Outcomes of Endoscopic Ultrasound-Guided Biliary Drainage in a General Hospital for Patients with Endoscopic Retrograde Cholangiopancreatography-Difficult Transpapillary Biliary Drainage
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Patient Characteristics
3.2. Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mallery, S.; Matlock, J.; Freeman, M.L. EUS-Guided Rendezvous Drainage of Obstructed Biliary and Pancreatic Ducts: Report of 6 Cases. Gastrointest. Endosc. 2004, 59, 100–107. [Google Scholar] [CrossRef]
- Kawakubo, K.; Isayama, H.; Kato, H.; Itoi, T.; Kawakami, H.; Hanada, K.; Ishiwatari, H.; Yasuda, I.; Kawamoto, H.; Itokawa, F.; et al. Multicenter Retrospective Study of Endoscopic Ultrasound-Guided Biliary Drainage for Malignant Biliary Obstruction in Japan. J. Hepato-Biliary-Pancreat. Sci. 2014, 21, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Kuraoka, N.; Hara, K.; Okuno, N.; Kuwahara, T.; Mizuno, N.; Shimizu, Y.; Niwa, Y.; Terai, S. Outcomes of EUS-Guided Choledochoduodenostomy as Primary Drainage for Distal Biliary Obstruction With Covered Self-Expandable Metallic Stents. Endosc. Int. Open 2020, 8, E861–E868. [Google Scholar] [CrossRef] [PubMed]
- Nakai, Y.; Isayama, H.; Kawakami, H.; Ishiwatari, H.; Kitano, M.; Ito, Y.; Yasuda, I.; Kato, H.; Matsubara, S.; Irisawa, A.; et al. Prospective Multicenter Study of Primary EUS-Guided Choledochoduodenostomy Using a Covered Metal Stent. Endosc. Ultrasound 2019, 8, 111–117. [Google Scholar] [PubMed]
- Bang, J.Y.; Navaneethan, U.; Hasan, M.; Hawes, R.; Varadarajulu, S. Stent Placement by EUS or ERCP for Primary Biliary Decompression in Pancreatic Cancer: A Randomized Trial (With Videos). Gastrointest. Endosc. 2018, 88, 9–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paik, W.H.; Lee, T.H.; Park, D.H.; Choi, J.H.; Kim, S.O.; Jang, S.; Kim, D.U.; Shim, J.H.; Song, T.J.; Lee, S.S.; et al. EUS-Guided Biliary Drainage Versus ERCP for the Primary Palliation of Malignant Biliary Obstruction: A Multicenter Randomized Clinical Trial. Am. J. Gastroenterol. 2018, 113, 987–997. [Google Scholar] [CrossRef] [PubMed]
- Oken, M.M.; Creech, R.H.; Tormey, D.C.; Horton, J.; Davis, T.E.; McFadden, E.T.; Carbone, P.P. Toxicity and Response Criteria of the Eastern Cooperative Oncology Group. Am. J. Clin. Oncol. 1982, 5, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Cotton, P.B.; Eisen, G.M.; Aabakken, L.; Baron, T.H.; Hutter, M.M.; Jacobson, B.C.; Mergener, K.; Nemcek, A.; Petersen, B.T.; Petrini, J.L.; et al. A Lexicon for Endoscopic Adverse Events: Report of an ASGE Workshop. Gastrointest. Endosc. 2010, 71, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Wiersema, M.J.; Sandusky, D.; Carr, R.; Wiersema, L.M.; Erdel, W.C.; Frederick, P.K. Endosonography-Guided Cholangiopancreatography. Gastrointest. Endosc. 1996, 43 Pt 1, 102–106. [Google Scholar] [CrossRef]
- Giovannini, M.; Moutardier, V.; Pesenti, C.; Bories, E.; Lelong, B.; Delpero, J.R. Endoscopic Ultrasound-Guided Bilioduodenal Anastomosis: A New Technique for Biliary Drainage. Endoscopy 2001, 33, 898–900. [Google Scholar] [CrossRef] [PubMed]
- Paik, W.H.; Park, D.H.; Choi, J.H.; Choi, J.H.; Lee, S.S.; Seo, D.W.; Lee, S.K.; Kim, M.H.; Lee, J.B. Simplified Fistula Dilation Technique and Modified Stent Deployment Maneuver for EUS-Guided Hepaticogastrostomy. World J. Gastroenterol. 2014, 20, 5051–5059. [Google Scholar] [CrossRef] [PubMed]
- Artifon, E.L.A.; Marson, F.P.; Gaidhane, M.; Kahaleh, M.; Otoch, J.P. Hepaticogastrostomy or Choledochoduodenostomy for Distal Malignant Biliary Obstruction after Failed ERCP: Is There Any Difference? Gastrointest. Endosc. 2015, 81, 950–959. [Google Scholar] [CrossRef] [PubMed]
- Nakai, Y.; Isayama, H.; Yamamoto, N.; Matsubara, S.; Ito, Y.; Sasahira, N.; Hakuta, R.; Umefune, G.; Takahara, N.; Hamada, T.; et al. Safety and Effectiveness of a Long, Partially Covered Metal Stent for Endoscopic Ultrasound-Guided Hepaticogastrostomy in Patients With Malignant Biliary Obstruction. Endoscopy 2016, 48, 1125–1128. [Google Scholar] [CrossRef] [PubMed]
- Ogura, T.; Chiba, Y.; Masuda, D.; Kitano, M.; Sano, T.; Saori, O.; Yamamoto, K.; Imaoka, H.; Imoto, A.; Takeuchi, T.; et al. Comparison of the Clinical Impact of Endoscopic Ultrasound-Guided Choledochoduodenostomy and Hepaticogastrostomy for Bile Duct Obstruction With Duodenal Obstruction. Endoscopy 2016, 48, 156–163. [Google Scholar] [PubMed]
- Anderloni, A.; Fugazza, A.; Troncone, E.; Auriemma, F.; Carrara, S.; Semeraro, R.; Maselli, R.; Di Leo, M.; D’Amico, F.; Sethi, A.; et al. Single-Stage EUS-Guided Choledochoduodenostomy Using a Lumen-Apposing Metal Stent for Malignant Distal Biliary Obstruction. Gastrointest. Endosc. 2019, 89, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, T.; Teoh, A.Y.B.; Itoi, T.; Yamao, K.; Hara, K.; Nakai, Y.; Isayama, H.; Kitano, M. Long-Term Outcomes of EUS-Guided Choledochoduodenostomy Using a Lumen-Apposing Metal Stent for Malignant Distal Biliary Obstruction: A Prospective Multicenter Study. Gastrointest. Endosc. 2018, 87, 1138–1146. [Google Scholar] [CrossRef] [PubMed]
- El Chafic, A.H.; Shah, J.N.; Hamerski, C.; Binmoeller, K.F.; Irani, S.; James, T.W.; Baron, T.H.; Nieto, J.; Romero, R.V.; Evans, J.A.; et al. EUS-Guided Choledochoduodenostomy for Distal Malignant Biliary Obstruction Using Electrocautery-Enhanced Lumen-Apposing Metal Stents: First US, Multicenter Experience. Dig. Dis. Sci. 2019, 64, 3321–3327. [Google Scholar] [CrossRef] [PubMed]
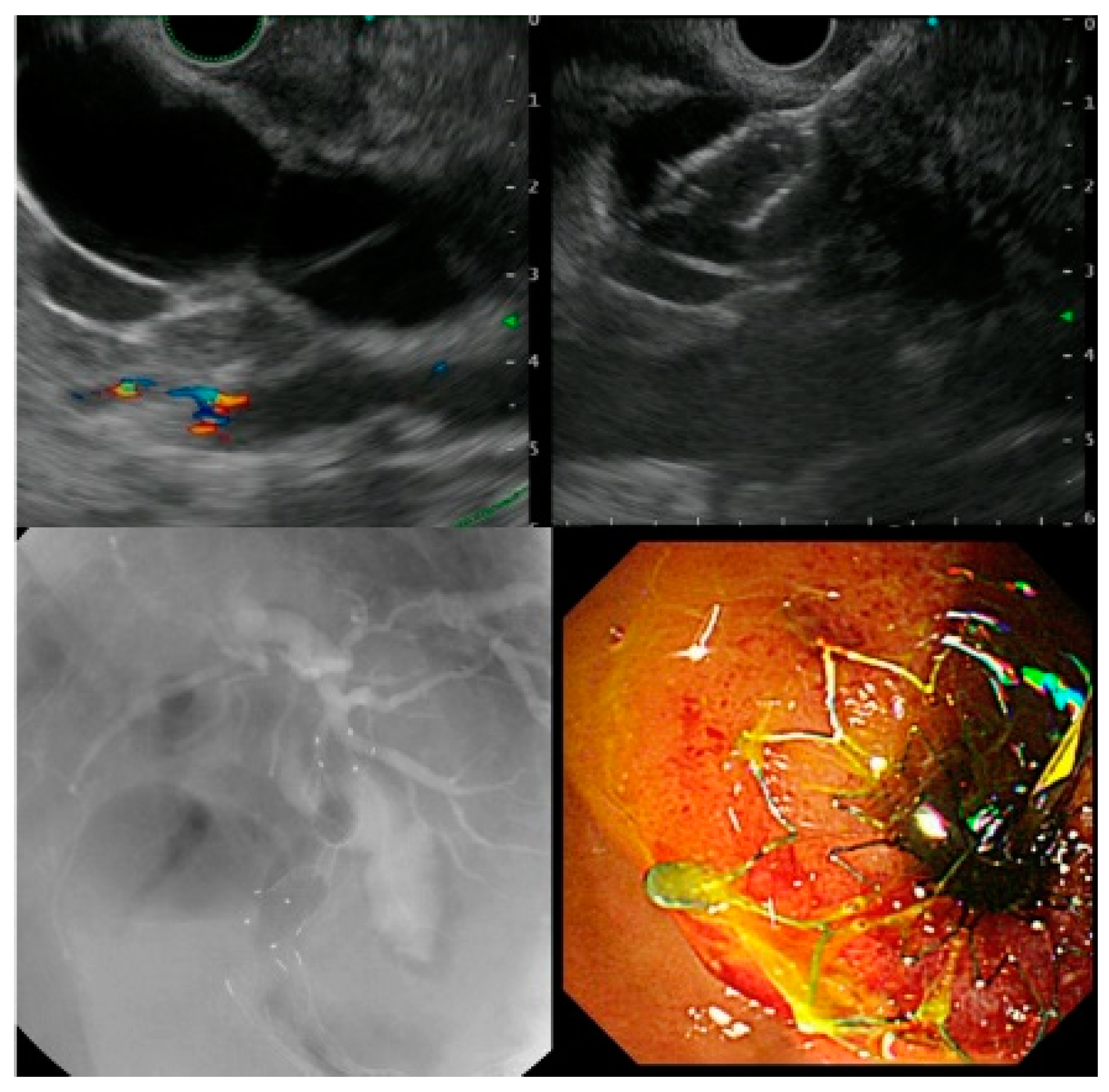
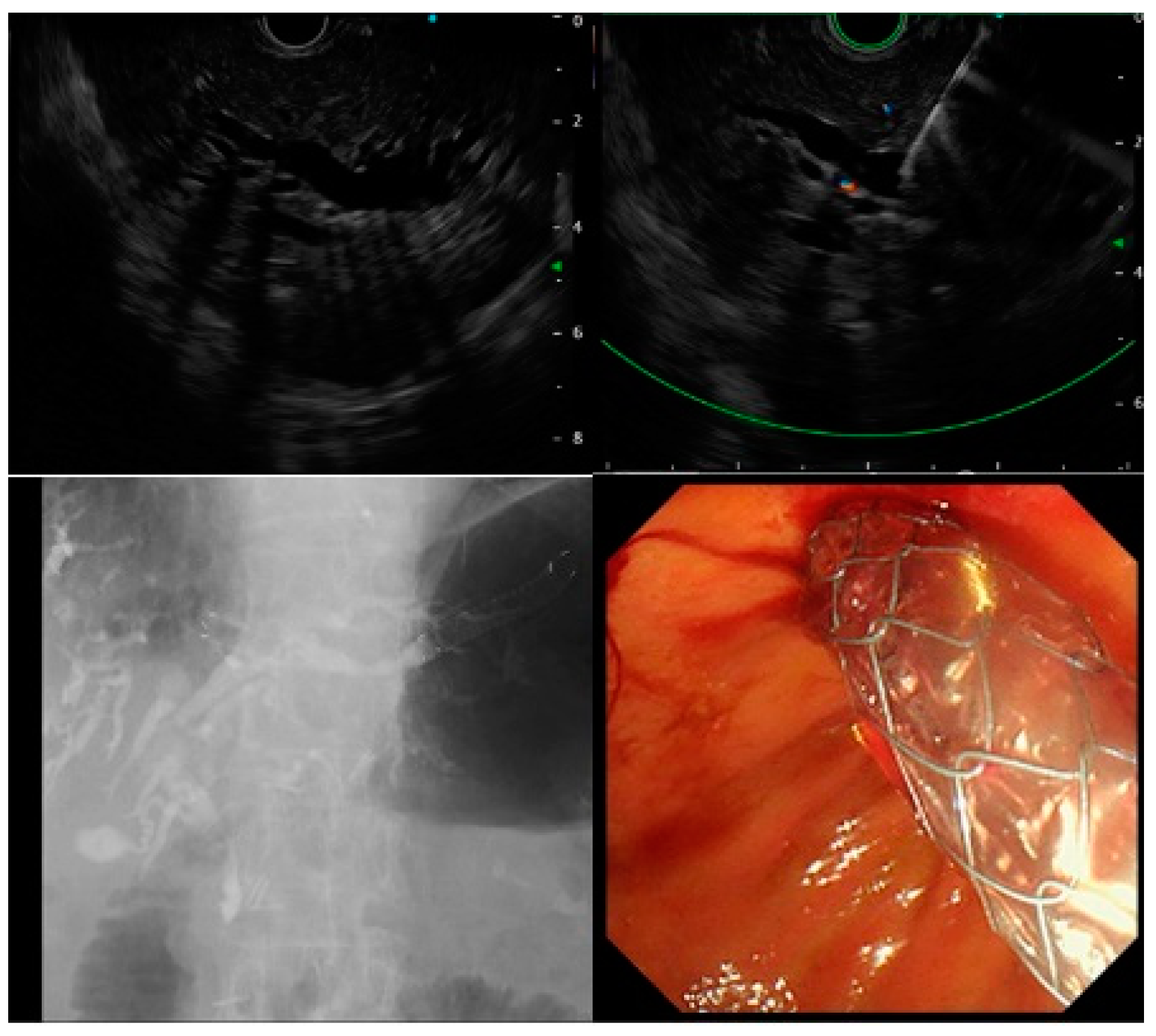
| EUS-CDS (n = 12) | EUS-HGS (n = 12) | |
|---|---|---|
| Median age (range) | 76.5 (57–75) | 76.5 (60–85) |
| Sex, male, n (%) | 4 (33.3) | 7 (58.3) |
| Diagnosis, n (%) | ||
| Pancreatic cancer | 9 (66.7) | 7 (58.3) |
| Cholangiocarcinoma | 0 | 1 (8.3) |
| Dissemination of cancer | 3 (33.3) | 2 (16.7) |
| Bile duct stones | 0 | 2 (16.7) |
| Pre-total Bilirubin, mean, mg/dL (range) | 9.49 (3.29–16.06) | 6.72 (0.48–13.65) |
| Performance status (PS) | ||
| Performance status < 2, n (%) | 7 (58.3) | 8 (66.7) |
| Performance status > 3, n (%) | 5 (41.7) | 4 (33.3) |
| Diameter of bile duct | ||
| Extrahepatic bile duct, mean, mm (range) | 14.9 (11–20) | N/A |
| Intrahepatic bile duct, mean, mm (range) | N/A | 5.1 (2.6–7) |
| Size of needle | ||
| 19G, n (%) | 12 (100) | 10 (83.3) |
| 22G, n (%) | 0 | 2 (16.7) |
| EUS-CDS (n = 12) | EUS-HGS (n = 12) | |
|---|---|---|
| Technical success, n (%) | 10/12 (83.3) | 11/12 (91.7) |
| Clinical success, n (%) | 10/12 (83.3) | 11/12 (91.7) |
| Deployment of SEMS, n (%) | 9 (75) | 4 (33.3) |
| Deployment of PS | 1 (8.3) | 7 (58.3) |
| Dilatation | ||
| Electrocautery dilation, n (%) | 10 (83.3) | 0 |
| Non-electrocautery dilation, n (%) | 1 (8.3) | 8 (66.7) |
| Non-dilation, n (%) | 0 | 3 (25) |
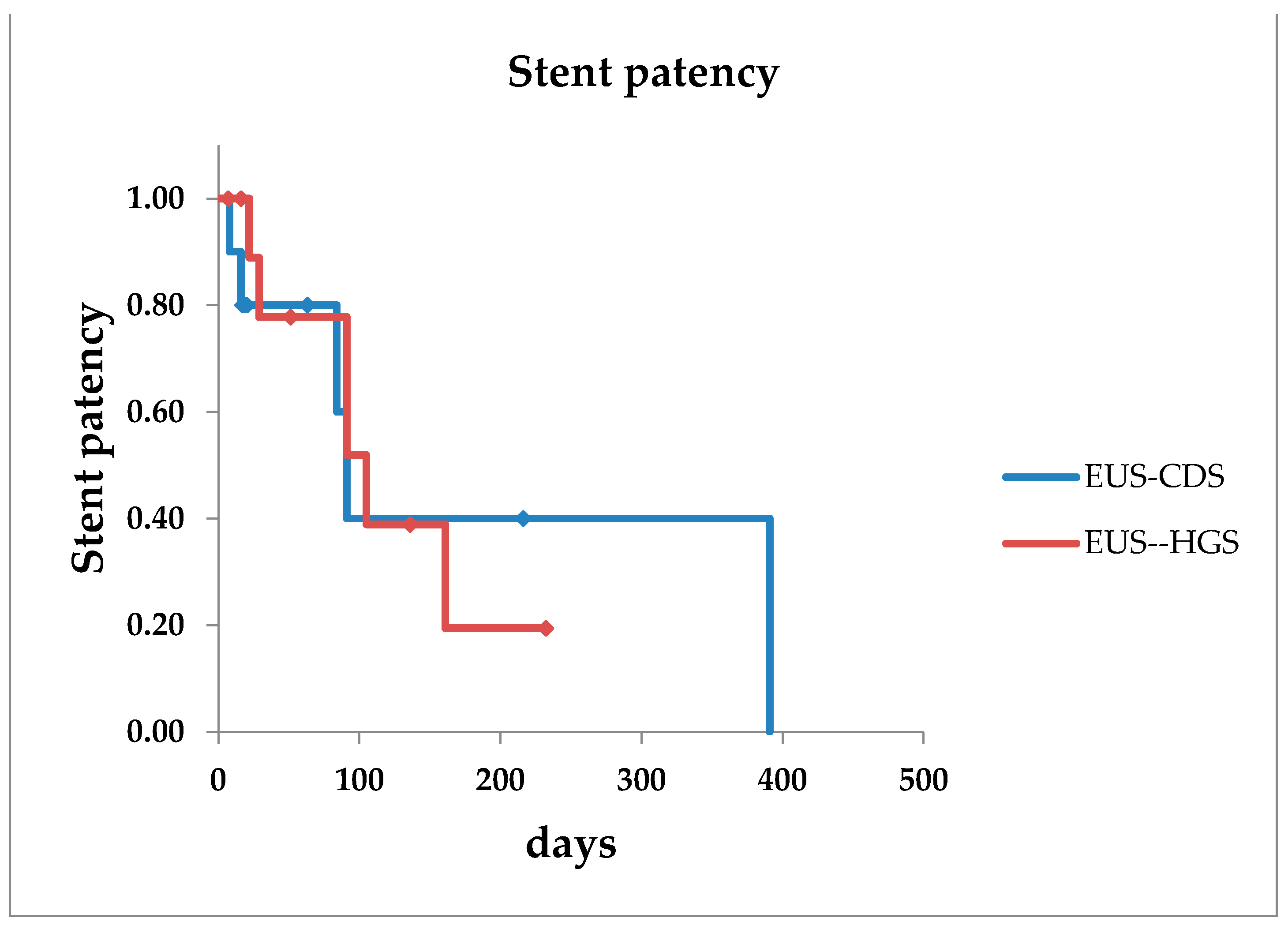
| Stent patency, median days | 91 | 101 |
| EUS-CDS (n = 12) | EUS-HGS (n = 12) | |
|---|---|---|
| Overall adverse events, n (%) | 1/12 (8.3) | 0/12 (0) |
| Type of adverse events, grade | bile peritonitis, severe | |
| Early adverse events, n (%) | 1/12 (8.3) | 0/12 (0) |
| Late adverse events, n (%) | 0/12 (0) | 0/12 (0) |
| EUS-CDS (n = 12) | EUS-HGS (n = 12) | |
|---|---|---|
| Treatment | ||
| Resection, n (%) | 0 | 2/12 (16.7) |
| Chemotherapy | 5/12 (41.7) | 5/12 (41.7) |
| Re-intervention, n (%) | 5 (41.7) | 6 (50) |
| Technical success | 5/5 (80) | 6/6 (100) |
| Re-intervention, n (%) | ||
| Stent exchange | 3/5 (60) | 5/6 (83.3) |
| Stent direction change | 2/5 (40) | 0 |
| Another EUS-BD | 0 | 1/6 (16.7) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuraoka, N.; Hashimoto, S.; Matsui, S.; Terai, S. Outcomes of Endoscopic Ultrasound-Guided Biliary Drainage in a General Hospital for Patients with Endoscopic Retrograde Cholangiopancreatography-Difficult Transpapillary Biliary Drainage. J. Clin. Med. 2021, 10, 4105. https://doi.org/10.3390/jcm10184105
Kuraoka N, Hashimoto S, Matsui S, Terai S. Outcomes of Endoscopic Ultrasound-Guided Biliary Drainage in a General Hospital for Patients with Endoscopic Retrograde Cholangiopancreatography-Difficult Transpapillary Biliary Drainage. Journal of Clinical Medicine. 2021; 10(18):4105. https://doi.org/10.3390/jcm10184105
Chicago/Turabian StyleKuraoka, Naosuke, Satoru Hashimoto, Shigeru Matsui, and Shuji Terai. 2021. "Outcomes of Endoscopic Ultrasound-Guided Biliary Drainage in a General Hospital for Patients with Endoscopic Retrograde Cholangiopancreatography-Difficult Transpapillary Biliary Drainage" Journal of Clinical Medicine 10, no. 18: 4105. https://doi.org/10.3390/jcm10184105
APA StyleKuraoka, N., Hashimoto, S., Matsui, S., & Terai, S. (2021). Outcomes of Endoscopic Ultrasound-Guided Biliary Drainage in a General Hospital for Patients with Endoscopic Retrograde Cholangiopancreatography-Difficult Transpapillary Biliary Drainage. Journal of Clinical Medicine, 10(18), 4105. https://doi.org/10.3390/jcm10184105






