Complement Component C1q as a Potential Diagnostic Tool for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Subtyping
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Measures
2.2.1. Fatigue Impact Scale
2.2.2. Composite Autonomic Symptom Score
2.2.3. Pittsburgh Sleep Quality Index
2.2.4. Short-Form-36 Health Survey
2.3. Blood Collection and Processing
2.4. Blood Analytics
2.5. Cluster Analysis
2.6. Statistical Analysis and Plotting
3. Results
3.1. Demographics and Clinical Characteristics of the Participants
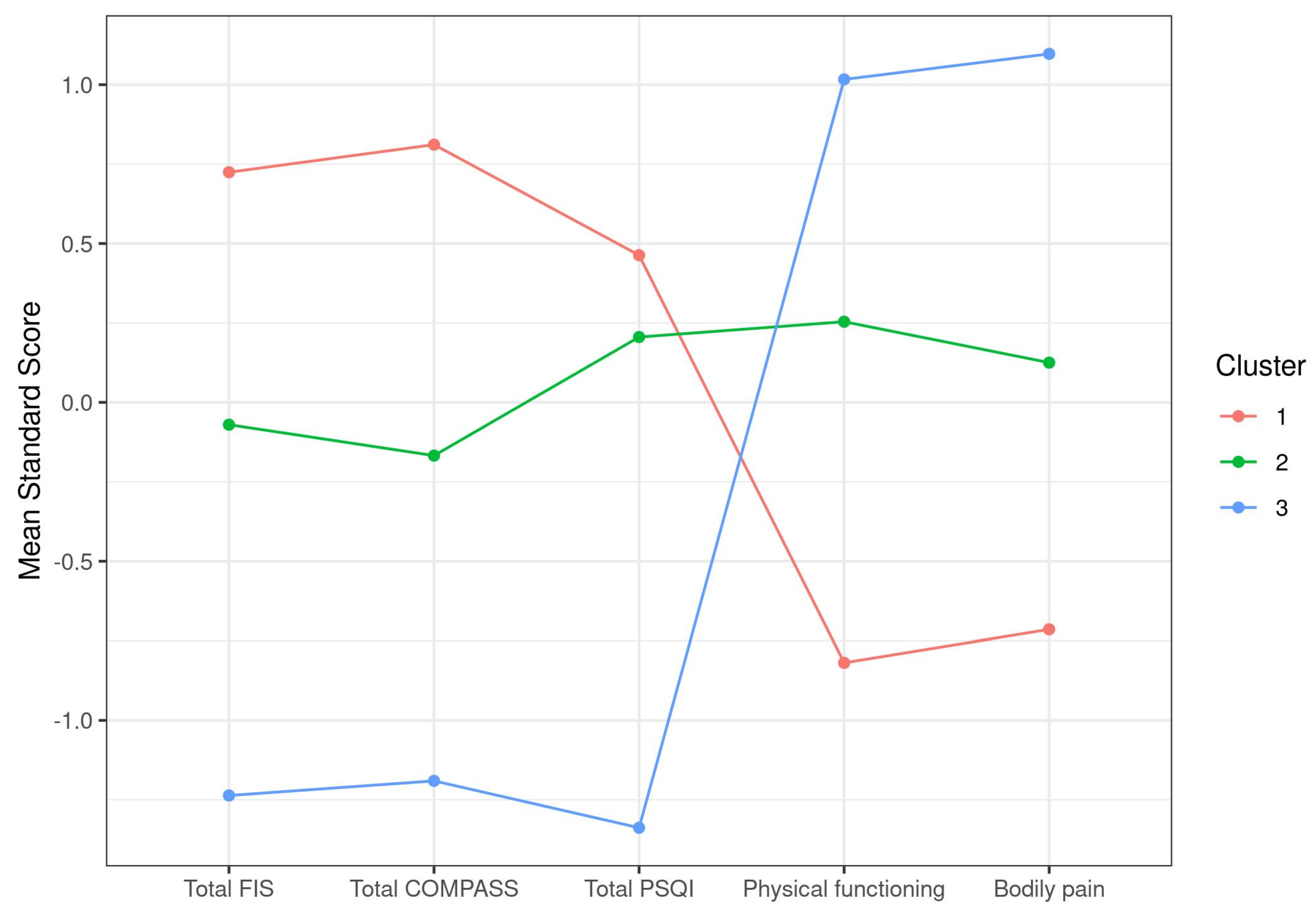
3.2. Exploratory Case Cluster Analysis Based on Symptoms
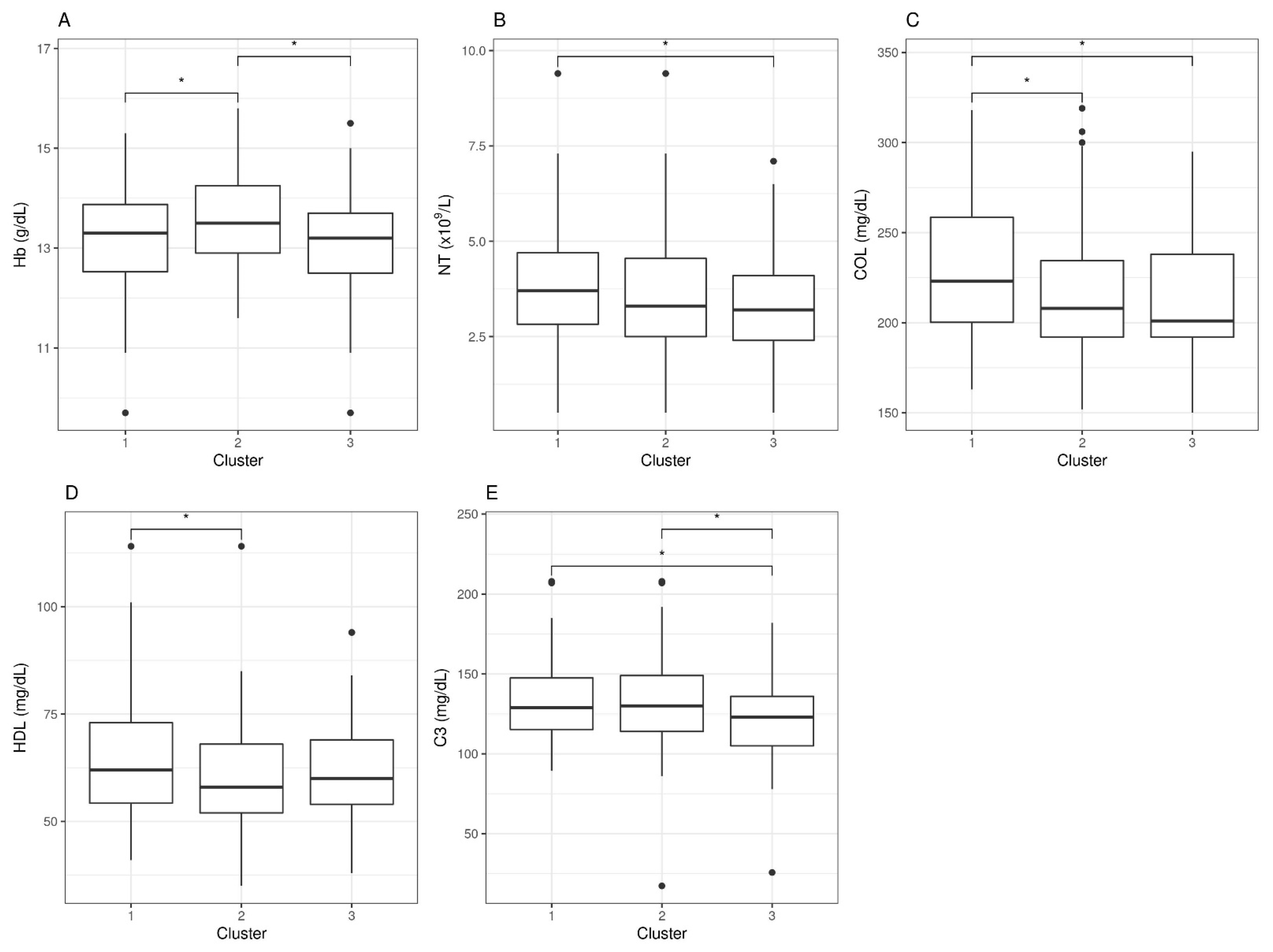
3.3. Cluster-Based Differential Analysis of Blood Parameters
3.4. Stratified Analysis
3.4.1. Outstanding Blood Parameters with Abnormal Values
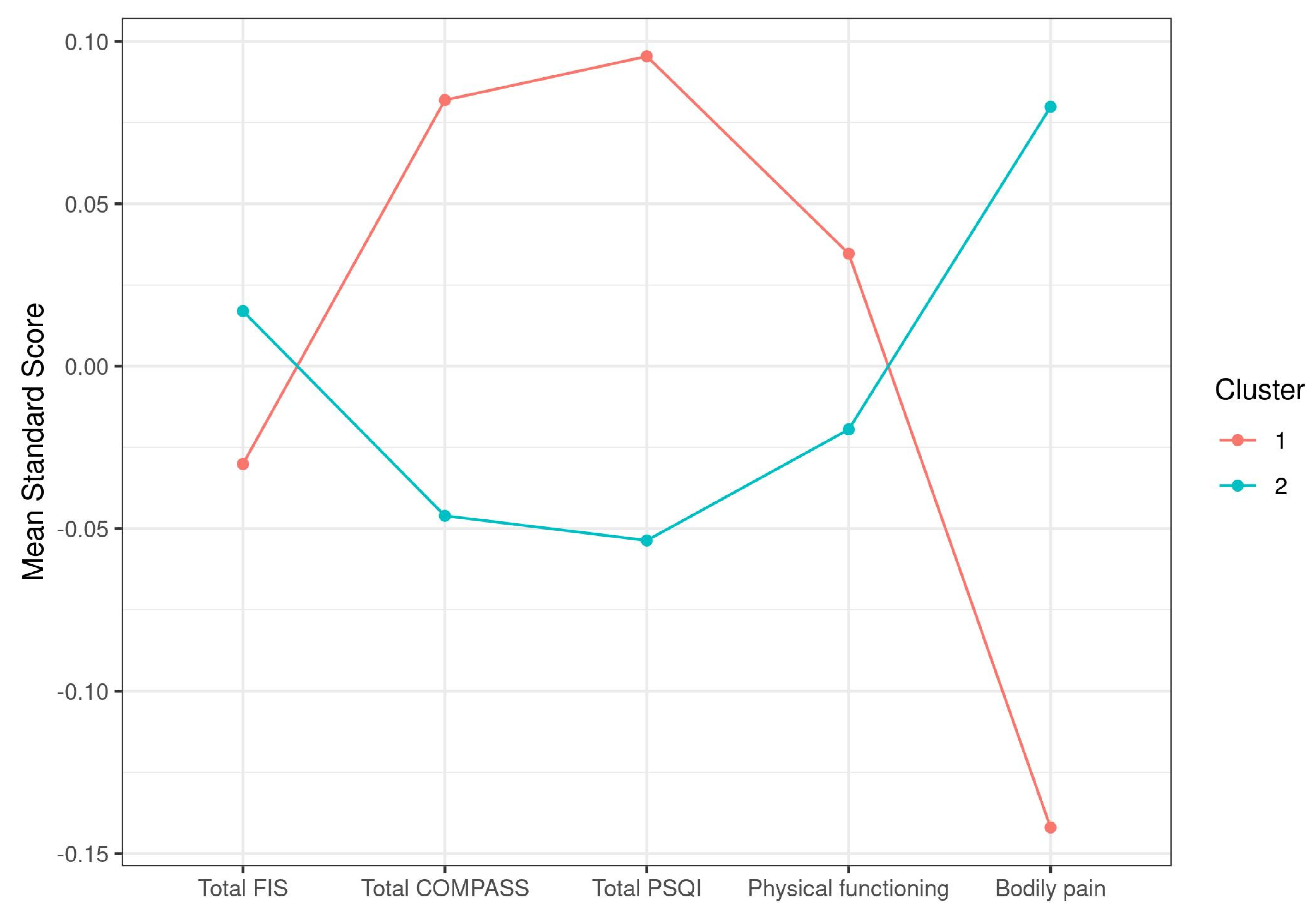
3.4.2. Symptom Differences across C1q Case Clusters
3.4.3. Blood analytic Differences across C1q Case Clusters
4. Discussion
Additional Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. ICD-10: International Classification of Statistical Classification of Diseases and Related Health Problems; World Health Organization: Geneva, Switzerland, 2004. [Google Scholar]
- World Health Organization; Committee on the Diagnostic Criteria for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome; Board on the Health of Select Populations; Institute of Medicine. Beyond Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Redefining an Illness; National Academies Press: Washington, DC, USA, 2015. [Google Scholar]
- Estévez-López, F.; Castro-Marrero, J.; Wang, X.; Bakken, I.J.; Ivanovs, A.; Nacul, L.; Sepúlveda, N.; Strand, E.B.; Pheby, D.; Alegre, J.; et al. European Network on ME/CFS (EUROMENE). Prevalence and incidence of myalgic encephalomyelitis/chronic fatigue syndrome in Europe: The Euro-EpiME study from the European network on ME/CFS (EUROMENE): A protocol for a systematic review. BMJ Open 2018, 8, e020817. [Google Scholar] [CrossRef] [PubMed]
- Clayton, E.W. Beyond myalgic encephalomyelitis/chronic fatigue syndrome: An IOM report on redefining an illness. JAMA 2015, 313, 1101–1102. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, K.; Straus, S.E.; Hickie, I.; Sharpe, M.C.; Dobbins, J.G.; Komaroff, A.; Schluederberg, A.; Jones, J.F.; Lloyd, A.R.; Wessely, S.; et al. The chronic fatigue syndrome: A comprehensive approach to its definition and study. Ann. Intern. Med. 1994, 121, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Carruthers, B.M.; Jain, A.K.; De Meirleir, K.L.; Peterson, D.L.; Klimas, N.G.; Lerner, A.; Bested, A.C.; Flor-Henry, P.; Joshi, P.; Powles, A.C.P.; et al. Myalgic encephalomyelitis/chronic fatigue syndrome: Clinical working case definition, diagnostic and treatment protocols. J. Chron. Fatigue Syndr. 2003, 11, 7–115. [Google Scholar] [CrossRef]
- Carruthers, B.M.; van de Sande, M.I.; De Meirleir, K.L.; Klimas, N.G.; Broderick, G.; Mitchell, T.; Staines, D.; Powles, A.C.P.; Speight, N.; Vallings, R.; et al. Myalgic encephalomyelitis: International Consensus Criteria. J. Intern. Med. 2011, 270, 3273–3278. [Google Scholar] [CrossRef] [Green Version]
- Lidbury, B.A.; Fisher, P.R. Biomedical Insights that Inform the Diagnosis of ME/CFS. Diagnostics 2020, 10, 92. [Google Scholar] [CrossRef] [Green Version]
- Nacul, L.; de Barros, B.; Kingdon, C.C.; Cliff, J.M.; Clark, T.G.; Mudie, K.; Dockrell, H.M.; Lacerda, E.M. Evidence of Clinical Pathology Abnormalities in People with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) from an Analytic Cross-Sectional Study. Diagnostics 2019, 9, 41. [Google Scholar] [CrossRef] [Green Version]
- Almenar-Pérez, E.; Sarria, L.; Nathanson, L.; Oltra, E. Assessing diagnostic value of microRNAs from peripheral blood mononuclear cells and extracellular vesicles in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Sci. Rep. 2020, 10, 2064. [Google Scholar] [CrossRef] [Green Version]
- Kitami, T.; Fukuda, S.; Kato, T.; Yamaguti, K.; Nakatomi, Y.; Yamano, E.; Kataoka, Y.; Mizuno, K.; Tsuboi, Y.; Kogo, Y.; et al. Deep phenotyping of myalgic encephalomyelitis/chronic fatigue syndrome in Japanese population. Sci. Rep. 2020, 10, 19933. [Google Scholar] [CrossRef]
- Fisk, J.D.; Ritvo, P.G.; Ross, L.; Haase, D.A.; Marrie, T.J.; Schlech, W.F. Measuring the functional impact of fatigue: Initial validation of the fatigue impact scale. Clin. Infect. Dis. 1994, 18, S79–S83. [Google Scholar] [CrossRef] [PubMed]
- Sletten, D.M.; Suarez, G.A.; Low, P.A.; Mandrekar, J.; Singer, W. COMPASS 31: A refined and abbreviated Composite Autonomic Symptom Score. Mayo. Clin. Proc. 2012, 87, 1196–1201. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Alonso, J.; Prieto, L.; Antó, J.M. The Spanish version of the SF-36 Health Survey: An instrument for measuring clinical results. Med. Clin. 1995, 104, 771–776. [Google Scholar]
- Ward, J.H., Jr. Hierarchical Grouping to Optimize an Objective Function. J. Am. Stat. Assoc. 1963, 58, 236–244. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org (accessed on 4 September 2020).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Obese Chooi, Y.C.; Ding, C.; Magkos, F. The epidemiology of obesity. Metabolism 2019, 92, 6–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrari, D.; Lombardi, G.; Banfi, G. Concerning the vitamin D reference range: Pre-analytical and analytical variability of vitamin D measurement. Biochem. Med. 2017, 27, 030501. [Google Scholar] [CrossRef] [Green Version]
- Nikolac Gabaj, N.; Unic, A.; Miler, M.; Pavicic, T.; Culej, J.; Bolanca, I.; Herman Mahecic, D.; Milevoj Kopcinovic, L.; Vrtaric, A. In sickness and in health: Pivotal role of vitamin D. Biochem Med. 2020, 30, 020501. [Google Scholar] [CrossRef]
- Surdu, A.M.; Pînzariu, O.; Ciobanu, D.M.; Negru, A.G.; Căinap, S.S.; Lazea, C.; Iacob, D.; Săraci, G.; Tirinescu, D.; Borda, I.M.; et al. Vitamin D and Its Role in the Lipid Metabolism and the Development of Atherosclerosis. Biomedicines 2021, 9, 172. [Google Scholar] [CrossRef] [PubMed]
- Yarparvar, A.; Elmadfa, I.; Djazayery, A.; Abdollahi, Z.; Salehi, F. The Association of Vitamin D Status with Lipid Profile and Inflammation Biomarkers in Healthy Adolescents. Nutrients 2020, 12, 590. [Google Scholar] [CrossRef] [Green Version]
- Trendelenburg, M. Autoantibodies against complement component C1q in systemic lupus erythematosus. Clin. Transl. Immunol. 2021, 10, e1279. [Google Scholar] [CrossRef]
- Słomko, J.; Estévez-López, F.; Kujawski, S.; Zawadka-Kunikowska, M.; Tafil-Klawe, M.; Klawe, J.J.; Morten, K.J.; Szrajda, J.; Murovska, M.; Newton, J.L.; et al. Autonomic Phenotypes in Chronic Fatigue Syndrome Are Associated with Illness Severity: A Cluster Analysis. J. Clin. Med. 2020, 9, 2531. [Google Scholar] [CrossRef]
- Merle, N.S.; Church, S.E.; Fremeaux-Bacchi, V.; Roumenina, L.T. Complement System Part I—Molecular Mechanisms of Activation and Regulation. Front Immunol. 2015, 6, 262. [Google Scholar] [CrossRef] [Green Version]
- Merle, N.S.; Noe, R.; Halbwachs-Mecarelli, L.; Fremeaux-Bacchi, V.; Roumenina, L.T. Complement System Part II: Role in Immunity. Front Immunol. 2015, 6, 257. [Google Scholar] [CrossRef] [Green Version]
- Roumenina, L.T.; Popov, K.T.; Bureeva, S.V.; Kojouharova, M.; Gadjeva, M.; Rabheru, S.; Thakrar, R.; Kaplun, A.; Kishore, U. Interaction of the globular domain of human C1q with Salmonella typhimurium lipopolysaccharide. Biochim. Biophys. Acta 2008, 1784, 1271–1276. [Google Scholar] [CrossRef]
- Gaboriaud, C.; Frachet, P.; Thielens, N.M.; Arlaud, G.J. The human c1q globular domain: Structure and recognition of non-immune self-ligands. Front Immunol. 2012, 2, 92. [Google Scholar] [CrossRef] [Green Version]
- Païdassi, H.; Tacnet-Delorme, P.; Lunardi, T.; Arlaud, G.J.; Thielens, N.M.; Frachet, P. The lectin-like activity of human C1q and its implication in DNA and apoptotic cell recognition. FEBS Lett. 2008, 582, 3111–3116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Defendi, F.; Thielens, N.M.; Clavarino, G.; Cesbron, J.Y.; Dumestre-Pérard, C. The Immunopathology of Complement Proteins and Innate Immunity in Autoimmune Disease. Clin. Rev. Allergy Immunol. 2020, 58, 229–251. [Google Scholar] [CrossRef]
- Morris, G.; Berk, M.; Galecki, P.; Maes, M. The emerging role of autoimmunity in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). Mol Neurobiol. 2014, 49, 741–756. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.; Berk, M.; Klein, H.; Walder, K.; Galecki, P.; Maes, M. Nitrosative Stress, Hypernitrosylation, and Autoimmune Responses to Nitrosylated Proteins: New Pathways in Neuroprogressive Disorders Including Depression and Chronic Fatigue Syndrome. Mol. Neurobiol. 2017, 54, 4271–4291. [Google Scholar] [CrossRef] [PubMed]
- Benavente, F.; Piltti, K.M.; Hooshmand, M.J.; Nava, A.A.; Lakatos, A.; Feld, B.G.; Creasman, D.; Gershon, P.D.; Anderson, A. Novel C1q receptor-mediated signaling controls neural stem cell behavior and neurorepair. Elife 2020, 9, e55732. [Google Scholar] [CrossRef] [PubMed]
- Noble, M.; Pröschel, C. The many roles of C1q. Elife 2020, 9, e61599. [Google Scholar] [CrossRef] [PubMed]
- Kouser, L.; Madhukaran, S.P.; Shastri, A.; Saraon, A.; Ferluga, J.; Al-Mozaini, M.; Kishore, U. Emerging and Novel Functions of Complement Protein C1q. Front Immunol. 2015, 6, 317. [Google Scholar] [CrossRef] [Green Version]
- Cho, K. Emerging Roles of Complement Protein C1q in Neurodegeneration. Aging Dis. 2019, 10, 652–663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bialas, A.R.; Stevens, B. TGF-β signaling regulates neuronal C1q expression and developmental synaptic refinement. Nat. Neurosci. 2013, 16, 1773–1782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Färber, K.; Cheung, G.; Mitchell, D.; Wallis, R.; Weihe, E.; Schwaeble, W.; Kettenmann, H. C1q, the recognition subcomponent of the classical pathway of complement, drives microglial activation. J. Neurosci. Res. 2009, 87, 644–652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lynch, N.J.; Willis, C.L.; Nolan, C.C.; Roscher, S.; Fowler, M.J.; Weihe, E.; Ray, D.E.; Schwaeble, W.J. Microglial activation and increased synthesis of complement component C1q precedes blood-brain barrier dysfunction in rats. Mol. Immunol. 2004, 40, 709–716. [Google Scholar] [CrossRef]
- Burckhardt, C.S.; Clark, S.R.; Bennett, R.M. The fibromyalgia impact questionnaire: Development and validation. J. Rheumatol. 1991, 18, 728–733. [Google Scholar] [PubMed]
- Rivera, J.; González, T. The Fibromyalgia Impact Questionnaire: A validated Spanish version to assess the health status in women with fibromyalgia. Clin. Exp. Rheumatol. 2004, 22, 554–560. [Google Scholar]
- Patel, K.V.; Amtmann, D.; Jensen, M.P.; Smith, S.M.; Veasley, C.; Turk, D.C. Clinical outcome assessment in clinical trials of chronic pain treatments. Pain Rep. 2021, 6, e784. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [Green Version]
- Ross, A.C.; Manson, J.E.; Abrams, S.A.; Aloia, J.F.; Brannon, P.M.; Clinton, S.K.; Durazo-Arvizu, R.A.; Gallagher, J.C.; Gallo, R.L.; Jones, G.; et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: What clinicians need to know. J. Clin. Endocrinol. Metab. 2011, 96, 53–58. [Google Scholar] [CrossRef] [PubMed]
| Variables | ME/CFS (n = 250) |
|---|---|
| Age, years | 45.9 ± 7.02 |
| BMI, kg/m2 † | 24.5 ± 4.72 |
| SBP, mmHg | 125.8 ± 2.5 |
| DBP (mmHg) | 76.3 ± 1.6 |
| Medication, n (%) | |
| NSAIDs | 9 (42.9) |
| Hypnotics | 5 (23.8) |
| Antidepressants | 6 (28.6) |
| Antipsychotics | 4 (19.0) |
| Opioids | 11 (52.4) |
| Measures | |
| FIS-40 | |
| Global score (0–160) | |
| Physical | 35.4 ± 2.4 |
| Cognitive | 34.0 ± 3.4 |
| Psychosocial | 63.9 ± 2.4 |
| COMPASS-31 | |
| Global score (0–100) | 53.6 ± 3.5 |
| Orthostatic intolerance | 24.3 ± 2.1 |
| Vasomotor | 1.4 ± 2.7 |
| Secretomotor | 9.3 ± 3.4 |
| Gastrointestinal | 11.6 ± 2.9 |
| Bladder | 3.5 ± 4.1 |
| Pupillomotor | 3.7 ± 3.4 |
| PSQI | |
| Global score (0–21) | 14.0 ± 0.7 |
| Subjective sleep quality | 1.9 ± 0.1 |
| Sleep latency | 2.2 ± 0.1 |
| Sleep duration | 1.5 ± 0.1 |
| Habitual sleep efficiency | 1.9 ± 0.2 |
| Sleep disturbances | 2.4 ± 0.1 |
| Sleeping medication | 1.9 ± 0.2 |
| Daytime dysfunction | 2.2 ± 0.1 |
| SF-36 | |
| Physical functioning | 26.9 ± 0.6 |
| Physical role | 3.7 ± 0.81 |
| Bodily pain | 16.2 ± 1.55 |
| General health perception | 21.3 ± 2.18 |
| Vitality | 17.0 ± 1.58 |
| Social role functioning | 28.2 ± 1.87 |
| Emotional role functioning | 30.5 ± 2.78 |
| Mental health | 41.4 ± 3.12 |
| Cluster 1 | Cluster 2 | Cluster 3 | p-Value | |
|---|---|---|---|---|
| Total FIS | 147.93 (9.88) | 131.79 (13.48) | 108.1 (21.83) | <0.0001 |
| Total COMPASS | 66.82 (10.52) | 50.9 (10.77) | 34.26 (12.71) | <0.0001 |
| Total PSQI | 15.82 (3.4) | 14.75 (2.94) | 8.33 (2.78) | <0.0001 |
| Physical functioning | 12.82 (8.82) | 31.27 (12.75) | 44.39 (16.79) | <0.0001 |
| Bodily pain | 6.3 (7.77) | 17.95 (11.79) | 31.45 (11.83) | <0.0001 |
| Size | 94 | 107 | 49 |
| Cluster 1 | Cluster 2 | Cluster 3 | p-Value | |
|---|---|---|---|---|
| Hb (g/dL) | 13.16 (1.05) | 13.57 (0.94) | 13.07 (1.12) | 0.0033 |
| NT (×/L) | 3.99 (1.65) | 3.58 (1.63) | 3.3 (1.48) | 0.0365 |
| COL (mg/dL) | 229.57 (36.69) | 216.6 (37.42) | 211.84 (33.73) | 0.0077 |
| HDL (mg/dL) | 64.71 (14.44) | 59.71 (12.48) | 63.12 (12.65) | 0.0292 |
| C3 (mg/dL) | 132.32 (24.65) | 132.02 (29.96) | 119.89 (28.09) | 0.0246 |
| Variables | n (%) |
|---|---|
| 25(OH).Vit.D3 | 151 (60.4) |
| LDL | 140 (56) |
| C1q | 107 (42.8) |
| 25(OH).Vit.D3, LDL | 81 (32.4) |
| 25(OH).Vit.D3, C1q | 72 (28.8) |
| COL | 66 (26.4) |
| C1q, LDL | 60 (24) |
| COL, LDL | 58 (23.2) |
| PMV | 56 (22.4) |
| Cluster 1 | Cluster 2 | p-Value | |
|---|---|---|---|
| Total FIS | 132.6 (22.83) | 133.56 (18.81) | 0.7358 |
| Total COMPASS | 54.96 (16.84) | 52.88 (15.94) | 0.3401 |
| Total PSQI | 14.29 (4.06) | 13.67 (4.21) | 0.2544 |
| Physical functioning | 27.5 (18.36) | 26.57 (16.56) | 0.6907 |
| Bodily pain | 14.24 (13.63) | 17.32 (13.95) | 0.0906 |
| Size | 90 | 160 |
| Cluster 1 | Cluster 2 | p-Value | |
|---|---|---|---|
| RBC (×/L) | 4.62 (0.32) | 4.53 (0.38) | 0.0431 |
| PT (g/dL) | 7.24 (0.39) | 7.1 (0.42) | 0.0093 |
| IgG3/IgG | 6.29 (3.64) | 8.91 (13.47) | 0.0219 |
| IgG4/IgG | 2.75 (1.7) | 3.32 (2.56) | 0.0343 |
| C1inh (mg/dL) | 25.56 (5.28) | 27.56 (5.58) | 0.0055 |
| C3 (mg/dL) | 137.44 (27.47) | 125.43 (27.48) | 0.0011 |
| C4 (mg/dL) | 30.73 (8.57) | 27.7 (7.72) | 0.006 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro-Marrero, J.; Zacares, M.; Almenar-Pérez, E.; Alegre-Martín, J.; Oltra, E. Complement Component C1q as a Potential Diagnostic Tool for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Subtyping. J. Clin. Med. 2021, 10, 4171. https://doi.org/10.3390/jcm10184171
Castro-Marrero J, Zacares M, Almenar-Pérez E, Alegre-Martín J, Oltra E. Complement Component C1q as a Potential Diagnostic Tool for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Subtyping. Journal of Clinical Medicine. 2021; 10(18):4171. https://doi.org/10.3390/jcm10184171
Chicago/Turabian StyleCastro-Marrero, Jesús, Mario Zacares, Eloy Almenar-Pérez, José Alegre-Martín, and Elisa Oltra. 2021. "Complement Component C1q as a Potential Diagnostic Tool for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Subtyping" Journal of Clinical Medicine 10, no. 18: 4171. https://doi.org/10.3390/jcm10184171
APA StyleCastro-Marrero, J., Zacares, M., Almenar-Pérez, E., Alegre-Martín, J., & Oltra, E. (2021). Complement Component C1q as a Potential Diagnostic Tool for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Subtyping. Journal of Clinical Medicine, 10(18), 4171. https://doi.org/10.3390/jcm10184171







