Lactoferrin: Cytokine Modulation and Application in Clinical Practice
Abstract
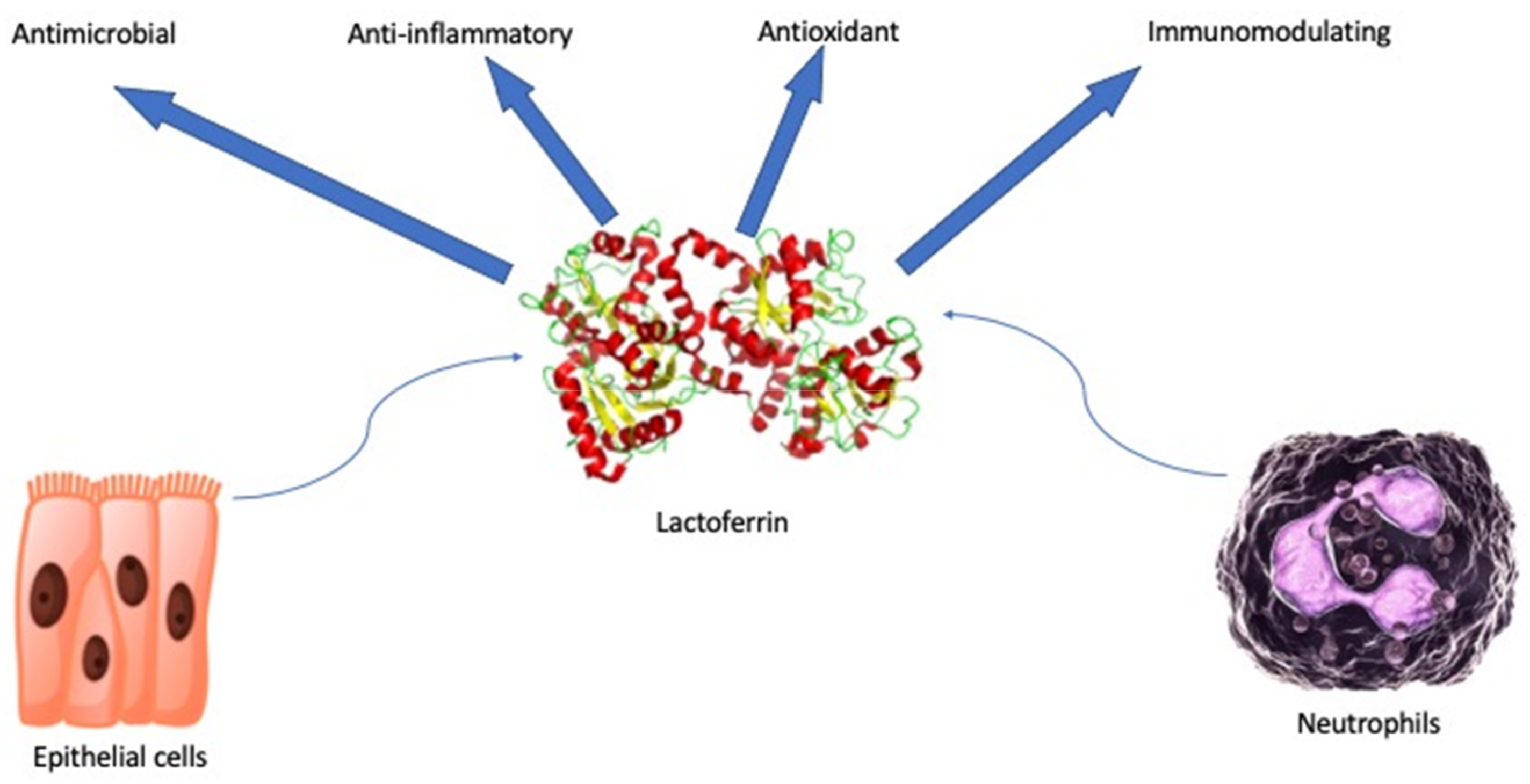
:1. Introduction
Cytokine Modulation and Immunological Effects
2. Materials and Methods
3. Results
3.1. Lactoferrin in Neonatology
3.2. Lactoferrin and Respiratory Viruses
3.3. Lactoferrin and Gastrointestinal Disease
3.4. Lactoferrin and Onco-Hematologic Disorders
3.5. Lactoferrin and Allergic Disorders
3.6. Others
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Berlutti, F.; Pantanella, F.; Natalizi, T.; Frioni, A.; Paesano, R.; Polimeni, A.; Valenti, P. Antiviral properties of lactoferrin—A natural immunity molecule. Molecules 2011, 16, 6992–7018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baker, E.N.; Baker, H.M. Molecular structure, binding properties and dynamics of lactoferrin. Cell Mol. Life Sci. 2005, 62, 2531–2539. [Google Scholar] [CrossRef]
- Rosa, L.; Cutone, A.; Lepanto, M.S.; Paesano, R.; Valenti, P. Lactoferrin: A Natural Glycoprotein Involved in Iron and Inflammatory Homeostasis. Int. J. Mol. Sci. 2017, 18, 1985. [Google Scholar] [CrossRef]
- Lepanto, M.S.; Rosa, L.; Paesano, R.; Valenti, P.; Cutone, A. Lactoferrin in Aseptic and Septic Inflammation. Molecules 2019, 24, 1323. [Google Scholar] [CrossRef] [Green Version]
- Telang, S. Lactoferrin: A Critical Player in Neonatal Host Defense. Nutrients 2018, 10, 1228. [Google Scholar] [CrossRef] [Green Version]
- Artym, J.; Zimecki, M.; Paprocka, M.; Kruzel, M.L. Orally administered lactoferrin restores humoral immune response in immunocompromised mice. Immunol. Lett. 2003, 89, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Artym, J.; Zimecki, M.; Kruzel, M.L. Effect of lactoferrin on the methotrexate-induced suppression of the cellular and humoral immune response in mice. Anticancer Res. 2004, 24, 3831–3836. [Google Scholar] [PubMed]
- Artym, J.; Zimecki, M.; Kuryszko, J.; Kruzel, M.L. Lactoferrin accelerates reconstitution of the humoral and cellular immune response during chemotherapy-induced immunosuppression and bone marrow transplant in mice. Stem. Cells Dev. 2005, 14, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Latorre, D.; Berlutti, F.; Valenti, P.; Gessani, S.; Puddu, P. Lf immunomodulatory strategies: Mastering bacterial endotoxin. Biochem. Cell Biol. 2012, 90, 269–278. [Google Scholar] [CrossRef]
- Legrand, D. Lactoferrin, a key molecule in immune and inflammatory processes. Biochem. Cell Biol. 2012, 90, 252–268. [Google Scholar] [CrossRef]
- Artym, J.; Zimecki, M.; Kruzel, M.L. Enhanced clearance of Escherichia coli and Staphylococcus aureus in mice treated with cyclophosphamide and lactoferrin. Int. Immunopharmacol. 2004, 4, 1149–1157. [Google Scholar] [CrossRef] [PubMed]
- Actor, J.K.; Hwang, S.-A.; Kruzel, M.L. Lactoferrin as a natural immune modulator. Curr. Pharm. Des. 2009, 15, 1956–1973. [Google Scholar] [CrossRef] [Green Version]
- Legrand, D.; Elass, E.; Pierce, A.; Mazurier, J. Lactoferrin and host defence: An overview of its immuno-modulating and anti-inflammatory properties. Biometals 2004, 17, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Legrand, D. Overview of Lactoferrin as a Natural Immune Modulator. J. Pediatr. 2016, 173, S10–S15. [Google Scholar] [CrossRef] [Green Version]
- De la Rosa, G.; Yang, D.; Tewary, P.; Varadhachary, A.; Oppenheim, J.J. Lactoferrin acts as an alarmin to promote the recruitment and activation of APCs and antigen-specific immune responses. J Immunol. 2008, 180, 6868–6876. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.-M.; Yeh, C.-C.; Chen, H.-L.; Lai, C.W.; Kuo, M.F.; Yeh, M.H.; Lin, W.; Tu, M.Y.; Cheng, H.C.; Chen, C.M. Porcine lactoferrin administration enhances peripheral lymphocyte proliferation and assists infectious bursal disease vaccination in native chickens. Vaccine 2010, 28, 2895–2902. [Google Scholar] [CrossRef]
- Redwan, E.M.; Uversky, V.N.; El-Fakharany, E.M.; Al-Mehdar, H. Potential lactoferrin activity against pathogenic viruses. Comptes Rendus Biol. 2014, 337, 581–595. [Google Scholar] [CrossRef]
- Bournazou, I.; Mackenzie, K.J.; Duffin, R.; Rossi, A.G.; Gregory, C.D. Inhibition of eosinophil migration by lactoferrin. Immunol. Cell Biol. 2010, 88, 220–223. [Google Scholar] [CrossRef]
- Sherman, M.P. Lactoferrin and Necrotizing Enterocolitis. Clin. Perinatol. 2013, 40, 79–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaczyńska, E.; Kocięba, M.; Śliwińska, E.; Zimecki, M. Bovine lactoferrin enhances proliferation of human peripheral blood lymphocytes and induces cytokine production in whole blood cultures. Adv. Clin. Exp. Med. 2014, 23, 871–876. [Google Scholar] [CrossRef] [Green Version]
- Drago-Serrano, M.E.; Campos-Rodriguez, R.; Carrero, J.C.; de la Garza, M. Lactoferrin and Peptide-derivatives: Antimicrobial Agents with Potential Use in Nonspecific Immunity Modulation. Curr. Pharm. Des. 2018, 24, 1067–1078. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhang, H.; Xie, Y.; Wang, Y. Recombinant expression of porcine lactoferrin peptide LF-6 with intein technology and its immunomodulatory function in ETEC K88-infected mice. Int. Immunopharmacol. 2016, 39, 181–191. [Google Scholar] [CrossRef]
- Manzoni, P. Bovine Lactoferrin Supplementation for Prevention of Late-Onset Sepsis in Very Low-Birth-Weight Neonates: A Randomized Trial. JAMA 2009, 302, 1421. [Google Scholar] [CrossRef]
- Manzoni, P.; Meyer, M.; Stolfi, I.; Rinaldi, M.; Cattani, S.; Pugni, L.; Romeo, M.G.; Messner, H.; Decembrino, L.; Laforgiaj, N.; et al. Bovine lactoferrin supplementation for prevention of necrotizing enterocolitis in very-low-birth-weight neonates: A randomized clinical trial. Early Hum. Dev. 2014, 90, S60–S65. [Google Scholar] [CrossRef]
- Pammi, M.; Gautham, K.S. Enteral lactoferrin supplementation for prevention of sepsis and necrotizing enterocolitis in preterm infants. Cochrane Database Syst. Rev. 2020, 3, CD007137. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Cao, L.; Yu, J. Prophylactic lactoferrin for preventing late-onset sepsis and necrotizing enterocolitis in preterm infants: A PRISMA-compliant systematic review and meta-analysis. Medicine 2018, 97, e11976. [Google Scholar] [CrossRef] [PubMed]
- Manzoni, P.; García Sánchez, R.; Meyer, M.; Stolfi, I.; Pugni, L.; Messner, H.; Cattani, S.; Betta, P.M.; Memo, L.; Decembrino, L.; et al. Exposure to Gastric Acid Inhibitors Increases the Risk of Infection in Preterm Very Low Birth Weight Infants but Concomitant Administration of Lactoferrin Counteracts This Effect. J. Pediatr. 2018, 193, 62–67.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnston, W.H.; Ashley, C.; Yeiser, M.; Harris, C.L.; Stolz, S.I.; Wampler, J.L.; Wittke, A.; Cooper, T.R. Growth and tolerance of formula with lactoferrin in infants through one year of age: Double-blind, randomized, controlled trial. BMC Pediatr. 2015, 15, 173. [Google Scholar] [CrossRef] [Green Version]
- Barrington, K.J.; Assaad, M.-A.; Janvier, A. The Lacuna Trial: A double-blind randomized controlled pilot trial of lactoferrin supplementation in the very preterm infant. J. Perinatol. 2016, 36, 666–669. [Google Scholar] [CrossRef]
- Ochoa, T.J.; Sizonenko, S.V. Lactoferrin and prematurity: A promising milk protein? Biochem. Cell. Biol. 2017, 95, 22–30. [Google Scholar] [CrossRef] [Green Version]
- Ginet, V.; van de Looij, Y.; Petrenko, V.; Toulotte, A.; Kiss, J.; Hüppi, P.S.; Sizonenko, S.V. Lactoferrin during lactation reduces lipopolysaccharide-induced brain injury. Biofactors 2016, 42, 323–336. [Google Scholar] [CrossRef]
- Van de Looij, Y.; Ginet, V.; Chatagner, A.; Toulotte, A.; Somm, E.; Hüppi, P.S.; Sizonenko, S.V. Lactoferrin during lactation protects the immature hypoxic-ischemic rat brain. Ann. Clin. Transl. Neurol. 2014, 1, 955–967. [Google Scholar] [CrossRef]
- Chen, K.; Chai, L.; Li, H.; Zhang, Y.; Xie, H.M.; Shang, J.; Tian, W.; Yang, P.; Jiang, A.C. Effect of bovine lactoferrin from iron-fortified formulas on diarrhea and respiratory tract infections of weaned infants in a randomized controlled trial. Nutrition 2016, 32, 222–227. [Google Scholar] [CrossRef]
- King, J.C.; Cummings, G.E.; Guo, N.; Trivedi, L.; Readmond, B.X.; Keane, V.; Feigelman, S.; de Waard, R. A double-blind, placebo-controlled, pilot study of bovine lactoferrin supplementation in bottle-fed infants. J. Pediatr. Gastroenterol. Nutr. 2007, 44, 245–251. [Google Scholar] [CrossRef] [Green Version]
- Vitetta, L.; Coulson, S.; Beck, S.L.; Gramotnev, H.; Du, S.; Lewis, S. The clinical efficacy of a bovine lactoferrin/whey protein Ig-rich fraction (Lf/IgF) for the common cold: A double blind randomized study. Complement. Ther. Med. 2013, 21, 164–171. [Google Scholar] [CrossRef]
- Lang, J.; Yang, N.; Deng, J.; Liu, K.; Yang, P.; Zhang, G.; Jiang, C. Inhibition of SARS pseudovirus cell entry by lactoferrin binding to heparan sulfate proteoglycans. PLoS ONE 2011, 6, e23710. [Google Scholar] [CrossRef] [PubMed]
- Parisi, G.F.; Carota, G.; Castruccio Castracani, C.; Spampinato, M.; Manti, S.; Papale, M.; Di Rosa, M.; Barbagallo, I.; Leonardi, S. Nutraceuticals in the Prevention of Viral Infections, including COVID-19, among the Pediatric Population: A Review of the Literature. Int. J. Mol. Sci. 2021, 22, 2465. [Google Scholar] [CrossRef]
- Campione, E.; Lanna, C.; Cosio, T.; Rosa, L.; Conte, M.P.; Iacovelli, F.; Romeo, A.; Falconi, M.; Del Vecchio, C.; Franchin, E.; et al. Lactoferrin Against SARS-CoV-2: In Vitro and In Silico Evidences. Front. Pharmacol. 2021, 12, 666600. [Google Scholar] [CrossRef] [PubMed]
- Campione, E.; Cosio, T.; Rosa, L.; Lanna, C.; Di Girolamo, S.; Gaziano, R.; Valenti, P.; Bianchi, L. Lactoferrin as Protective Natural Barrier of Respiratory and Intestinal Mucosa against Coronavirus Infection and Inflammation. Int. J. Mol. Sci. 2020, 21, 4903. [Google Scholar] [CrossRef] [PubMed]
- Sinopoli, A.; Isonne, C.; Santoro, M.M.; Baccolini, V. The effects of orally administered lactoferrin in the prevention and management of viral infections: A systematic review. Rev. Med. Virol. 2021, e2261. [Google Scholar] [CrossRef]
- Ochoa, T.J.; Chea-Woo, E.; Baiocchi, N.; Pecho, I.; Campos, M.; Prada, A.; Valdiviezo, G.; Lluque, A.; Lai, D.; Cleary, T.G. Randomized double-blind controlled trial of bovine lactoferrin for prevention of diarrhea in children. J. Pediatr. 2013, 162, 349–356. [Google Scholar] [CrossRef] [Green Version]
- Tsukahara, T.; Fujimori, A.; Misawa, Y.; Oda, H.; Yamauchi, K.; Abe, F.; Nomiyama, T. The Preventive Effect of Lactoferrin-Containing Yogurt on Gastroenteritis in Nursery School Children-Intervention Study for 15 Weeks. Int. J. Environ. Res. Public Health 2020, 17, 2534. [Google Scholar] [CrossRef] [Green Version]
- Egashira, M.; Takayanagi, T.; Moriuchi, M.; Moriuchi, H. Does daily intake of bovine lactoferrin-containing products ameliorate rotaviral gastroenteritis? Acta. Paediatr. 2007, 96, 1242–1244. [Google Scholar] [CrossRef]
- Zavaleta, N.; Figueroa, D.; Rivera, J.; Sánchez, J.; Alfaro, S.; Lönnerdal, B. Efficacy of rice-based oral rehydration solution containing recombinant human lactoferrin and lysozyme in Peruvian children with acute diarrhea. J. Pediatr. Gastroenterol. Nutr. 2007, 44, 258–264. [Google Scholar] [CrossRef]
- Ochoa, T.J.; Chea-Woo, E.; Campos, M.; Pecho, I.; Prada, A.; McMahon, R.J.; Cleary, T.G. Impact of Lactoferrin Supplementation on Growth and Prevalence of Giardia Colonization in Children. Clin. Infect. Dis. 2008, 46, 1881–1883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, W.D.; Wold, K.J.; Bollinger, L.B.; Ordiz, M.I.; Shulman, R.J.; Maleta, K.M.; Manary, M.J.; Trehan, I. Supplementation with Lactoferrin and Lysozyme Ameliorates Environmental Enteric Dysfunction: A Double-Blind, Randomized, Placebo-Controlled Trial. Am. J. Gastroenterol. 2019, 114, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Mizuki, M.; Tsukahara, T.; Oda, H.; Tanaka, M.; Yamauchi, K.; Abe, F.; Nomiyama, T. Effects of Lactoferrin on Prevention of Acute Gastrointestinal Symptoms in Winter: A Randomized, Double-Blinded, Placebo-Controlled Trial for Staff of Kindergartens and Nursery Schools in Japan. Int. J. Environ. Res. Public Health 2020, 17, 9582. [Google Scholar] [CrossRef] [PubMed]
- Laffan, A.M.; McKenzie, R.; Forti, J.; Conklin, D.; Marcinko, R.; Shrestha, R.; Bellantoni, M.; Greenough, W.B., III. Lactoferrin for the prevention of post-antibiotic diarrhoea. J. Health Popul. Nutr. 2011, 29, 547–551. [Google Scholar] [CrossRef] [Green Version]
- Mikulic, N.; Uyoga, M.A.; Mwasi, E.; Stoffel, N.U.; Zeder, C.; Karanja, S.; Zimmermann, M.B. Iron Absorption is Greater from Apo-Lactoferrin and is Similar Between Holo-Lactoferrin and Ferrous Sulfate: Stable Iron Isotope Studies in Kenyan Infants. J. Nutr. 2020, 150, 3200–3207. [Google Scholar] [CrossRef]
- Chen, K.; Zhang, G.; Chen, H.; Cao, Y.; Dong, X.; Li, H.; Liu, C. Dose Effect of Bovine Lactoferrin Fortification on Iron Metabolism of Anemic Infants. J. Nutr. Sci. Vitaminol. 2020, 66, 24–31. [Google Scholar] [CrossRef] [Green Version]
- Rezk, M.; Dawood, R.; Abo-Elnasr, M.; Al Halaby, A.; Marawan, H. Lactoferrin versus ferrous sulphate for the treatment of iron deficiency anemia during pregnancy: A randomized clinical trial. J. Matern. Fetal Neonatal Med. 2016, 29, 1387–1390. [Google Scholar] [CrossRef]
- Paesano, R.; Berlutti, F.; Pietropaoli, M.; Goolsbee, W.; Pacifici, E.; Valenti, P. Lactoferrin efficacy versus ferrous sulfate in curing iron disorders in pregnant and non-pregnant women. Int. J. Immunopathol. Pharmacol. 2010, 23, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Parisi, G.F.; Cannata, E.; Manti, S.; Papale, M.; Meli, M.; Russo, G.; Di Cataldo, A.; Leonardi, S. Lung clearance index: A new measure of late lung complications of cancer therapy in children. Pediatr. Pulmonol. 2020, 55, 3450–3456. [Google Scholar] [CrossRef]
- Wang, A.; Duncan, S.E.; Lesser, G.J.; Ray, W.K.; Dietrich, A.M. Effect of lactoferrin on taste and smell abnormalities induced by chemotherapy: A proteome analysis. Food Funct. 2018, 9, 4948–4958. [Google Scholar] [CrossRef]
- Kozu, T.; Iinuma, G.; Ohashi, Y.; Saito, Y.; Akasu, T.; Saito, D.; Alexander, D.B.; Iigo, M.; Kakizoe, T.; Tsuda, H. Effect of orally administered bovine lactoferrin on the growth of adenomatous colorectal polyps in a randomized, placebo-controlled clinical trial. Cancer Prev. Res. 2009, 2, 975–983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iigo, M.; Alexander, D.B.; Xu, J.; Futakuchi, M.; Suzui, M.; Kozu, T.; Akasu, T.; Saito, D.; Kakizoe, T.; Yamauchi, K.; et al. Inhibition of intestinal polyp growth by oral ingestion of bovine lactoferrin and immune cells in the large intestine. Biometals 2014, 27, 1017–1029. [Google Scholar] [CrossRef] [Green Version]
- Elrod, K.C.; Moore, W.R.; Abraham, W.M.; Tanaka, R.D. Lactoferrin, a potent tryptase inhibitor, abolishes late-phase airway responses in allergic sheep. Am. J. Respir. Crit. Care Med. 1997, 156, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, S.; Parisi, G.; Papale, M.; Zicari, A.M.; Olcese, R.; Licari, A.; Marseglia, G.; Ciprandi, G. Small airways in children with allergic rhinoconjunctivitis: The potential role of a multicomponent nutraceutical. Acta Biomed. 2020, 91, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Acıoğlu, E.; Yigit, O.; Alkan, Z.; Azizli, E.; Gelişgen, R.; Uzun, H. The effects of corticosteroid on tissue lactoferrin in patients with nasal polyposis. Am. J. Rhinol. Allergy 2012, 26, e28–e31. [Google Scholar] [CrossRef]
- Wang, S.B.; Deng, Y.Q.; Ren, J.; Xiao, B.K.; Chen, Z.; Tao, Z.Z. Lactoferrin administration into the nostril alleviates murine allergic rhinitis and its mechanisms. Scand. J. Immunol. 2013, 78, 507–515. [Google Scholar] [CrossRef] [Green Version]
- Kruzel, M.L.; Bacsi, A.; Choudhury, B.; Sur, S.; Boldogh, I. Lactoferrin decreases pollen antigen-induced allergic airway inflammation in a murine model of asthma. Immunology 2006, 119, 159–166. [Google Scholar] [CrossRef]
- Griffiths, C.E.; Cumberbatch, M.; Tucker, S.C.; Dearman, R.J.; Andrew, S.; Headon, D.R.; Kimber, I. Exogenous topical lactoferrin inhibits allergen-induced Langerhans cell migration and cutaneous inflammation in humans. Br. J. Dermatol. 2001, 144, 715–725. [Google Scholar] [CrossRef]
- Tong, P.L.; West, N.P.; Cox, A.J.; Gebski, V.J.; Watts, A.M.; Dodds, A.; de St Groth, B.F.; Cripps, A.W.; Shumack, S. Oral supplementation with bovine whey-derived Ig-rich fraction and lactoferrin improves SCORAD and DLQI in atopic dermatitis. J. Dermatol. Sci. 2017, 85, 143–146. [Google Scholar] [CrossRef]
- Russo, R.; Superti, F.; Karadja, E.; De Seta, F. Randomised clinical trial in women with Recurrent Vulvovaginal Candidiasis: Efficacy of probiotics and lactoferrin as maintenance treatment. Mycoses 2019, 62, 328–335. [Google Scholar] [CrossRef]
- Russo, R.; Karadja, E.; De Seta, F. Evidence-based mixture containing Lactobacillus strains and lactoferrin to prevent recurrent bacterial vaginosis: A double blind, placebo controlled, randomised clinical trial. Benef. Microbes 2019, 10, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Ko, Y.; Park, Y.-K.; Kim, N.-I.; Ha, W.-K.; Cho, Y. Dietary effect of lactoferrin-enriched fermented milk on skin surface lipid and clinical improvement of acne vulgaris. Nutrition 2010, 26, 902–909. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.; Chan, G.; Santos, J.; Dee, K.; Co, J.K. A randomized, double-blind, placebo-controlled trial to determine the efficacy and safety of lactoferrin with vitamin E and zinc as an oral therapy for mild to moderate acne vulgaris. Int. J. Dermatol. 2017, 56, 686–690. [Google Scholar] [CrossRef] [PubMed]
- Nakano, M.; Yoshida, A.; Wakabayashi, H.; Tanaka, M.; Yamauchi, K.; Abe, F.; Masuda, Y. Effect of tablets containing lactoferrin and lactoperoxidase on gingival health in adults: A randomized, double-blind, placebo-controlled clinical trial. J. Periodontal. Res. 2019, 54, 702–708. [Google Scholar] [CrossRef]
| Area | Results |
|---|---|
| Neonatology | Prevention of LOS, urinary tract infections, decreased length of hospital stay. Prevention of NEC stage II or III if associated with probiotics. Less time to achieve FEF. Fewer incidence of hospital-acquired infections. Reduction of infection-related mortality |
| Pulmunology | In infants: fewer incidence of lower respiratory tract illness. Prevention of rhinorrhea, cough, and wheezing. In adults: lower incidence of cold-related symptoms |
| Gastroenterology | In children: amelioration of symptoms and severity of gastroenteritis, less days of vomiting and diarrhea. Lower prevalence of Giardia species colonization. In adults: prevention of acute gastrointestinal symptoms. Efficacy in post-antibiotic diarrhea. |
| Allergic disorders | Reduction of Th2 inflammation, lower accumulation of eosinophils, and decreased cellular oxidative stress levels in mice with allergic airway inflammation. In humans, topical inhibits allergen-induced mobilization and migration of epidermal Langerhans cells. Improvement of the SCORAD and DLQI scores in patients with atopic dermatitis |
| Onco-Hematology | Improvement of Hb in anemic children and pregnant women. Improvement of taste and smell abnormalities in patients receiving chemotherapy. Retardation of adenomatous polyp growth |
| Dermatology | Improvement of acne vulgaris lesions |
| Gynecology | Improvement of symptoms in women with VVC and bacterial vaginosis |
| Dentistry | Improvement of gingival inflammation |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Presti, S.; Manti, S.; Parisi, G.F.; Papale, M.; Barbagallo, I.A.; Li Volti, G.; Leonardi, S. Lactoferrin: Cytokine Modulation and Application in Clinical Practice. J. Clin. Med. 2021, 10, 5482. https://doi.org/10.3390/jcm10235482
Presti S, Manti S, Parisi GF, Papale M, Barbagallo IA, Li Volti G, Leonardi S. Lactoferrin: Cytokine Modulation and Application in Clinical Practice. Journal of Clinical Medicine. 2021; 10(23):5482. https://doi.org/10.3390/jcm10235482
Chicago/Turabian StylePresti, Santiago, Sara Manti, Giuseppe Fabio Parisi, Maria Papale, Ignazio Alberto Barbagallo, Giovanni Li Volti, and Salvatore Leonardi. 2021. "Lactoferrin: Cytokine Modulation and Application in Clinical Practice" Journal of Clinical Medicine 10, no. 23: 5482. https://doi.org/10.3390/jcm10235482
APA StylePresti, S., Manti, S., Parisi, G. F., Papale, M., Barbagallo, I. A., Li Volti, G., & Leonardi, S. (2021). Lactoferrin: Cytokine Modulation and Application in Clinical Practice. Journal of Clinical Medicine, 10(23), 5482. https://doi.org/10.3390/jcm10235482








