 | 0. | 0.52 | 0.92 | 0 |
| Instrumental ADL | 1.01 | 1.43 | 0 | 1.05 | 1.51 | 0 | 1.01 | 1.40 | 0 | 1.01 | 1.49 | 0 |
| Outcome | Start of Period | After 6 Months | Difference in Overall Trend Between Control and Follow-Up; p-Value | |
|---|---|---|---|---|
| Quality of life: SF-36 * | Estimated mean (95%CI) | |||
| Physical functioning | Control: Intervention: | 52.3 (47.2, 57.4) 52.8 (47.6, 57.9) | 53.8 (48.3, 59.2) 52.0 (46.9, 57.1) | 0.057 |
| Social functioning | Control: Intervention: | 80.8 (70.3, 91.3) 68.0 (61.6, 74.5) | 74.0 (66.9, 81.1) 69.0 (63.1, 75.0) | 0.071 (**) |
| Role physical | Control: Intervention: | 45.4 (34.7, 56.0) 43.7 (32.9, 54.4) | 44.1 (32.5, 55.6) 42.9 (32.3, 53.5) | 0.855 |
| Role emotional | Control: Intervention: | 77.0 (67.3, 86.7) 72.5 (62.7, 82.3) | 74.4 (63.8, 85.0) 72.5 (62.8, 82.1) | 0.327 |
| Mental Health | Control: Intervention: | 80.9 (74.5, 87.3) 71.6 (67.5, 75.7) | 74.8 (70.3, 79.3) 71.3 (67.5, 75.1) | 0.009 (**) |
| Vitality | Control: Intervention: | 58.0 (54.0, 61.9) 57.9 (53.9, 61.9) | 58.1 (53.8, 62.3) 56.8 (52.9, 60.8) | 0.246 |
| Pain | Control: Intervention: | 71.2 (65.5, 76.9) 71.3 (65.5, 77.0) | 70.9 (64.8, 77.1) 70.2 (64.6, 75.9) | 0.570 |
| General health | Control: Intervention: | 49.9 (46.0, 53.7) 49.3 (45.4, 53.2) | 50.2 (46.1, 54.4) 48.8 (45.0, 52.7) | 0.373 |
| Quality of Life * (EQ-5D) | Control: Intervention: | 6.7 (6.2, 7.2) 6.7 (6.2, 7.2) | 6.8 (6.3, 7.3) 6.7 (6.2, 7.1) | 0.082 |
| Activities of daily living (ADL) † | ||||
| ADL | Control: Intervention: | 0.97 (0.79, 1.16) 0.99 (0.81, 1.17) | 0.98 (0.78, 1.17) 1.02 (0.83, 1.12) | 0.551 |
| Instrumental ADL | Control: Intervention: | 1.82 (1.54, 2.09) 1.84 (1.57, 2.12) | 1.87 (1.58, 2.16) 1.86 (1.59, 2.14) | 0.608 |
| Start Intervention N = 770 | End Intervention N = 745 | Follow-Up # | ||
|---|---|---|---|---|
| Intermediate N = 603 | End of Study N = 597 | |||
| Total number of medications *: | 5469 | 5527 | 4407 | 4318 |
| Average number of medications *: | 7.4 sd 2.5 | 7.6 sd 2.5 | 7.3 sd 2.4 | 7.2 sd 2.5 |
| Medication according to category | ||||
| Cardiovascular | 3030 (4.1 sd 1.8) | 3042 (4.1 sd 1.9) | 2452 (4.1 sd 1.8) | 2411 (4.0 sd 1.8) |
| Diabetes Mellitus | 395 (0.5 sd 0.9) | 404 (0.5 sd 0.8) | 357 (0.6 sd 0.9) | 339 (0.6 sd 0.9) |
| Digestive tract | 447 (0.6 sd 0.7) | 451 (0.6 sd 0.7) | 372 (0.6 sd 0.7) | 351 (0.6 sd 0.9) |
| Lung diseases | 308 (0.4 sd 0.9) | 324 (0.4 sd 0.9) | 240 (0.4 sd 0.9) | 252 (0.4 sd 0.9) |
| Psychotropic drugs | 212 (0.3 sd 0.6) | 201 (0.3 sd 0.5) | 148 (0.2 sd 0.5) | 148 (0.2 sd 0.5) |
| Analgesics | 178 (0.2 sd 0.5) | 173 (0.2 sd 0.5) | 120 (0.2 sd 0.5) | 123 (0.2 sd 0.5) |
| Other | 899 (1.2 sd 1.3) | 932 (1.3 sd 1.4) | 718 (1.2 sd 1.3) | 694 (1.2 sd 1.3) |
| Median medication adherence ** 8 (min-max) | 97.7 (74–100) | - | 98.2 (33–100) | |
| End Intervention | Follow-Up | ||
|---|---|---|---|
| 6 Months | 12 Months | ||
| Changes made to medication | |||
| None | 4473 (70.7%) | 3367 (69.8%) | 3367 (69.8%) |
| Medication stopped | 798 (12.6%) | 673 (14.9%) | 616 (14.2%) |
| New medication added | 339 (5.4%) | 451 (8.9%) | 437 (8.9%) |
| Dose changed | 482 (7.6%) | 305 (6.7%) | 247 (5.6%) |
| Restarted | 237 (3.7%) | 103 (2.0%) | 158 (3.2%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bosch-Lenders, D.; Jansen, J.; Stoffers, H.E.J.H.; Winkens, B.; Aretz, K.; Twellaar, M.; Schols, J.M.G.A.; van der Kuy, P.-H.M.; Knottnerus, J.A.; van den Akker, M. The Effect of a Comprehensive, Interdisciplinary Medication Review on Quality of Life and Medication Use in Community Dwelling Older People with Polypharmacy. J. Clin. Med. 2021, 10, 600. https://doi.org/10.3390/jcm10040600
Bosch-Lenders D, Jansen J, Stoffers HEJH, Winkens B, Aretz K, Twellaar M, Schols JMGA, van der Kuy P-HM, Knottnerus JA, van den Akker M. The Effect of a Comprehensive, Interdisciplinary Medication Review on Quality of Life and Medication Use in Community Dwelling Older People with Polypharmacy. Journal of Clinical Medicine. 2021; 10(4):600. https://doi.org/10.3390/jcm10040600
Chicago/Turabian StyleBosch-Lenders, Donna, Jesse Jansen, Henri E. J. H. (Jelle) Stoffers, Bjorn Winkens, Karin Aretz, Mascha Twellaar, Jos M. G. A. Schols, Paul-Hugo M. van der Kuy, J. André Knottnerus, and Marjan van den Akker. 2021. "The Effect of a Comprehensive, Interdisciplinary Medication Review on Quality of Life and Medication Use in Community Dwelling Older People with Polypharmacy" Journal of Clinical Medicine 10, no. 4: 600. https://doi.org/10.3390/jcm10040600
APA StyleBosch-Lenders, D., Jansen, J., Stoffers, H. E. J. H., Winkens, B., Aretz, K., Twellaar, M., Schols, J. M. G. A., van der Kuy, P.-H. M., Knottnerus, J. A., & van den Akker, M. (2021). The Effect of a Comprehensive, Interdisciplinary Medication Review on Quality of Life and Medication Use in Community Dwelling Older People with Polypharmacy. Journal of Clinical Medicine, 10(4), 600. https://doi.org/10.3390/jcm10040600









