Assessment of Physical Activity Using Waist-Worn Accelerometers in Hospitalized Heart Failure Patients and Its Relationship with Kansas City Cardiomyopathy Questionnaire
Abstract
:1. Introduction
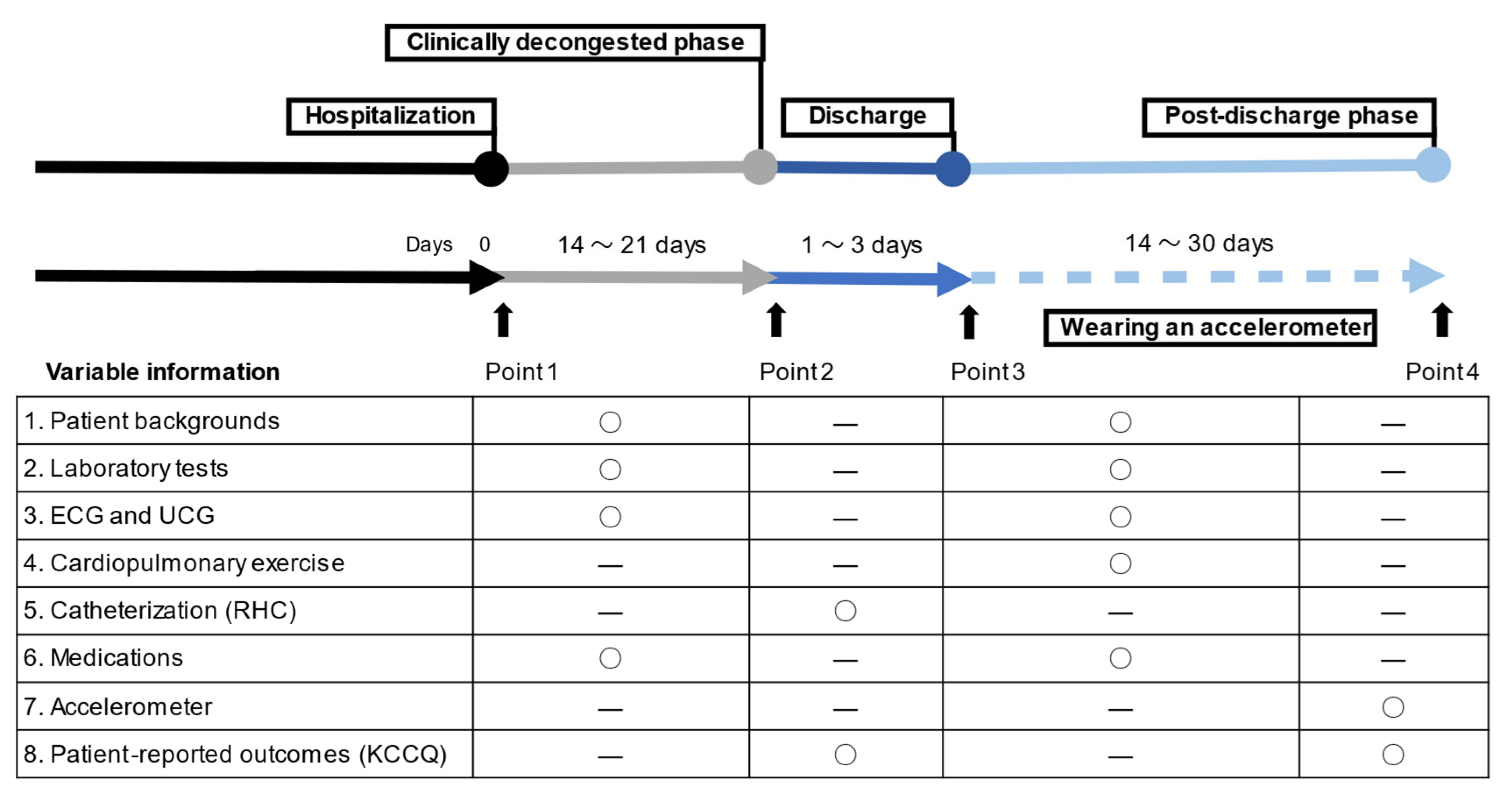
2. Materials and Methods
2.1. Study Cohort and Sample
2.2. Clinical Data Acquisition
2.3. Accelerometry
2.4. Health-Related Quality of Life
2.5. Cardiopulmonary Exercise Testing
2.6. Right Heart Catheterization
2.7. Statistical Analysis
3. Results
3.1. Patients’ Characteristics
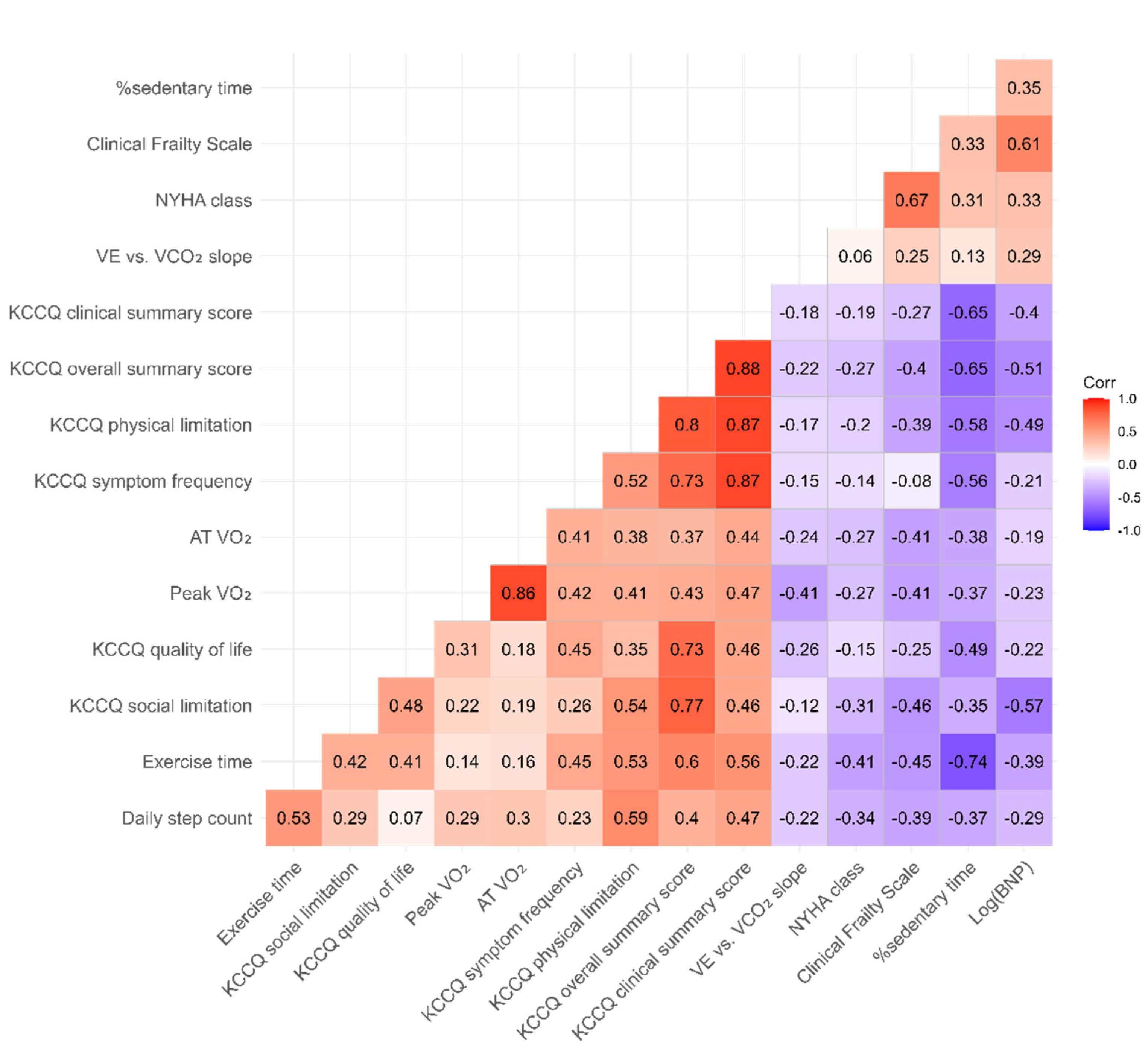
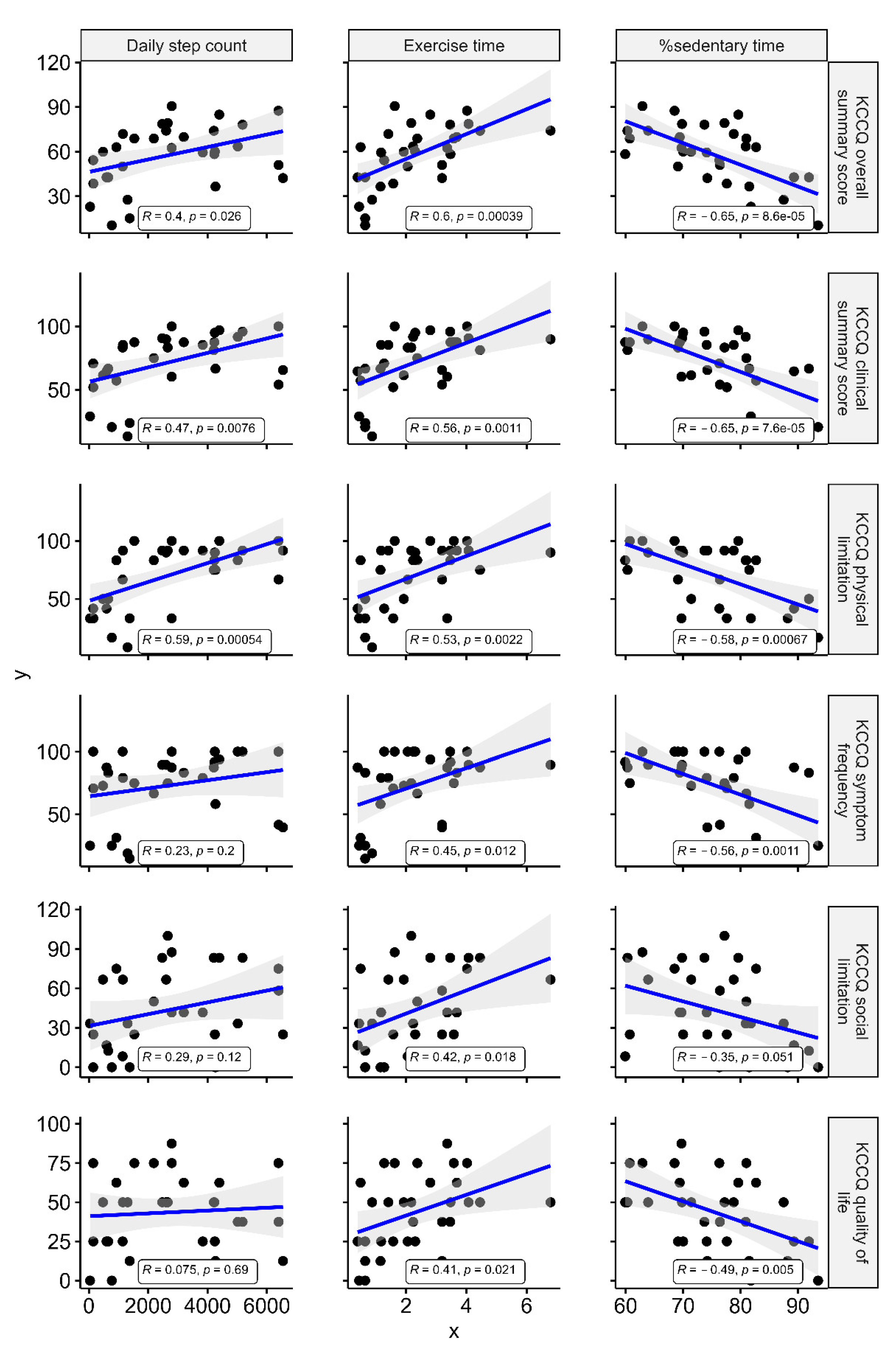
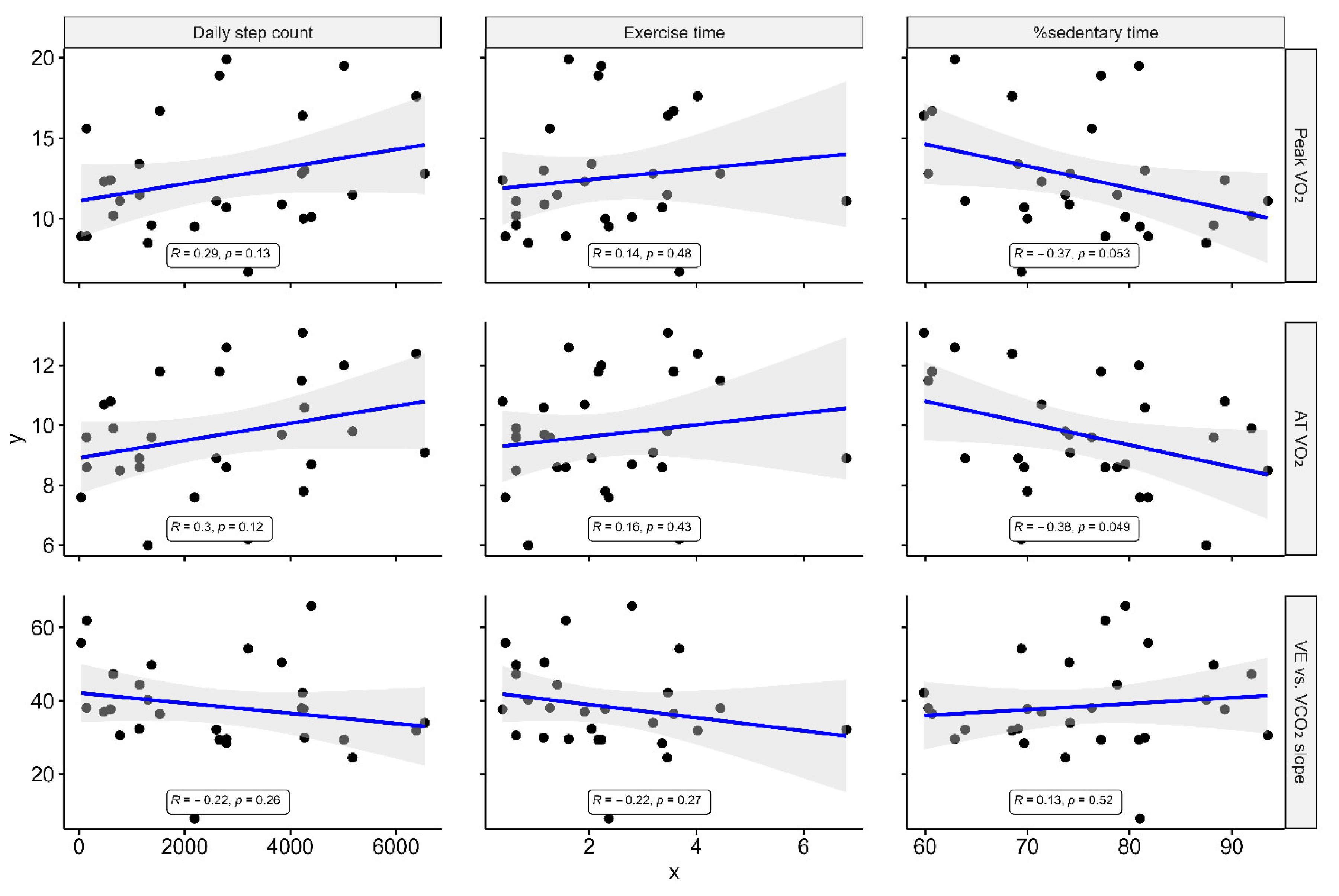
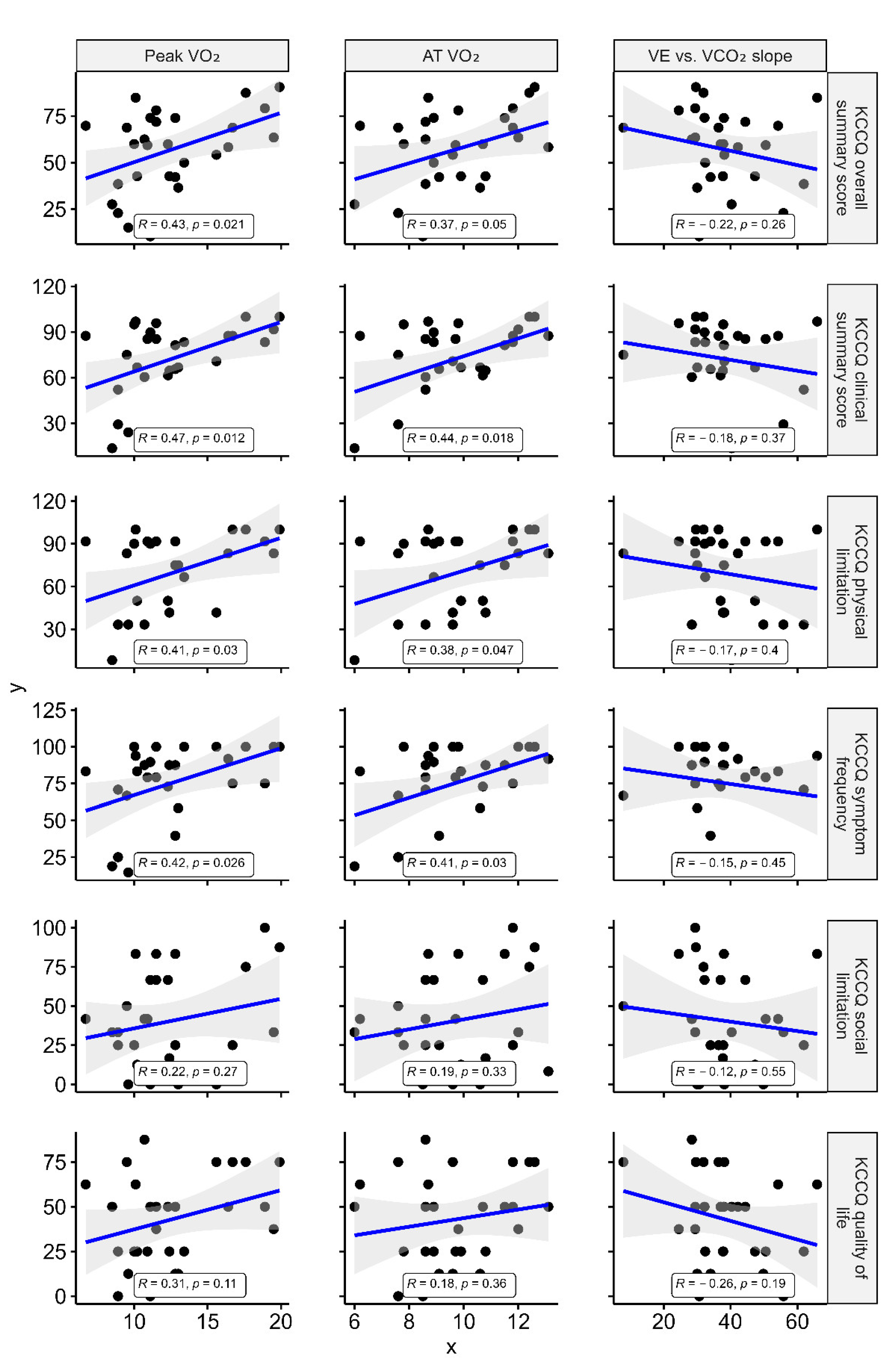
3.2. Association between KCCQ, Accelerometer-Measured Parameters, and CPX Data
3.3. Subgroup Analysis
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lobelo, F.; Rohm Young, D.; Sallis, R.; Garber, M.D.; Billinger, S.A.; Duperly, J.; Hutber, A.; Pate, R.R.; Thomas, R.J.; Widlansky, M.E.; et al. Routine Assessment and Promotion of Physical Activity in Healthcare Setting: A Scientific Statement from the American Heart Association. Circulation 2018, 137, e495–e522. [Google Scholar] [CrossRef]
- Thomas, R.J.; Beatty, A.L.; Beckie, T.M.; Brewer, L.C.; Brown, T.M.; Forman, D.E.; Franklin, B.A.; Keteyian, S.J.; Kitzman, D.W.; Regensteiner, J.G.; et al. Home-Based Cardiac Rehabilitation: A Scientific Statement from the American Association of Cardiovascular and Pulmonary Rehabilitation from the American Heart Association, and the American College of Cardiology. Circulation 2019, 140, e69–e89. [Google Scholar] [CrossRef] [PubMed]
- Pelliccia, A.; Sharma, S.; Gati, S.; Bäck, M.; Börjesson, M.; Caselli, S.; Collet, J.P.; Corrado, D.; Drezner, J.A.; Halle, M.; et al. 2020 ESC Guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur. Heart J. 2021, 42, 17–96. [Google Scholar] [PubMed]
- Klompstra, L.; Kyriakou, M.; Lambrinou, E.; Piepoli, M.F.; Coats, A.J.; Cohen-Solal, A.; Cornelis, J.; Gellen, B.; Marques-Sule, E.; Niederseer, D.; et al. Measuring physical activity with activity monitors in patients with heart failure: From literature to practice. A position paper from the Committee on Exercise Physiology and Training of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2021, 23, 83–91. [Google Scholar] [CrossRef]
- Reddy, Y.N.; Rikhi, A.; Obokata, M.; Shah, S.J.; Lewis, G.D.; AbouEzzedine, O.F.; Dunlay, S.; McNulty, S.; Chakraborty, H.; Stevenson, L.W.; et al. Quality of life in heart failure with preserved ejection fraction: Importance of obesity, functional capacity, and physical inactivity. Eur. J. Heart Fail. 2020, 22, 1009–1018. [Google Scholar] [CrossRef]
- Werhahn, S.M.; Dathe, H.; Rottmann, T.; Franke, T.; Vahdat, D.; Hasenfuß, G.; Seidler, T. Designing meaningful outcome parameters using mobile technology: A new mobile application for telemonitoring of patients with heart failure. ESC Heart Fail. 2019, 6, 516–525. [Google Scholar] [CrossRef]
- Vetrovsky, T.; Clark, C.C.; Bisi, M.C.; Siranec, M.; Linhart, A.; Tufano, J.J.; Duncan, M.J.; Belohlavek, J. Advanced in accelerometry for cardiovascular patients: A systematic review with practical recommendatoins. ESC Heart Fail. 2020, 7, 2021–2031. [Google Scholar] [CrossRef]
- Shiraishi, Y.; Kohsaka, S.; Abe, T.; Mizuno, A.; Goda, A.; Izumi, Y.; Yagawa, M.; Akita, K.; Sawano, M.; Inohara, T.; et al. Validation of the Get with The Guideline-Heart Failure risk score in Japanese patients and the potential improvement of its discrimination ability by the inclusion of B-type natriuretic peptide level. Am. Heart J. 2016, 171, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, Y.; Kohsaka, S.; Nagai, T.; Goda, A.; Mizuno, A.; Nagatomo, Y.; Sujino, Y.; Fukuoka, R.; Sawano, M.; Kohno, T.; et al. Validation and recalibration of Seattle heart failure model in Japanese acute heart failure patients. J. Card. Fail. 2019, 25, 561–567. [Google Scholar] [CrossRef] [PubMed]
- McKee, P.A.; Castelli, W.P.; McNamara, P.M.; Kannel, W.B. The natural history of congestive heart failure: The Framingham study. N. Engl. J. Med. 1971, 285, 1441–1446. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, B.; Coats, A.J.; Tsutsui, H.; Abdelhamid, C.M.; Adamopoulos, S.; Albert, N.; Anker, S.D.; Atherton, J.; Böhm, M.; Butler, J.; et al. Universal Definition and Classification of Heart Failure: A Report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure. J. Card. Fail. 2021, 23, 352–380. [Google Scholar]
- Rockwood, K.; Song, X.; MacKnight, C.; Bergman, H.; Hogan, D.B.; McDowell, I.; Mitnitski, A. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005, 173, 489–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oshima, Y.; Kawaguchi, K.; Tanaka, S.; Ohkawara, K.; Hikihara, Y.; Ishikawa-Takata, K.; Tabata, I. Classifying household and locomotive activities using a triaxial accelerometer. Gait Posture 2010, 31, 370–374. [Google Scholar] [CrossRef]
- Ohkawara, K.; Oshima, Y.; Hikihara, Y.; Ishikawa, K.; Tabata, I.; Tanaka, S. Real-time estimation of daily physical activity intensity by a triaxial accelerometer and a gravity-removal classification algorithm. Br. J. Nutr. 2011, 105, 1681–1691. [Google Scholar] [CrossRef]
- Migueles, J.H.; Cadenas-Sanchez, C.; Ekelund, U.; Nyström, C.D.; Mora-Gonzalez, J.; Löf, M.; Labayen, I.; Ruiz, J.R.; Ortega, F.B. Accelerometer data collection and processing criteria to assess physical activity and other outcomes: A systematic review and practical considerations. Sports Med. 2017, 47, 1821–1845. [Google Scholar] [CrossRef]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.P.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef]
- Green, C.P.; Porter, C.B.; Bresnahan, D.R.; Spertus, J.A. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: A new health status measure for heart failure. J. Am. Coll. Cardiol. 2000, 35, 1245–1255. [Google Scholar] [CrossRef] [Green Version]
- Spertus, J.A.; Jones, P.G. Development and Validation of a Short Version of the Kansas City Cardiomyopathy Questionnaire. Circ. Cardiovasc. Qual Outcomes 2015, 8, 469–476. [Google Scholar] [CrossRef]
- US Food and Drug Administration. Clinical Outcome Assessment (COA) Qualification Submissions Office of Cardiology H, Endocrinology, and Nephrology (OCEHM) Division of Cardiovascular Nephrology (DCN). DDT COA #000084: Kansas City Cardiomyopathy Questionnaire (KCCQ). Available online: https://www.fda.gov/drugs/clinical-outcome-assessment-coa-qualification-program/ddt-coa-000084-kansas-city-cardiomyopathy-questionnaire-kccq (accessed on 5 February 2021).
- Gottlieb, S.; Federal Drug Administration Medical Device Development Tool (MDDT) Program. Statement from FDA Commission Scott Gottlieb, MDM, on New Steps to Advance Medical Device Innovation and Help Patients Gain Faster Access to Beneficial Technologies. Available online: https://www.fda.gov/news-events/press-announcements/statement-fda-commissioner-scott-gottlieb-md-new-steps-advance-medical-device-innovation-and-help (accessed on 5 February 2021).
- Clark, A.L.; Poole-Wilson, P.A.; Coats, A.J. Relation between ventilation and carbon dioxide production in patients with chronic heart failure. J. Am. Coll. Cardiol. 1992, 20, 1326–1332. [Google Scholar] [CrossRef] [Green Version]
- Francis, D.P.; Shamim, W.; Davies, L.C.; Piepoli, M.F.; Ponikowski, P.; Anker, S.D.; Coats, A.J. Cardiopulmonary exercise testing for prognosis in chronic heart failure: Continuous and independent prognostic value form VE/VCO2 slope and peak VO2. Eur. Heart J. 2000, 21, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Kitzman, D.W.; Brubaker, P.; Morgan, T.; Haykowsky, M.; Hundley, G.; Kraus, W.E.; Eggebeen, J.; Nicklas, B.J. Effect of Calooric Restriction or Aerobic Exercise Training on Peak Oxygen Consumption and Quality of Life in Obese Older Patients with Heart Failure With Preserved Ejection Fraction: A Randomized Clinical Trial. JAMA 2016, 315, 36–46. [Google Scholar] [CrossRef]
- Nolte, K.; Herrmann-Lingen, C.; Wachter, R.; Gelbrich, G.; Düngen, H.D.; Duvinage, A.; Hoischen, N.; von Oehsen, K.; Schwarz, S.; Hasenfuss, G.; et al. Effects of exercise training on different quality of life dimensions in heart failure with preserved ejection fraction: The Ex-DHF-P trial. Eur. J. Prev. Cardiol. 2015, 22, 582–593. [Google Scholar] [CrossRef]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: Executive Summary: A Report of the America College of Cariology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 140, e563–e595. [Google Scholar] [CrossRef]
- Snipelisky, D.; Kelly, J.; Levine, J.A.; Koepp, G.A.; Anstrom, K.J.; McNulty, S.E.; Zakeri, R.; Felker, G.M.; Hernandez, A.F.; Braunwald, E.; et al. Accelerometer-Measured daily Activity in Heart Failure with Preserved Ejection Fraction: Clinical Correlates and Association With Standard Heart Failure Severity Indices. Circ. Heart Fail. 2017, 10, e003878. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.; Omar, M.; Kistorp, C.; Poulsen, M.K.; Tuxen, C.; Gustafsson, I.; Køber, L.; Gustafsson, F.; Faber, J.; Fosbøl, E.L.; et al. Twelve weeks of treatment with empagliflozin in patients with heart failure and reduced ejection fraction: A double-blinded, randomized, and placebo-controlled trial. Am. Hear. J. 2020, 228, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Piepoli, M.F.; Hussain, R.I.; Comin-Colet, J.; Dosantos, R.; Ferber, P.; Jaarsma, T.; Edelmann, F. OUTSTEP-HF: Randomized controlled trial comparing short-term effects of sacubitril/valsartan versus enalapril on daily physical activity in patients with chronic heart failure with reduced ejection fraction. Eur. J. Heart Fail. 2020, 23, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Alosco, M.L.; Spitznagel, M.B.; Cohen, R.; Sweet, L.H.; Hayes, S.M.; Josephson, R.; Hughes, J.; Gunstad, J. Decreases in daily physical activity predict acute decline in attention and executive function in heart failure. J. Card. Fail. 2015, 21, 339–346. [Google Scholar] [CrossRef] [Green Version]
- Dibben, G.O.; Gandhi, M.M.; Taylor, R.S.; Dalal, H.M.; Metcalf, B.; Doherty, P.; Tang, L.H.; Kelson, M.; Hillsdon, M. Physical activity assessment by accelerometry in people with heart failure. BMC Sports Sci. Med. Rehabil. 2020, 12, 47. [Google Scholar] [CrossRef]
- McMurray, J.J.; Packer, M.; Desai, A.S.; Gong, J.; Lefkowitz, M.P.; Rizkala, A.R.; Rouleau, J.L.; Shi, V.C.; Solomon, S.D.; Swedberg, K.; et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N. Engl. J. Med. 2014, 371, 993–1004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McMurray, J.J.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Variable | All Patients n = 31 | KCCQ-OSS ≥ 60 n = 16 | KCCQ-OSS < 60 n = 15 | p-Value |
|---|---|---|---|---|
| Background | ||||
| Age, years | 63 (51–78) | 63 (44–69) | 72 (56–78) | 0.151 |
| Men, n (%) | 20 (65) | 11 (69) | 9 (60) | 0.611 |
| Body mass index, kg/m2 | 20.8 (18.9–24.9) | 24.2 (21.2–27.8) | 20.3 (18.5–20.7) | 0.001 |
| Systolic blood pressure, mm Hg | 97 (90–105) | 98 (85–105) | 97 (91–112) | 0.599 |
| Heart rate, beat per minute | 70 (60–75) | 70 (60–76) | 71 (60–75) | 0.770 |
| LVEF, % | 32 (24–42) | 27 (24–37) | 37 (29–58) | 0.041 |
| LVDd, mm | 58 (52–66) | 58 (54–67) | 56 (42–61) | 0.161 |
| LVDs, mm | 50 (41–58) | 52 (44–64) | 46 (29–54) | 0.045 |
| NYHA functional class, n (%) | 0.354 | |||
| II | 8 (26) | 3 (19) | 5 (33) | |
| III | 23 (74) | 13 (81) | 10 (67) | |
| Clinical frailty scale, n (%) | 0.038 | |||
| 2 (fit) | 12 (39) | 10 (62) | 2 (13) | |
| 3 (managing well) | 11 (36) | 4 (25) | 7 (47) | |
| 4 (very mild frailty) | 7 (23) | 2 (12) | 5 (33) | |
| 5 (mild frailty) | 1 (1) | 0 (0) | 1 (7) | |
| Triggers of acute decompensation | 0.610 | |||
| Nonadherence to diet, n (%) | 4 (13) | 2 (13) | 2 (13) | |
| Nonadherence to medication, n (%) | 1 (3) | 1 (6) | 0 (0) | |
| Overwork, n (%) | 4 (13) | 1 (19) | 3 (20) | |
| Arrhythmia, n (%) | 5 (16) | 3 (19) | 2 (13) | |
| Coronary ischemia, n (%) | 2 (7) | 1 (6) | 1 (6) | |
| Infection, n (%) | 3 (10) | 2 (13) | 1 (6) | |
| Others or none, n (%) | 15 (48) | 8 (57) | 7 (47) | |
| Comorbidities | ||||
| ≥1 HF hospitalization in the past year, n (%) | 21 (68) | 12 (75) | 9 (60) | 0.372 |
| Coronary artery disease, n (%) | 6 (19) | 2 (13) | 4 (25) | 0.411 |
| Atrial fibrillation, n (%) | 10 (32) | 5 (31) | 5 (33) | 0.901 |
| Hypertension, n (%) | 12 (39) | 9 (56) | 3 (20) | 0.038 |
| Diabetes mellitus, n (%) | 8 (26) | 3 (19) | 5 (33) | 0.354 |
| Stroke, n (%) | 7 (23) | 4 (25) | 3 (20) | 0.739 |
| COPD, n (%) | 2 (7) | 1 (6) | 1 (7) | 0.962 |
| Laboratory tests | ||||
| Hemoglobin, g/dL | 12.8 (11.3–14.2) | 13.0 (11.4–14.9) | 12.4 (11.2–13.8) | 0.572 |
| Creatinine, mg/L | 1.1 (0.9–1.5) | 1.1 (0.9–1.4) | 1.2 (0.9–1.5) | 0.520 |
| Blood urea nitrogen, mg/L | 24.6 (19.2–34.4) | 20.6 (14.8–30.5) | 30.0 (24.6–35.5) | 0.027 |
| Sodium, mEq/L | 138.9 (136.6–140.6) | 138.7 (136.7–140.0) | 138.9 (136.1–140.9) | 0.545 |
| Potassium, mEq/L | 4.3 (4·0–4·6) | 4.3 (4.2–4.5) | 4.3 (3.9–4.6) | 0.545 |
| Total bilirubin, mg/L | 0.9 (0.7–1.3) | 0.9 (0.7–1.2) | 0.9 (0.6–1.3) | 0.861 |
| Albumin, mg/L | 3.9 (3.6–4.1) | 4.0 (3.8–4.1) | 3.8 (3.5–4.1) | 0.318 |
| BNP at hospitalization, pg/mL | 1118 (447–1498) | 835 (248–1173) | 1478 (573–1958) | 0.022 |
| BNP at discharge, pg/mL | 370 (244–437) | 253 (160–397) | 413 (334–788) | 0.001 |
| BNP improvement, % | 58.4 (28.4–74.9) | 65.1 (26.8–78.8) | 56.0 (20.9–71.8) | 0.458 |
| HF treatment | ||||
| Loop diuretics, n (%) | 27 (88) | 12 (75) | 15 (100) | 0.038 |
| ACEI or ARB, n (%) | 23 (74) | 12 (75) | 11 (73) | 0.916 |
| ARNI, n (%) | 0 (0) | 0 (0) | 0 (0) | – |
| Beta blocker, n (%) | 27 (88) | 14 (88) | 13 (87) | 0.945 |
| MRA, n (%) | 20 (65) | 11 (69) | 9 (60) | 0.611 |
| SGLT2i, n (%) | 11 (35) | 5 (31) | 6 (40) | 0.611 |
| ICD, n (%) | 3 (10) | 2 (13) | 1 (7) | 0.583 |
| CRT, n (%) | 3 (10) | 2 (13) | 1 (7) | 0.583 |
| CPX parameters * | ||||
| Peak VO2, mL/kg/min | 11.5 (10.0–15.0) | 11.5 (10.1–17.9) | 11.7 (9.4–13.1) | 0.427 |
| AT VO2, mL/kg/min | 9.6 (8.6–11.3) | 9.4 (8.4–11.9) | 9.6 (8.6–10.6) | 0.701 |
| VE vs. VCO2 slope | 37.4 (30.2–46.6) | 32.0 (29.2–39.6) | 39.2 (33.6–50.0) | 0.050 |
| Accelerometer-measured parameters | ||||
| Daily step count, n/day | 2604 (926–4247) | 2792 (2259–4360) | 1138 (475–4230) | 0.049 |
| Exercise time | 2.2 (1.2–3.5) | 3.1 (2.2–3.9) | 1.2 (0.6–2.0) | 0.001 |
| %sedentary time, % | 76.3 (69.4–81.5) | 69.9 (65.1–79.4) | 77.6 (74.2–88.2) | 0.030 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shiraishi, Y.; Niimi, N.; Goda, A.; Takei, M.; Kimura, T.; Kohno, T.; Kawana, M.; Fukuda, K.; Kohsaka, S. Assessment of Physical Activity Using Waist-Worn Accelerometers in Hospitalized Heart Failure Patients and Its Relationship with Kansas City Cardiomyopathy Questionnaire. J. Clin. Med. 2021, 10, 4103. https://doi.org/10.3390/jcm10184103
Shiraishi Y, Niimi N, Goda A, Takei M, Kimura T, Kohno T, Kawana M, Fukuda K, Kohsaka S. Assessment of Physical Activity Using Waist-Worn Accelerometers in Hospitalized Heart Failure Patients and Its Relationship with Kansas City Cardiomyopathy Questionnaire. Journal of Clinical Medicine. 2021; 10(18):4103. https://doi.org/10.3390/jcm10184103
Chicago/Turabian StyleShiraishi, Yasuyuki, Nozomi Niimi, Ayumi Goda, Makoto Takei, Takehiro Kimura, Takashi Kohno, Masataka Kawana, Keiichi Fukuda, and Shun Kohsaka. 2021. "Assessment of Physical Activity Using Waist-Worn Accelerometers in Hospitalized Heart Failure Patients and Its Relationship with Kansas City Cardiomyopathy Questionnaire" Journal of Clinical Medicine 10, no. 18: 4103. https://doi.org/10.3390/jcm10184103
APA StyleShiraishi, Y., Niimi, N., Goda, A., Takei, M., Kimura, T., Kohno, T., Kawana, M., Fukuda, K., & Kohsaka, S. (2021). Assessment of Physical Activity Using Waist-Worn Accelerometers in Hospitalized Heart Failure Patients and Its Relationship with Kansas City Cardiomyopathy Questionnaire. Journal of Clinical Medicine, 10(18), 4103. https://doi.org/10.3390/jcm10184103






