Cerebral and Systemic Stress Parameters in Correlation with Jugulo-Arterial CO2 Gap as a Marker of Cerebral Perfusion during Carotid Endarterectomy
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Recruitment
2.2. Anesthesia and Neuromonitoring
2.3. Blood Sampling Protocol and Laboratory Measurements
2.4. Statistical Analysis
3. Results
3.1. Clinical Characteristics
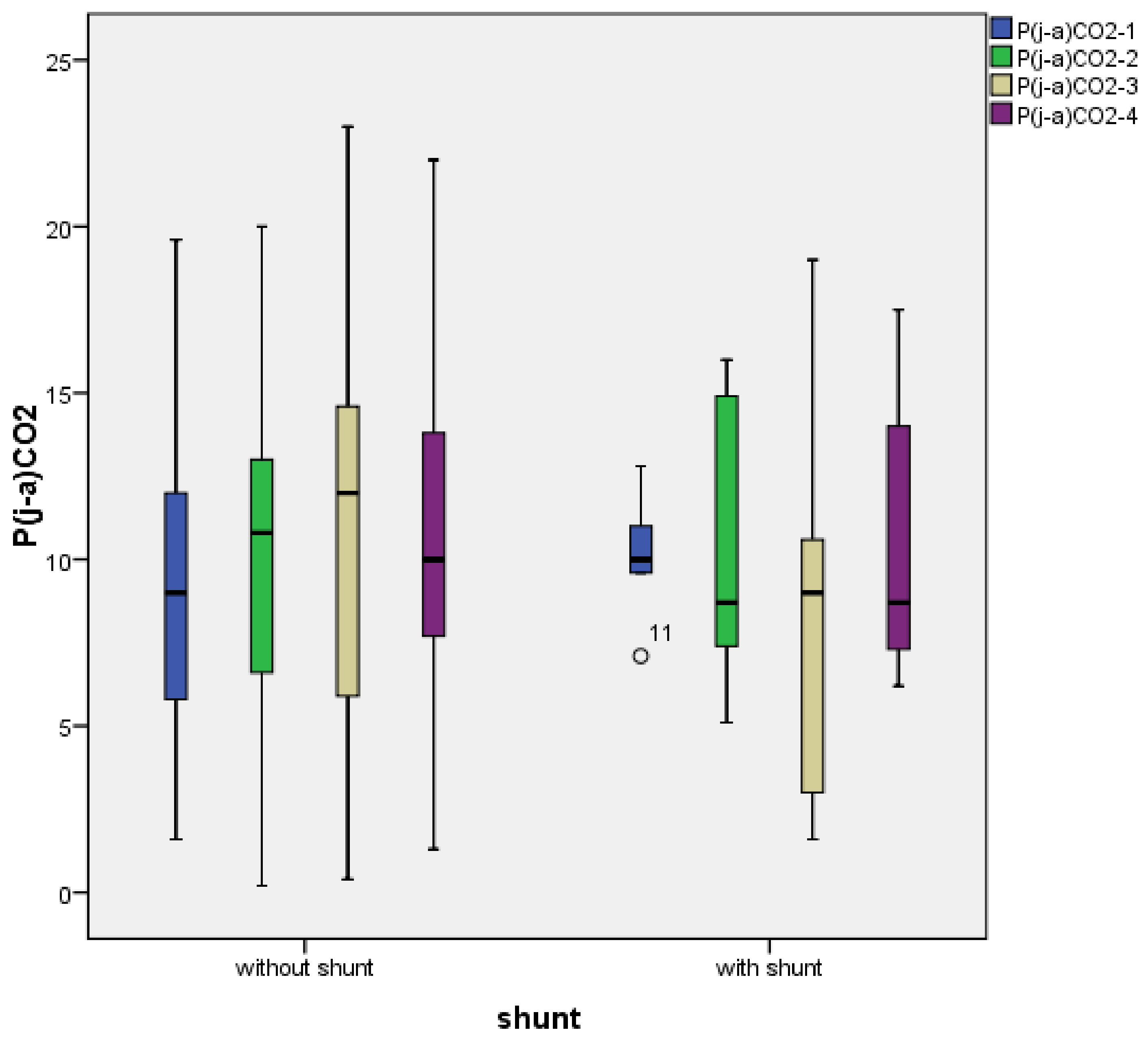
3.2. Temporal Characterization of Jugulo-Arterial Carbon Dioxide Tension Difference (P(j-a)CO2)
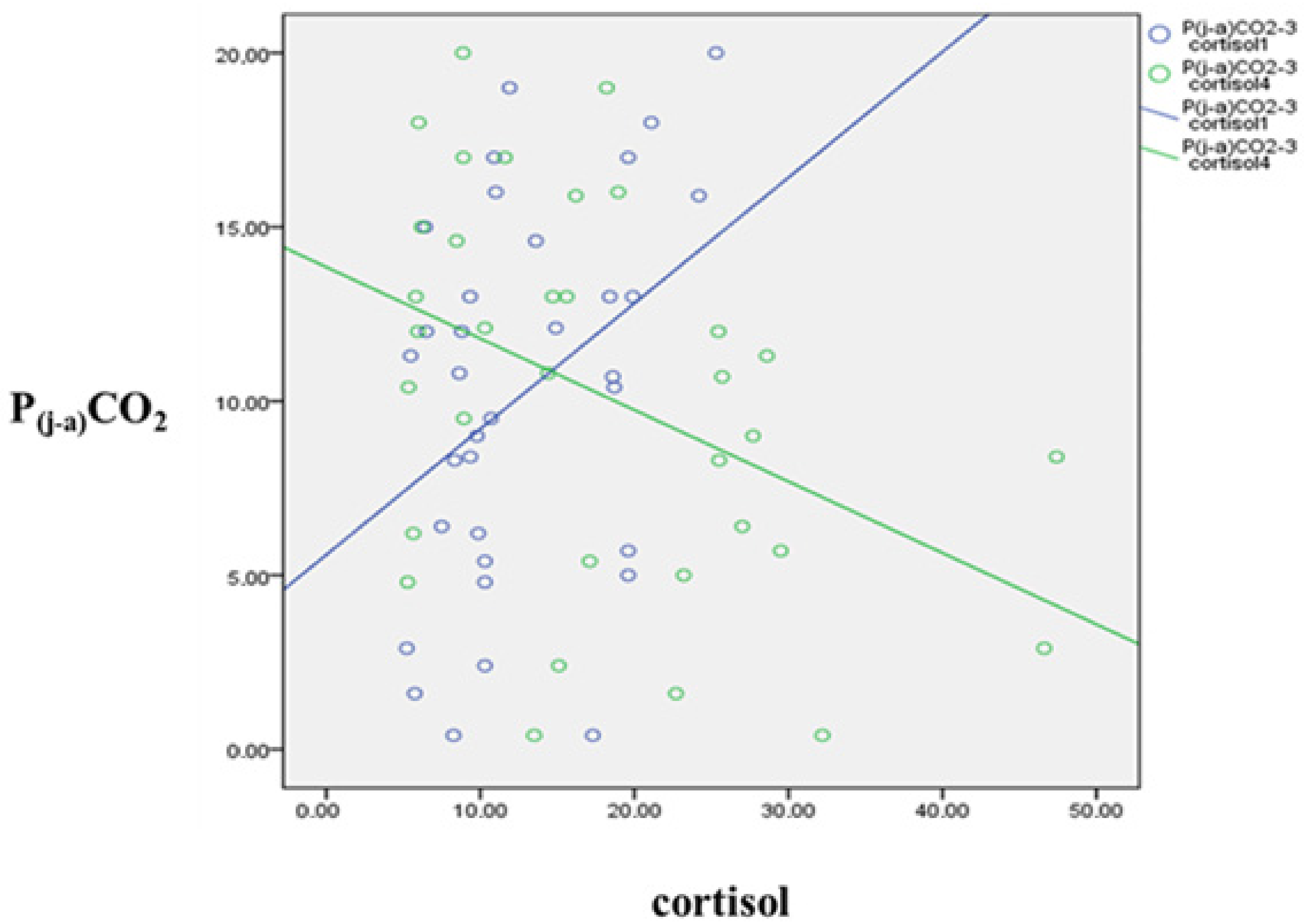
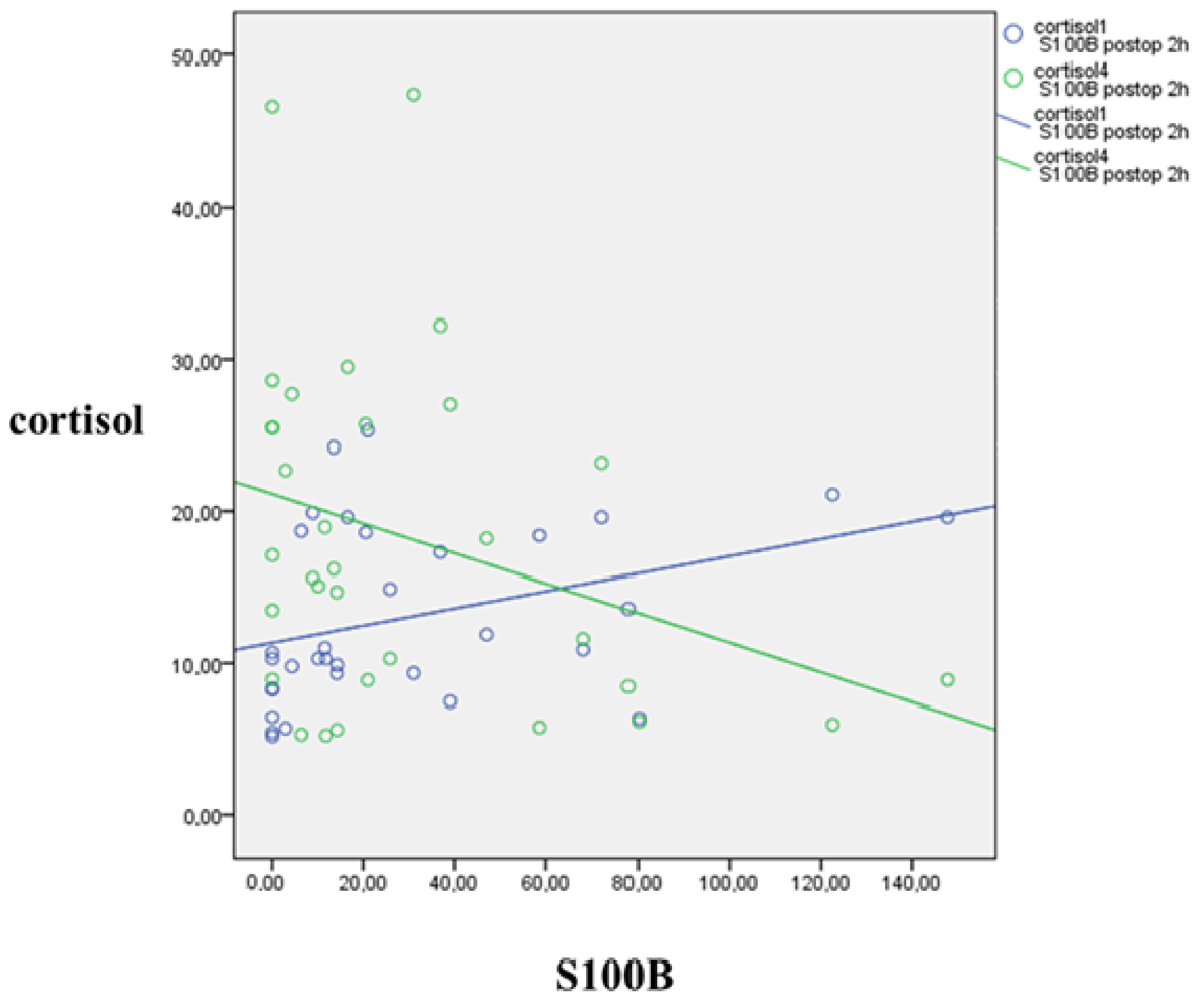
3.3. Jugulo-Arterial Carbon Dioxide Tension Difference (P(j-a)CO2) and Cortisol’s Relation
3.4. S100B’s Relation to Jugulo-Arterial Carbon Dioxide Gap (P(j-a)CO2) and Cortisol
3.5. Correlations between Lactate, S100B, Cortisol and L-Arginine
3.6. Correlation of Blood Pressure and P(j-a)CO2
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rerkasem, A.; Orrapin, S.; Howard, D.P.; Rerkasem, K. Carotid Endarterectomy for Symptomatic Carotid Stenosis. Cochrane Database Syst. Rev. 2020, 9, CD001081. [Google Scholar] [CrossRef]
- Marrocco-Trischitta, M.M.; Tiezzi, A.; Svampa, M.G.; Bandiera, G.; Camilli, S.; Stillo, F.; Petasecca, P.; Sampogna, F.; Abeni, D.; Guerrini, P. Perioperative Stress Response to Carotid Endarterectomy: The Impact of Anesthetic Modality. J. Vasc. Surg. 2004, 39, 1295–1304. [Google Scholar] [CrossRef] [PubMed]
- Unic-Stojanovic, D.; Jovic, M. Local Anaesthesia for Carotid Endarterectomy: Con: Decrease the Stress for All. Eur. J. Anaesthesiol. 2016, 33, 238–240. [Google Scholar] [CrossRef]
- Halandras, P.M. Vascular Surgery and Geriatric Patients. Clin. Geriatr. Med. 2019, 35, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Bourke, V.C.; Bourke, B.M.; Beiles, C.B. Operative Factors Associated with the Development of New Brain Lesions During Awake Carotid Endarterectomy. Eur. J. Vasc. Endovasc. Surg. 2016, 51, 167–173. [Google Scholar] [CrossRef][Green Version]
- Dellaretti, M.; de Vasconcelos, L.T.; Dourado, J.; de Souza, R.F.; Fontoura, R.R.; de Sousa, A.A. Locoregional Anesthesia for Carotid Endarterectomy: Identification of Patients with Intolerance to Cross-Clamping. World Neurosurg. 2016, 87, 61–64. [Google Scholar] [CrossRef]
- GALA Trial Collaborative Group; Lewis, S.C.; Warlow, C.P.; Bodenham, A.R.; Colam, B.; Rothwell, P.M.; Torgerson, D.; Dellagrammaticas, D.; Horrocks, M.; Liapis, C.; et al. General Anaesthesia versus Local Anaesthesia for Carotid Surgery (GALA): A Multicentre, Randomised Controlled Trial. Lancet 2008, 372, 2132–2142. [Google Scholar] [CrossRef] [PubMed]
- Bodenham, A.R.; Howell, S.J. General Anaesthesia vs. Local Anaesthesia: An Ongoing Story. Br. J. Anaesth. 2009, 103, 785–789. [Google Scholar] [CrossRef]
- Uno, M.; Takai, H.; Yagi, K.; Matsubara, S. Surgical Technique for Carotid Endarterectomy: Current Methods and Problems. Neurol. Med. Chir. 2020, 60, 419–428. [Google Scholar] [CrossRef]
- Kim, J.-S.; Ko, J.S.; Bang, S.; Kim, H.; Lee, S.Y. Cervical Plexus Block. Korean J. Anesthesiol. 2018, 71, 274–288. [Google Scholar] [CrossRef]
- Iwasaki, M.; Edmondson, M.; Sakamoto, A.; Ma, D. Anesthesia, Surgical Stress, and “Long-Term” Outcomes. Acta Anaesthesiol. Taiwan 2015, 53, 99–104. [Google Scholar] [CrossRef]
- Sternbach, Y.; Illig, K.A.; Zhang, R.; Shortell, C.K.; Rhodes, J.M.; Davies, M.G.; Lyden, S.P.; Green, R.M. Hemodynamic Benefits of Regional Anesthesia for Carotid Endarterectomy. J. Vasc. Surg. 2002, 35, 333–339. [Google Scholar] [CrossRef][Green Version]
- Jacques, F.; Elkouri, S.; Bracco, D.; Hemmerling, T.; Daniel, V.; Beaudoin, N.; Bruneau, L.; Blair, J.-F. Regional Anesthesia for Carotid Surgery: Less Intraoperative Hypotension and Vasopressor Requirement. Ann. Vasc. Surg. 2009, 23, 324–329. [Google Scholar] [CrossRef]
- Yücel, C.; Ketenciler, S.; Gürsoy, M.; Türkmen, S.; Kayalar, N. The Effect of Hemodynamic Parameters on Cerebral Oxygenization During Carotid Endarterectomy. Braz. J. Cardiovasc. Surg. 2021. online ahead of print. [Google Scholar] [CrossRef]
- Tauber, H.; Streif, W.; Gebetsberger, J.; Gasteiger, L.; Pierer, E.; Knoflach, M.; Fraedrich, G.; Gummerer, M.; Fritz, J.; Velik-Salchner, C. Cardiac Output and Cerebral Blood Flow during Carotid Surgery in Regional versus General Anesthesia: A Prospective Randomized Controlled Study. J. Vasc. Surg. 2021, 74, 930–937. [Google Scholar] [CrossRef]
- Prete, A.; Yan, Q.; Al-Tarrah, K.; Akturk, H.K.; Prokop, L.J.; Alahdab, F.; Foster, M.A.; Lord, J.M.; Karavitaki, N.; Wass, J.A.; et al. The Cortisol Stress Response Induced by Surgery: A Systematic Review and Meta-Analysis. Clin. Endocrinol. 2018, 89, 554–567. [Google Scholar] [CrossRef]
- Hoefer, J.; Pierer, E.; Rantner, B.; Stadlbauer, K.-H.; Fraedrich, G.; Fritz, J.; Kleinsasser, A.; Velik-Salchner, C. Ultrasound-Guided Regional Anesthesia for Carotid Endarterectomy Induces Early Hemodynamic and Stress Hormone Changes. J. Vasc. Surg. 2015, 62, 57–67. [Google Scholar] [CrossRef]
- Szabó, P.; Mayer, M.; Horváth-Szalai, Z.; Tóth, K.; Márton, S.; Menyhei, G.; Sínay, L.; Molnár, T. Awake Sedation with Propofol Attenuates Intraoperative Stress of Carotid Endarterectomy in Regional Anesthesia. Ann. Vasc. Surg. 2020, 63, 311–318. [Google Scholar] [CrossRef]
- Brooks, G.A. The Science and Translation of Lactate Shuttle Theory. Cell Metab. 2018, 27, 757–785. [Google Scholar] [CrossRef]
- Chong, Z.Z.; Changyaleket, B.; Xu, H.; Dull, R.O.; Schwartz, D.E. Identifying S100B as a Biomarker and a Therapeutic Target For Brain Injury and Multiple Diseases. Curr. Med. Chem. 2016, 23, 1571–1596. [Google Scholar] [CrossRef]
- Michetti, F.; D’Ambrosi, N.; Toesca, A.; Puglisi, M.A.; Serrano, A.; Marchese, E.; Corvino, V.; Geloso, M.C. The S100B Story: From Biomarker to Active Factor in Neural Injury. J. Neurochem. 2019, 148, 168–187. [Google Scholar] [CrossRef] [PubMed]
- Di Legge, S.; Di Piero, V.; Di Stani, F.; Perna, R.; Gattuso, R.; Reale, M.G.; Valentini, F.B.; Lenzi, G.L. Carotid Endarterectomy and Gliofibrillar S100b Protein Release. Neurol. Sci. 2003, 24, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Falkensammer, J.; Oldenburg, W.A.; Hendrzak, A.J.; Neuhauser, B.; Pedraza, O.; Ferman, T.; Klocker, J.; Biebl, M.; Hugl, B.; Meschia, J.F.; et al. Evaluation of Subclinical Cerebral Injury and Neuropsychologic Function in Patients Undergoing Carotid Endarterectomy. Ann. Vasc. Surg. 2008, 22, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Scarcello, E.; Morrone, F.; Piro, P.; Tarsitano, S.; Intrieri, F.; Vaccarella, S.; Guerra, E.; Serra, R.; de Franciscis, S. Protein S-100B as Biochemical Marker of Brain Ischemic Damage after Treatment of Carotid Stenosis. Ann. Vasc. Surg. 2011, 25, 975–978. [Google Scholar] [CrossRef]
- Arfvidsson, B.; Nilsson, T.K.; Norgren, L. S100B Concentrations Increase Perioperatively in Jugular Vein Blood despite Limited Metabolic and Inflammatory Response to Clinically Uneventful Carotid Endarterectomy. Clin. Chem. Lab. Med. 2015, 53, 111–117. [Google Scholar] [CrossRef]
- Makovec, M.; Kerin, K.; Skitek, M.; Jerin, A.; Klokočovnik, T. Association of Biomarker S100B and Cerebral Oximetry with Neurological Changes during Carotid Endarterectomy Performed in Awake Patients. Vasa 2020, 49, 285–293. [Google Scholar] [CrossRef]
- Changyaleket, B.; Xu, H.; Vetri, F.; Valyi-Nagy, T.; Paisansathan, C.; Chong, Z.Z.; Pelligrino, D.A.; Testai, F.D. Intracerebroventricular Application of S100B Selectively Impairs Pial Arteriolar Dilating Function in Rats. Brain Res. 2016, 1634, 171–178. [Google Scholar] [CrossRef]
- Mekontso-Dessap, A.; Castelain, V.; Anguel, N.; Bahloul, M.; Schauvliege, F.; Richard, C.; Teboul, J.-L. Combination of Venoarterial PCO2 Difference with Arteriovenous O2 Content Difference to Detect Anaerobic Metabolism in Patients. Intensive Care Med. 2002, 28, 272–277. [Google Scholar] [CrossRef]
- Huber, W.; Zanner, R.; Schneider, G.; Schmid, R.; Lahmer, T. Assessment of Regional Perfusion and Organ Function: Less and Non-Invasive Techniques. Front. Med. 2019, 6, 50. [Google Scholar] [CrossRef]
- Scheeren, T.W.L.; Wicke, J.N.; Teboul, J.-L. Understanding the Carbon Dioxide Gaps. Curr. Opin. Crit. Care 2018, 24, 181–189. [Google Scholar] [CrossRef]
- Mallat, J.; Lemyze, M.; Tronchon, L.; Vallet, B.; Thevenin, D. Use of Venous-to-Arterial Carbon Dioxide Tension Difference to Guide Resuscitation Therapy in Septic Shock. World J. Crit. Care Med. 2016, 5, 47–56. [Google Scholar] [CrossRef]
- Mahendran, S.; Nguyen, J.; Butler, E.; Aneman, A. Prospective, Observational Study of Carbon Dioxide Gaps and Free Energy Change and Their Association with Fluid Therapy Following Cardiac Surgery. Acta Anaesthesiol. Scand. 2020, 64, 202–210. [Google Scholar] [CrossRef]
- Rossi, S.; Colombo, A.; Magnoni, S.; Zanier, E.R.; Conte, V.; Stocchetti, N. Cerebral Veno-Arterial PCO2 Difference as an Estimator of Uncompensated Cerebral Hypoperfusion. Acta Neurochir. Suppl. 2002, 81, 201–204. [Google Scholar] [CrossRef]
- Chieregato, A.; Marchi, M.; Fainardi, E.; Targa, L. Cerebral Arterio-Venous PCO2 Difference, Estimated Respiratory Quotient, and Early Posttraumatic Outcome: Comparison with Arterio-Venous Lactate and Oxygen Differences. J. Neurosurg. Anesthesiol. 2007, 19, 222–228. [Google Scholar] [CrossRef]
- Moritz, S.; Kasprzak, P.; Woertgen, C.; Taeger, K.; Metz, C. The Accuracy of Jugular Bulb Venous Monitoring in Detecting Cerebral Ischemia in Awake Patients Undergoing Carotid Endarterectomy. J. Neurosurg. Anesthesiol. 2008, 20, 8–14. [Google Scholar] [CrossRef]
- Zanier, E.R.; Rossi, S.; Conte, V.; Colombo, A.; Nicolini, R.; Caironi, P.; Stocchetti, N.; Gattinoni, L. The Ratio between Arterio-Venous PCO2 Difference and Arterio-Jugular Oxygen Difference as Estimator of Critical Cerebral Hypoperfusion. Minerva Anestesiol. 2006, 72, 543–549. [Google Scholar]
- Nishiyama, Y.; Ueda, M.; Katsura, K.-I.; Otsuka, T.; Abe, A.; Nagayama, H.; Katayama, Y. Asymmetric Dimethylarginine (ADMA) as a Possible Risk Marker for Ischemic Stroke. J. Neurol. Sci. 2010, 290, 12–15. [Google Scholar] [CrossRef]
- Baky, N.A.A.; Zaidi, Z.F.; Fatani, A.J.; Sayed-Ahmed, M.M.; Yaqub, H. Nitric Oxide Pros and Cons: The Role of L-Arginine, a Nitric Oxide Precursor, and Idebenone, a Coenzyme-Q Analogue in Ameliorating Cerebral Hypoxia in Rat. Brain Res. Bull. 2010, 83, 49–56. [Google Scholar] [CrossRef]
- Meloni, B.P.; Brookes, L.M.; Clark, V.W.; Cross, J.L.; Edwards, A.B.; Anderton, R.S.; Hopkins, R.M.; Hoffmann, K.; Knuckey, N.W. Poly-Arginine and Arginine-Rich Peptides Are Neuroprotective in Stroke Models. J. Cereb. Blood Flow Metab. 2015, 35, 993–1004. [Google Scholar] [CrossRef]
- Molnar, T.; Pusch, G.; Papp, V.; Feher, G.; Szapary, L.; Biri, B.; Nagy, L.; Keki, S.; Illes, Z. The L-Arginine Pathway in Acute Ischemic Stroke and Severe Carotid Stenosis: Temporal Profiles and Association with Biomarkers and Outcome. J. Stroke Cerebrovasc. Dis. 2014, 23, 2206–2214. [Google Scholar] [CrossRef]
- Kielstein, J.T.; Donnerstag, F.; Gasper, S.; Menne, J.; Kielstein, A.; Martens-Lobenhoffer, J.; Scalera, F.; Cooke, J.P.; Fliser, D.; Bode-Böger, S.M. ADMA Increases Arterial Stiffness and Decreases Cerebral Blood Flow in Humans. Stroke 2006, 37, 2024–2029. [Google Scholar] [CrossRef] [PubMed]
- Pizzarelli, F.; Maas, R.; Dattolo, P.; Tripepi, G.; Michelassi, S.; D’Arrigo, G.; Mieth, M.; Bandinelli, S.; Ferrucci, L.; Zoccali, C. Asymmetric Dimethylarginine Predicts Survival in the Elderly. Age 2013, 35, 2465–2475. [Google Scholar] [CrossRef] [PubMed]
- Szabo, P.; Lantos, J.; Nagy, L.; Keki, S.; Volgyi, E.; Menyhei, G.; Illes, Z.; Molnar, T. L-Arginine Pathway Metabolites Predict Need for Intra-Operative Shunt During Carotid Endarterectomy. Eur. J. Vasc. Endovasc. Surg. 2016, 52, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Do, W.; Cho, A.-R.; Kim, E.-J.; Kim, H.-J.; Kim, E.; Lee, H.-J. Ultrasound-Guided Superficial Cervical Plexus Block under Dexmedetomidine Sedation versus General Anesthesia for Carotid Endarterectomy: A Retrospective Pilot Study. Yeungnam Univ. J. Med. 2018, 35, 45–53. [Google Scholar] [CrossRef]
- Watts, M.E.; Pocock, R.; Claudianos, C. Brain Energy and Oxygen Metabolism: Emerging Role in Normal Function and Disease. Front. Mol. Neurosci. 2018, 11, 216. [Google Scholar] [CrossRef]
- Hicks, J.W.; Wang, T. Arterial Blood Gases during Maximum Metabolic Demands: Patterns across the Vertebrate Spectrum. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2021, 254, 110888. [Google Scholar] [CrossRef]
- Li, J.; Shalabi, A.; Ji, F.; Meng, L. Monitoring Cerebral Ischemia during Carotid Endarterectomy and Stenting. J. Biomed. Res. 2017, 31, 11–16. [Google Scholar] [CrossRef]
- Richards, S.J.G.; Frizelle, F.A.; Geddes, J.A.; Eglinton, T.W.; Hampton, M.B. Frailty in Surgical Patients. Int. J. Colorectal Dis. 2018, 33, 1657–1666. [Google Scholar] [CrossRef]
- Drummond, J.C. Blood Pressure and the Brain: How Low Can You Go? Anesth. Analg. 2019, 128, 759–771. [Google Scholar] [CrossRef]
- Stoneham, M.D.; Thompson, J.P. Arterial Pressure Management and Carotid Endarterectomy. Br. J. Anaesth. 2009, 102, 442–452. [Google Scholar] [CrossRef]
- Miller, T.R.; Shivashankar, R.; Mossa-Basha, M.; Gandhi, D. Reversible Cerebral Vasoconstriction Syndrome, Part 1: Epidemiology, Pathogenesis, and Clinical Course. Am. J. Neuroradiol. 2015, 36, 1392–1399. [Google Scholar] [CrossRef]
- Gupta, D.; Morley, J.E. Hypothalamic-Pituitary-Adrenal (HPA) Axis and Aging. Compr. Physiol. 2014, 4, 1495–1510. [Google Scholar] [CrossRef]
- Molnar, T.; Horvath, A.; Szabo, Z.; Vamos, Z.; Dóczi, T.; Illes, Z. Detection of silent cerebral microcirculatory abnormalities in patients with manifest ischemic coronary disease: A perfusion brain MRI study combined with dipyridamole stress. Scand. Cardivasc. J. 2020, 55, 97–101. [Google Scholar] [CrossRef]

| Total Population n = 54 | Without Shunt n = 49 | Shunted Patients n = 5 | p-Value | |
|---|---|---|---|---|
| age | 65.59 ± 8.0 | 65.45 ± 8.31 | 67 ± 4.18 | 0.504 |
| male (%) | 43 (79.6) | 38 (77.55) | 5 (100) | 0.206 |
| BMI | 27.33 ± 4.57 | 27.12 ± 4.59 | 29.24 ± 4.37 | 0.353 |
| right sided operation (%) | 23 (42.59) | 22 (44.89) | 1 (20) | 0.658 |
| operated stenosis (%) | 84.72 ± 6.33 | 84.9 ± 6.25 | 83.0 ± 7.58 | 0.614 |
| contralateral stenosis (%) | 44.89 ± 25.74 | 45,35 ± 25.71 | 40 ± 29.44 | 0.746 |
| clamp time (minutes) | 22.11 ± 6.36 | 22.36 ± 6.17 | 19.66 ± 8.4 | 0.520 |
| creatinine (µmol/L) | 81.37 ± 22.25 | 80.53 ± 22.13 | 89.6 ± 24.2 | 0.460 |
| hemoglobin before surgery (g/dL) | 14.19 ± 1.09 | 14.14 ± 1.13 | 14.76 ± 0.54 | 0.062 |
| hemoglobin after surgery (g/dL) | 12.98 ± 1.02 | 12.95 ± 1.02 | 13.24 ± 1.12 | 0.607 |
| Previous stroke (%) | 20 (37) | 18 (36.7) | 2 (40) | 0.885 |
| Previous TIA (%) | 10 (18.5) | 9 (18.4) | 1 (20) | 0.929 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovács-Ábrahám, Z.; Aczél, T.; Jancsó, G.; Horváth-Szalai, Z.; Nagy, L.; Tóth, I.; Nagy, B.; Molnár, T.; Szabó, P. Cerebral and Systemic Stress Parameters in Correlation with Jugulo-Arterial CO2 Gap as a Marker of Cerebral Perfusion during Carotid Endarterectomy. J. Clin. Med. 2021, 10, 5479. https://doi.org/10.3390/jcm10235479
Kovács-Ábrahám Z, Aczél T, Jancsó G, Horváth-Szalai Z, Nagy L, Tóth I, Nagy B, Molnár T, Szabó P. Cerebral and Systemic Stress Parameters in Correlation with Jugulo-Arterial CO2 Gap as a Marker of Cerebral Perfusion during Carotid Endarterectomy. Journal of Clinical Medicine. 2021; 10(23):5479. https://doi.org/10.3390/jcm10235479
Chicago/Turabian StyleKovács-Ábrahám, Zoltán, Timea Aczél, Gábor Jancsó, Zoltán Horváth-Szalai, Lajos Nagy, Ildikó Tóth, Bálint Nagy, Tihamér Molnár, and Péter Szabó. 2021. "Cerebral and Systemic Stress Parameters in Correlation with Jugulo-Arterial CO2 Gap as a Marker of Cerebral Perfusion during Carotid Endarterectomy" Journal of Clinical Medicine 10, no. 23: 5479. https://doi.org/10.3390/jcm10235479
APA StyleKovács-Ábrahám, Z., Aczél, T., Jancsó, G., Horváth-Szalai, Z., Nagy, L., Tóth, I., Nagy, B., Molnár, T., & Szabó, P. (2021). Cerebral and Systemic Stress Parameters in Correlation with Jugulo-Arterial CO2 Gap as a Marker of Cerebral Perfusion during Carotid Endarterectomy. Journal of Clinical Medicine, 10(23), 5479. https://doi.org/10.3390/jcm10235479








