Real-World Data from a Multi-Center Study: Insights to Psoriatic Arthritis Care
Abstract
1. Introduction
2. Materials and Methods
2.1. Center, Provider, and Patient Recruitment
2.2. Data Gathering and Processing
2.3. Statistical Analysis
3. Results
3.1. Center and Physician-Level Estimates for the Population of Patients with Active Psoriatic Arthritis, including Inadequate-Responders and Patients Who Have Never Been Treated Systemically
“Please estimate how many adult patients, suffering from active psoriatic arthritis, are currently under [your care/the care of your center]”
“Please estimate how many adult patients, suffering from active psoriatic arthritis, currently under [your care/the care of your center], [have inadequate response to at least 2 csDMARDs/have not been treated with systemic agents to date]?”
3.2. Patient-Reported Difficulties Related to the Drug Reimbursement Procedure
“How long did you wait to be admitted to the facility to which you were referred to complete formalities related to qualification for biological treatment under the drug program (i.e., how much time passed since the visit at which you received referral)?”
“The period of time from the visit at which the procedure of qualifying a patient for active biological treatment of PsA (under the drug program) was started to the day the patient was given the first dose of a biological drug”
“What was troublesome or problematic for you while applying for biological treatment of PsA? (under the drug program, you can choose more than 1 answer)”
“How long does it usually take for you to visit a facility that provides biological treatment for PsA (as part of a drug program)?”
“In order to be administered the biological drug for PsA (under the drug program), do you ever have to take time off from work?”
“In order to visit a facility that provides biological treatment of PsA (under the drug program), do you ever have to engage the assistance of someone else?”
3.3. Availability and Barriers to Biologic Treatment—A Physician’s Perspective
“How do you think the availability of biological treatment for adult patients with active PsA has changed in the last 12 months?”
“In your opinion, what are the main barriers to inclusion of adult patients with active PsA into biological therapy (within the drug program)?” [Multiple responses were allowed.]
“The following list includes selected factors that may affect the availability of biological therapy for patients treated for active PsA. For each of them, please rate how much each factor currently constitutes a barrier to the enrollment of your patients in the biological treatment program.”
“Do you think the funds allocated to the biological treatment program for adult patients with active PsA are sufficient (taking into account the number of outpatient patients eligible for such treatment) or rather insufficient (i.e., treatment is lacking for a proportion of patients who are eligible)?”
“In what percentage of adult patients with active PsA admitted by you (in open treatment, who are eligible for biological treatment under the drug program) is treatment not initiated due to insufficient funding.”
“How often do you think it might occur that providers treating adult patients with active PsA give up the introduction of biological therapy due to bureaucratic difficulties and a significant workload (related to the qualification and monitoring of patients under the drug program)? Please think not only about your own experiences, but also about the situations your colleagues encounter.”
3.4. Common Causes for Withdrawal of bDMARD Therapy
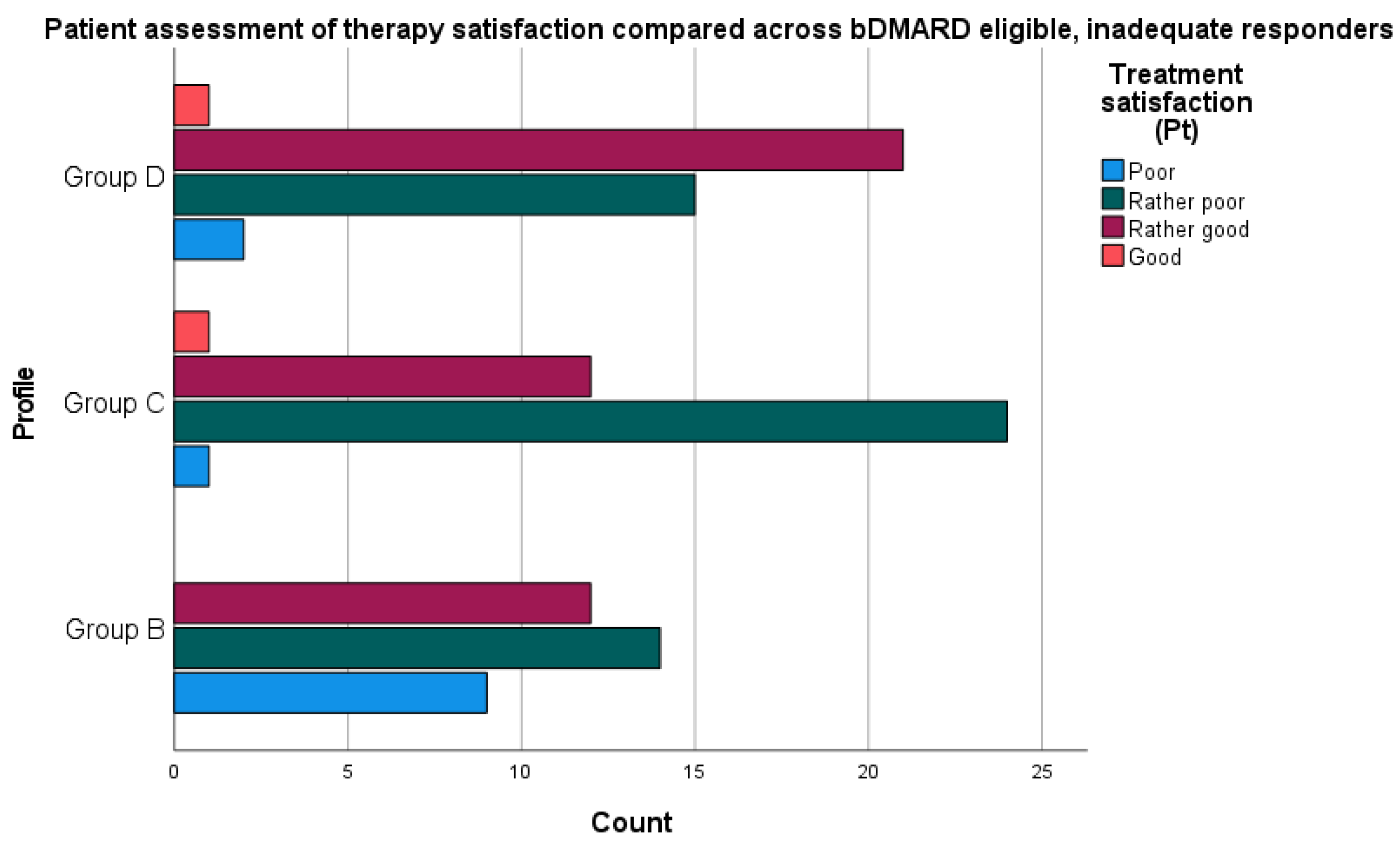
3.5. Patient Satisfaction in Treatment and Physician Assessment of Therapeutic Effectiveness—The Relationship with Current bDMARD Therapy
3.6. Comparison of bDMARD Users and Patients Who Qualify, Are Eligible, but Chose Not to Initiate Biologic Therapy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, S.; Mendelsohn, A.; Sarnes, E. The Burden of Psoriatic Arthritis: A Literature Review from a Global Health Systems Perspective. Pharm. Ther. 2010, 35, 680–689. [Google Scholar]
- Batko, B. Patient-Centered Care in Psoriatic Arthritis-A Perspective on Inflammation, Disease Activity, and Psychosocial Factors. J. Clin. Med. 2020, 9, 3103. [Google Scholar] [CrossRef] [PubMed]
- Betteridge, N.; Boehncke, W.-H.; Bundy, C.; Gossec, L.; Gratacós, J.; Augustin, M. Promoting Patient-Centred Care in Psoriatic Arthritis: A Multidisciplinary European Perspective on Improving the Patient Experience. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Boehncke, W.-H.; Menter, A. Burden of Disease: Psoriasis and Psoriatic Arthritis. Am. J. Clin. Dermatol. 2013, 14, 377–388. [Google Scholar] [CrossRef]
- Gossec, L.; de Wit, M.; Kiltz, U.; Braun, J.; Kalyoncu, U.; Scrivo, R.; Maccarone, M.; Carton, L.; Otsa, K.; Sooäär, I.; et al. A Patient-Derived and Patient-Reported Outcome Measure for Assessing Psoriatic Arthritis: Elaboration and Preliminary Validation of the Psoriatic Arthritis Impact of Disease (PsAID) Questionnaire, a 13-Country EULAR Initiative. Ann. Rheum. Dis. 2014, 73, 1012–1019. [Google Scholar] [CrossRef] [PubMed]
- Reich, K.; Krüger, K.; Mössner, R.; Augustin, M. Epidemiology and Clinical Pattern of Psoriatic Arthritis in Germany: A Prospective Interdisciplinary Epidemiological Study of 1511 Patients with Plaque-Type Psoriasis. Br. J. Dermatol. 2009, 160, 1040–1047. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, S.; Couderc, M.; Pereira, B.; Dubost, J.-J.; Malochet-Guinamand, S.; Tournadre, A.; Soubrier, M.; Moisset, X. Prevalence of Migraine and Neuropathic Pain in Rheumatic Diseases. J. Clin. Med. 2020, 9, 1890. [Google Scholar] [CrossRef] [PubMed]
- Cometi, L.; Bruni, C.; Chiti, N.; Tofani, L.; Nacci, F.; Bartoli, F.; Bellando-Randone, S.; Melchiorre, D.; Fiori, G.; Guiducci, S.; et al. Effect of Dysmetabolisms and Comorbidities on the Efficacy and Safety of Biological Therapy in Chronic Inflammatory Joint Diseases. J. Clin. Med. 2020, 9, 1310. [Google Scholar] [CrossRef]
- Olejniczak-Staruch, I.; Ciążyńska, M.; Sobolewska-Sztychny, D.; Narbutt, J.; Skibińska, M.; Lesiak, A. Alterations of the Skin and Gut Microbiome in Psoriasis and Psoriatic Arthritis. Int. J. Mol. Sci. 2021, 22, 3998. [Google Scholar] [CrossRef]
- Coates, L.C.; Orbai, A.-M.; Morita, A.; Benichou, O.; Kerr, L.; Adams, D.H.; Shuler, C.L.; Birt, J.; Helliwell, P.S. Achieving Minimal Disease Activity in Psoriatic Arthritis Predicts Meaningful Improvements in Patients’ Health-Related Quality of Life and Productivity. BMC Rheumatol. 2018, 2, 24. [Google Scholar] [CrossRef]
- Armstrong, A.W.; Robertson, A.D.; Wu, J.; Schupp, C.; Lebwohl, M.G. Undertreatment, Treatment Trends, and Treatment Dissatisfaction among Patients with Psoriasis and Psoriatic Arthritis in the United States: Findings from the National Psoriasis Foundation Surveys, 2003–2011. JAMA Dermatol. 2013, 149, 1180–1185. [Google Scholar] [CrossRef]
- Tveit, K.S.; Duvetorp, A.; Østergaard, M.; Skov, L.; Danielsen, K.; Iversen, L.; Seifert, O. Treatment Use and Satisfaction among Patients with Psoriasis and Psoriatic Arthritis: Results from the NORdic PAtient Survey of Psoriasis and Psoriatic Arthritis (NORPAPP). J. Eur. Acad. Dermatol. Venereol. 2019, 33, 340–354. [Google Scholar] [CrossRef]
- Lofland, J.H.; Johnson, P.T.; Ingham, M.P.; Rosemas, S.C.; White, J.C.; Ellis, L. Shared Decision-Making for Biologic Treatment of Autoimmune Disease: Influence on Adherence, Persistence, Satisfaction, and Health Care Costs. Patient Prefer. Adherence 2017, 11, 947–958. [Google Scholar] [CrossRef]
- Jang, D.; Lee, A.-H.; Shin, H.-Y.; Song, H.-R.; Park, J.-H.; Kang, T.-B.; Lee, S.-R.; Yang, S.-H. The Role of Tumor Necrosis Factor Alpha (TNF-α) in Autoimmune Disease and Current TNF-α Inhibitors in Therapeutics. Int. J. Mol. Sci. 2021, 22, 2719. [Google Scholar] [CrossRef]
- Coates, L.C.; Kavanaugh, A.; Mease, P.J.; Soriano, E.R.; Laura Acosta-Felquer, M.; Armstrong, A.W.; Bautista-Molano, W.; Boehncke, W.-H.; Campbell, W.; Cauli, A.; et al. Group for Research and Assessment of Psoriasis and Psoriatic Arthritis 2015 Treatment Recommendations for Psoriatic Arthritis. Arthritis Rheumatol. 2016, 68, 1060–1071. [Google Scholar] [CrossRef] [PubMed]
- Smolen, J.S.; Braun, J.; Dougados, M.; Emery, P.; Fitzgerald, O.; Helliwell, P.; Kavanaugh, A.; Kvien, T.K.; Landewé, R.; Luger, T.; et al. Treating Spondyloarthritis, Including Ankylosing Spondylitis and Psoriatic Arthritis, to Target: Recommendations of an International Task Force. Ann. Rheum. Dis. 2014, 73, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Batko, B.; Korkosz, M.; Juś, A.; Wiland, P. Management of Rheumatoid Arthritis in Poland–Where Daily Practice Might Not Always Meet Evidence-Based Guidelines. Arch. Med. Sci. 2019, 17, 1286–1293. [Google Scholar] [CrossRef]
- Sagan, A.; Panteli, D.; Borkowski, W.; Dmowski, M.; Domanski, F.; Czyzewski, M.; Gorynski, P.; Karpacka, D.; Kiersztyn, E.; Kowalska, I.; et al. Poland Health System Review. Health Syst. Transit. 2011, 13, 1–193. [Google Scholar]
- Coates, L.C.; Moverley, A.R.; McParland, L.; Brown, S.; Navarro-Coy, N.; O’Dwyer, J.L.; Meads, D.M.; Emery, P.; Conaghan, P.G.; Helliwell, P.S. Effect of Tight Control of Inflammation in Early Psoriatic Arthritis (TICOPA): A UK Multicentre, Open-Label, Randomised Controlled Trial. Lancet 2015, 386, 2489–2498. [Google Scholar] [CrossRef]
- Ogdie, A.; de Wit, M.; Callis Duffin, K.; Campbell, W.; Chau, J.; Coates, L.C.; Eder, L.; Elmamoun, M.; FitzGerald, O.; Gladman, D.D.; et al. Defining Outcome Measures for Psoriatic Arthritis: A Report from the GRAPPA-OMERACT Working Group. J. Rheumatol. 2017, 44, 697–700. [Google Scholar] [CrossRef]
- Conaghan, P.G.; Alten, R.; Deodhar, A.; Sullivan, E.; Blackburn, S.; Tian, H.; Gandhi, K.; Jugl, S.M.; Strand, V. Relationship of Pain and Fatigue with Health-Related Quality of Life and Work in Patients with Psoriatic Arthritis on TNFi: Results of a Multi-National Real-World Study. RMD Open 2020, 6, e001240. [Google Scholar] [CrossRef] [PubMed]
- Conaghan, P.G.; Strand, V.; Alten, R.; Sullivan, E.; Blackburn, S.; Huneault, L.; Tian, H.; Gandhi, K.; Jugl, S. OP0107 Pain Still Remains a High Unmet Need among Psoriatic Arthritis Patients Receiving Existing Biologic Treatment: Results from a MultiNational Real-World Survey. Ann. Rheum. Dis. 2017, 76, 96–97. [Google Scholar] [CrossRef]
- Rencz, F.; Kemény, L.; Gajdácsi, J.Z.; Owczarek, W.; Arenberger, P.; Tiplica, G.S.; Stanimirović, A.; Niewada, M.; Petrova, G.; Marinov, L.T.; et al. Use of Biologics for Psoriasis in Central and Eastern European Countries. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 2222–2230. [Google Scholar] [CrossRef]
- Naldi, L.; Cazzaniga, S.; Di Mercurio, M.; Grossi, E.; Addis, A. Psocare study centres Inequalities in Access to Biological Treatments for Psoriasis: Results from the Italian Psocare Registry. Br. J. Dermatol. 2017, 176, 1331–1338. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Sudharshan, L.; Hsu, M.-A.; Koenig, A.S.; Cappelleri, J.C.; Liu, W.F.; Smith, T.W.; Pasquale, M.K. Patient Preferences Associated with Therapies for Psoriatic Arthritis: A Conjoint Analysis. Am. Health Drug Benefits 2018, 11, 408–417. [Google Scholar] [PubMed]
- Bolge, S.C.; Eldridge, H.M.; Lofland, J.H.; Ravin, C.; Hart, P.J.; Ingham, M.P. Patient Experience with Intravenous Biologic Therapies for Ankylosing Spondylitis, Crohn’s Disease, Psoriatic Arthritis, Psoriasis, Rheumatoid Arthritis, and Ulcerative Colitis. Patient Prefer. Adherence 2017, 11, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Putrik, P.; Ramiro, S.; Keszei, A.P.; Hmamouchi, I.; Dougados, M.; Uhlig, T.; Kvien, T.K.; Boonen, A. Lower Education and Living in Countries with Lower Wealth Are Associated with Higher Disease Activity in Rheumatoid Arthritis: Results from the Multinational COMORA Study. Ann. Rheum. Dis. 2016, 75, 540–546. [Google Scholar] [CrossRef]
- Hifinger, M.; Putrik, P.; Ramiro, S.; Keszei, A.P.; Hmamouchi, I.; Dougados, M.; Gossec, L.; Boonen, A. In Rheumatoid Arthritis, Country of Residence Has an Important Influence on Fatigue: Results from the Multinational COMORA Study. Rheumatology 2016, 55, 735–744. [Google Scholar] [CrossRef][Green Version]
- Lebwohl, M.G.; Bachelez, H.; Barker, J.; Girolomoni, G.; Kavanaugh, A.; Langley, R.G.; Paul, C.F.; Puig, L.; Reich, K.; van de Kerkhof, P.C.M. Patient Perspectives in the Management of Psoriasis: Results from the Population-Based Multinational Assessment of Psoriasis and Psoriatic Arthritis Survey. J. Am. Acad. Dermatol. 2014, 70, 871–881.e30. [Google Scholar] [CrossRef]
- Aletaha, D.; Husni, M.E.; Merola, J.F.; Ranza, R.; Bertheussen, H.; Lippe, R.; Young, P.M.; Cappelleri, J.C.; Brown, T.M.; Ervin, C.; et al. Treatment Mode Preferences in Psoriatic Arthritis: A Qualitative Multi-Country Study. Patient Prefer. Adherence 2020, 14, 949–961. [Google Scholar] [CrossRef]


| Inclusion Criteria for Each Patient Group | A | B | C | D |
|---|---|---|---|---|
| Active psoriatic arthritis according to specialist | + | + | + | + |
| Inadequate response to at least 2 csDMARDs | + | + | + | + |
| Ongoing biologic treatment | + | − | − | − |
| Prior biologic treatment without achievement of at least low-disease activity up to present day | − | + | − | − |
| Fulfilling biologic reimbursement criteria | + | + | + | − |
| Biologic therapy eligible under specialist assessment | + | + | + | + |
| Variable | Group A | Group C | p-Value | |
|---|---|---|---|---|
| Age in years, Mean (SD) (n = 68) | 44.96 (11.3) | 46.90 (11.9) | 0.464 | |
| Gender, female (%) (n = 75) | 15 (44.1) | 19 (55.9) | 0.540 | |
| Duration of PsA in years, Median (IQR) (n = 68) | 10 (8) | 7.5 (6) | 0.088 | |
| Education level, count (%) (n = 75) | High | 17 (73.9) | 6 (26.1) | 0.007 |
| Moderate | 18 (38.3) | 29 (61.7) | ||
| Basic | 1 (20) | 4 (80) | ||
| Residence, count (%) (n = 75) | Rural or small city | 23 (46.4) | 24 (53.6) | 0.833 |
| Large city | 13 (48.9) | 15 (51.1) | ||
| Employed, count (%) (n = 74) | Yes | 25 (46.3) | 29 (53.7) | 0.777 |
| No | 10 (50) | 10 (50) | ||
| High (above average PL salary) income, count (%) (n = 75) | Approx. >1300 USD | 11 (64.7) | 6 (35.3) | 0.117 |
| Approx. <1300 USD | 25 (43.1) | 33 (56.9) | ||
| Patient satisfaction with treatment, count (%) (n = 74) | Dissatisfied | 0 (0) | 25 (100) | <0.001 |
| Satisfied | 36 (73.5) | 13 (26.5) | ||
| Provider assessment of therapeutic status, count (%) (n = 70) | Poor | 0 (0) | 29 (100) | <0.001 |
| Good | 34 (82.9) | 7 (17.1) | ||
| NSAIDs currently (n = 63) | Yes | 16 (36.4) | 28 (63.6) | 0.019 |
| No | 13 (68.4) | 6 (31.6) | ||
| NSAIDs Currently | Therapeutic Status (Provider) | Patient Treatment Satisfaction | ||||
|---|---|---|---|---|---|---|
| Good | Poor | p-Value | Good | Poor | p-Value | |
| Yes | 34 (35.4%) | 62 (64.6%) | 0.010 | 54 (52.4%) | 49 (47.6%) | 0.464 |
| No | 19 (63.3%) | 11 (36.7%) | 18 (60%) | 12 (40%) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batko, B.; Kucharz, E.; Stajszczyk, M.; Brzosko, M.; Samborski, W.; Żuber, Z. Real-World Data from a Multi-Center Study: Insights to Psoriatic Arthritis Care. J. Clin. Med. 2021, 10, 4106. https://doi.org/10.3390/jcm10184106
Batko B, Kucharz E, Stajszczyk M, Brzosko M, Samborski W, Żuber Z. Real-World Data from a Multi-Center Study: Insights to Psoriatic Arthritis Care. Journal of Clinical Medicine. 2021; 10(18):4106. https://doi.org/10.3390/jcm10184106
Chicago/Turabian StyleBatko, Bogdan, Eugeniusz Kucharz, Marcin Stajszczyk, Marek Brzosko, Włodzimierz Samborski, and Zbigniew Żuber. 2021. "Real-World Data from a Multi-Center Study: Insights to Psoriatic Arthritis Care" Journal of Clinical Medicine 10, no. 18: 4106. https://doi.org/10.3390/jcm10184106
APA StyleBatko, B., Kucharz, E., Stajszczyk, M., Brzosko, M., Samborski, W., & Żuber, Z. (2021). Real-World Data from a Multi-Center Study: Insights to Psoriatic Arthritis Care. Journal of Clinical Medicine, 10(18), 4106. https://doi.org/10.3390/jcm10184106






