Discriminant Analysis of Main Prognostic Factors Associated with Hemodynamically Significant PDA: Apgar Score, Silverman–Anderson Score, and NT-Pro-BNP Level
Abstract
1. Introduction
2. Materials and Methods
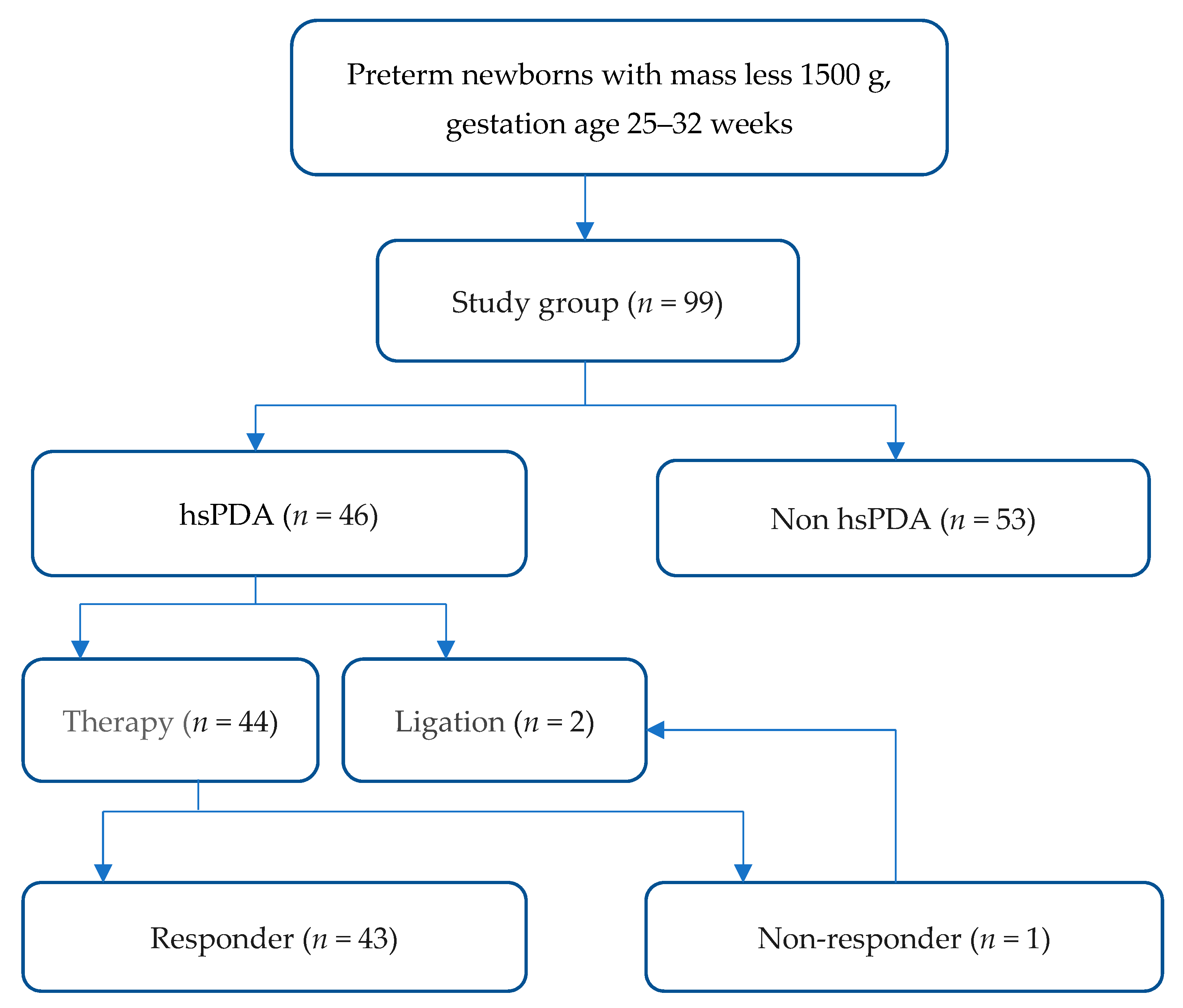
2.1. Subjects and Data Collection
2.2. Data Analysis
3. Results
3.1. Baseline Characteristics
3.2. Prediction Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Clyman, R.I.; Couto, J.; Murphy, G.M. Patent Ductus Arteriosus: Are Current Neonatal Treatment Options Better or Worse Than No Treatment at All? Semin. Perinatol. 2012, 36, 123–129. [Google Scholar] [CrossRef]
- Yoo, H.; Lee, J.A.; Oh, S.; Jung, Y.H.; Sohn, J.A.; Shin, S.H.; Choi, C.W.; Kim, E.K.; Kim, H.S.; Kim, B. Il Comparison of the mortality and in-hospital outcomes of preterm infants treated with ibuprofen for patent ductus arteriosus with or without clinical symptoms attributable to the patent ductus arteriosus at the time of ibuprofen Treatment. J. Korean Med. Sci. 2017, 32, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Parkerson, S.; Philip, R.; Talati, A.; Sathanandam, S. Management of Patent Ductus Arteriosus in Premature Infants in. Front Pediatr. 2021, 8, 1–11. [Google Scholar] [CrossRef]
- Dice, J.E.; Bhatia, J. Patent Ductus Arteriosus: An Overview. J. Pediatr. Pharmacol. Ther. 2007, 12, 138–146. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, H.J.; Park, H.K.; Ahn, J.H.; Kim, H.S.; Jang, H.J.; Ro, S.K.; Kim, H. Surgical ligation of patent ductus arteriosus in preterm neonates weighing less than 1500g: A 9-year single center experience. J. Cardiothorac. Surg. 2020, 15, 1–9. [Google Scholar] [CrossRef]
- Noori, S.; McCoy, M.; Friedlich, P.; Bright, B.; Gottipati, V.; Seri, I.; Sekar, K. Failure of ductus arteriosus closure is associated with increased mortality in preterm infants. Pediatrics 2009, 123, 2–4. [Google Scholar] [CrossRef]
- Backes, C.H.; Rivera, B.K.; Bridge, J.A.; Armstrong, A.K.; Boe, B.A.; Berman, D.P.; Fick, T.; Holzer, R.J.; Hijazi, Z.M.; Abadir, S.; et al. Percutaneous Patent Ductus Arteriosus (PDA) Closure During Infancy: A Meta-analysis. Pediatrics 2017, 139, e20162927. [Google Scholar] [CrossRef]
- Semberova, J.; Sirc, J.; Miletin, J.; Kucera, J.; Berka, I.; Sebkova, S.; O’Sullivan, S.; Franklin, O.; Stranak, Z. Spontaneous Closure of Patent Ductus Arteriosus in Infants ≤1500 g. Pediatrics 2017, 140, e20164258. [Google Scholar] [CrossRef]
- Benitz, W.E. Patent Ductus Arteriosus in Preterm Infants. Pediatrics 2016, 137, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Koch, J.; Hensley, G.; Roy, L.; Brown, S.; Ramaciotti, C.; Rosenfeld, C.R. Prevalence of Spontaneous Closure of the Ductus Arteriosus in Neonates at a Birth Weight of 1000 Grams or Less. Pediatrics 2006, 117, 1113–1121. [Google Scholar] [CrossRef] [PubMed]
- Nemerofsky, S.L.; Parravicini, E.; Bateman, D.; Kleinman, C.; Polin, R.A.; Lorenz, J.M. The ductus arteriosus rarely requires treatment in infants >1000 grams. Am. J. Perinatol. 2008, 25, 661–666. [Google Scholar] [CrossRef]
- Kabra, N.S.; Schmidt, B.; Roberts, R.S.; Doyle, L.W.; Papile, L.; Fanaroff, A. Neurosensory Impairment after Surgical Closure of Patent Ductus Arteriosus in Extremely Low Birth Weight Infants: Results from the Trial of Indomethacin Prophylaxis in Preterms. J. Pediatr. 2007, 150, 229.e1–234.e1. [Google Scholar] [CrossRef]
- Clyman, R.; Cassady, G.; Kirklin, J.K.; Collins, M.; Philips, J.B. The Role of Patent Ductus Arteriosus Ligation in Bronchopulmonary Dysplasia: Reexamining a Randomized Controlled Trial. J. Pediatr. 2009, 154, 873–876. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, J.L.; Noori, S. What is a hemodynamically significant PDA in preterm infants? Congenit. Heart Dis. 2019, 14, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.H.; Lee, B.S.; Jung, E.; Oh, M.Y.; Do, H.J.; Kim, E.A.R.; Kim, K.S. Plasma B-type natriuretic peptide cannot predict treatment response to ibuprofen in preterm infants with patent ductus arteriosus. Sci. Rep. 2020, 10, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Mänhardt, L.B.; Norozi, K.; Müller, C.; Willaschek, C.; Kostuch, B.; Buchhorn, R. NT-Pro-B-Type Natriuretic Peptide Levels in Infants with Failure to Thrive due to Caloric Deprivation. Int. J. Pediatr. 2010, 2010, 1–4. [Google Scholar] [CrossRef] [PubMed][Green Version]
- König, K.; Guy, K.J.; Drew, S.M.; Barfield, C.P. B-type and N-terminal pro-B-type natriuretic peptides are equally useful in assessing patent ductus arteriosus in very preterm infants. Acta Paediatr. Int. J. Paediatr. 2015, 104, e139–e142. [Google Scholar] [CrossRef]
- Sanjeev, S.; Pettersen, M.; Lua, J.; Thomas, R.; Shankaran, S.; L’Ecuyer, T. Role of plasma B-type natriuretic peptide in screening for hemodynamically significant patent ductus arteriosus in preterm neonates. J. Perinatol. 2005, 25, 709–713. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.A. Practice for preterm patent ductus arteriosus; focusing on the hemodynamic significance and the impact on the neonatal outcomes. Korean J. Pediatr. 2019, 62, 245–251. [Google Scholar] [CrossRef]
- Gillam-Krakauer, M.; Reese, J. Diagnosis and management of patent ductus arteriosus. Neoreviews 2018, 19, e394–e402. [Google Scholar] [CrossRef]
- Cuna, A.; Kandasamy, J.; Sims, B. B-type natriuretic peptide and mortality in extremely low birth weight infants with pulmonary hypertension: A retrospective cohort analysis. BMC Pediatr. 2014, 14, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Slaughter, J.L.; Cua, C.L.; Notestine, J.L.; Rivera, B.K.; Marzec, L.; Hade, E.M.; Maitre, N.L.; Klebanoff, M.A.; Ilgenfritz, M.; Le, V.T.; et al. Early prediction of spontaneous patent ductus arteriosus (PDA) closure and pdaassociated outcomes: A prospective cohort investigation. BMC Pediatr. 2019, 19, 1–17. [Google Scholar] [CrossRef]
- Sehgal, A.; Paul, E.; Menahem, S. Functional echocardiography in staging for ductal disease severity: Role in predicting outcomes. Eur. J. Pediatr. 2013, 172, 179–184. [Google Scholar] [CrossRef]
- Weisz, D.E.; McNamara, P.J.; El-Khuffash, A. Cardiac biomarkers and haemodynamically significant patent ductus arteriosus in preterm infants. Early Hum. Dev. 2017, 105, 41–47. [Google Scholar] [CrossRef]
- Jain, A.; Shah, P.S. Diagnosis, evaluation, and management of patent ductus arteriosus in preterm neonates. JAMA Pediatr. 2015, 169, 863–872. [Google Scholar] [CrossRef] [PubMed]
- Kleinrouweler, C.E.; Cheong-See, F.M.; Collins, G.S.; Kwee, A.; Thangaratinam, S.; Khan, K.S.; Mol, B.W.J.; Pajkrt, E.; Moons, K.G.M.; Schuit, E. Prognostic models in obstetrics: Available, but far from applicable. Am. J. Obstet. Gynecol. 2016, 214, 79.e36–90.e36. [Google Scholar] [CrossRef] [PubMed]
- Silverman, W.; Anderson, D. A controlled clinical trial of effects of water mist on obstructive respiratory signs, death rate and necropsy findings among premature infants. Pediatrics 1956, 17, 1–10. [Google Scholar]
- Hedstrom, A.B.; Gove, N.E.; Mayock, D.E.; Batra, M. Performance of the Silverman Anderson Respiratory Severity Score in predicting PCO2 and respiratory support in newborns: A prospective cohort study. J. Perinatol. 2018, 38, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.; El-Khuffash, A.F. Defining “haemodynamic significance” of the patent ductus arteriosus: Do we have all the answers? Neonatology 2020, 117, 225–232. [Google Scholar] [CrossRef]
- Mitra, S.; Disher, T. Early treatment versus expectant management of hemodynamically significant patent ductus arteriosus for preterm infants. Cochrane Database Syst. Rev. 2019, 12, CD013278. [Google Scholar] [CrossRef]
- Hagadorn, J.I.; Brownell, E.A.; Trzaski, J.M.; Johnson, K.R.; Lainwala, S.; Campbell, B.T.; Herbst, K.W. Trends and variation in management and outcomes of very low-birth-weight infants with patent ductus arteriosus. Pediatr. Res. 2016, 80, 785–792. [Google Scholar] [CrossRef]
- Rolland, A.; Shankar-Aguilera, S.; Diomandé, D.; Zupan-Simunek, V.; Boileau, P. Natural evolution of patent ductus arteriosus in the extremely preterm infant. Arch. Dis. Child. Fetal Neonatal Ed. 2015, 100, F55–F58. [Google Scholar] [CrossRef][Green Version]
- Sung, S.I.; Chang, Y.S.; Chun, J.Y.; Yoon, S.A.; Yoo, H.S.; Ahn, S.Y.; Park, W.S. Mandatory Closure Versus Nonintervention for Patent Ductus Arteriosus in Very Preterm Infants. J. Pediatr. 2016, 177, 66.e1–71.e1. [Google Scholar] [CrossRef]
- El-Khuffash, A.; Weisz, D.E.; McNamara, P.J. Reflections of the changes in patent ductus arteriosus management during the last 10 € years. Arch. Dis. Child. Fetal Neonatal Ed. 2016, 101, F474–F478. [Google Scholar] [CrossRef]
- El-Khuffash, A.; James, A.T.; Corcoran, J.D.; Dicker, P.; Franklin, O.; Elsayed, Y.N.; Ting, J.Y.; Sehgal, A.; Malikiwi, A.; Harabor, A.; et al. A Patent Ductus Arteriosus Severity Score Predicts Chronic Lung Disease or Death before Discharge. J. Pediatr. 2015, 167, 1354.e2–1361.e2. [Google Scholar] [CrossRef] [PubMed]
- Prescott, S.; Keim-Malpass, J. Patent Ductus Arteriosus in the Preterm Infant: Diagnostic and Treatment Options. Adv. Neonatal Care 2017, 17, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Coviello, C.; Tataranno, M.L.; Corsini, I.; Leonardi, V.; Longini, M.; Bazzini, F.; Buonocore, G.; Dani, C. Isoprostanes as Biomarker for Patent Ductus Arteriosus in Preterm Infants. Front. Pediatr. 2020, 8, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Bardanzellu, F.; Piras, C.; Atzei, A.; Neroni, P.; Fanos, V. Early Urinary Metabolomics in Patent Ductus Arteriosus Anticipates the Fate: Preliminary Data. Front. Pediatr. 2020, 8, 1–18. [Google Scholar] [CrossRef]
- Kindler, A.; Seipolt, B.; Heilmann, A.; Range, U.; Rüdiger, M.; Hofmann, S.R. Development of a diagnostic clinical score for hemodynamically significant patent ductus arteriosus. Front. Pediatr. 2017, 5, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Andersson, S.; Tikkanen, I.; Pesonen, E.; Meretoja, O.; Hynynen, M.; Fyhrquist, F. Atrial natriuretic peptide in patent ductus arteriosus. Pediatr. Res. 1987, 21, 396–398. [Google Scholar] [CrossRef]
- Holmström, H.; Hall, C.; Thaulow, E. Plasma levels of natriuretic peptides and hemodynamic assessment of patent ductus arteriosus in preterm infants. Acta Paediatr. Int. J. Paediatr. 2001, 90, 184–191. [Google Scholar] [CrossRef]
- Nuntnarumit, P.; Khositseth, A.; Thanomsingh, P. N-terminal probrain natriuretic peptide and patent ductus arteriosus in preterm infants. J. Perinatol. 2009, 29, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Gokulakrishnan, G.; Sathappan, V.S.; Kulkarni, M.; Leeflang, M.M.G.; Fernandes, C.J.; Price, J.; Pammi, M. Brain natriuretic peptide and N-terminal brain natriuretic peptide for the diagnosis of hemodynamically significant patent ductus arteriosus in preterm neonates. Cochrane Database Syst. Rev. 2018, 2018, CD013129. [Google Scholar] [CrossRef]
- El-Khuffash, A.F.; Amoruso, M.; Culliton, M.; Molloy, E.J. N-terminal pro-B-type natriuretic peptide as a marker of ductal haemodynamic significance in preterm infants: A prospective observational study. Arch. Dis. Child. Fetal Neonatal Ed. 2007, 92, F421–F422. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Letzner, J.; Berger, F.; Schwabe, S.; Benzing, J.; Morgenthaler, N.G.; Bucher, H.U.; Bührer, C.; Arlettaz, R.; Wellmann, S. Plasma C-terminal pro-endothelin-1 and the natriuretic pro-peptides NT-proBNP and MR-proANP in very preterm infants with patent ductus arteriosus. Neonatology 2012, 101, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Cambonie, G.; Dupuy, A.M.; Combes, C.; Vincenti, M.; Mesnage, R.; Cristol, J.P. Can a clinical decision rule help ductus arteriosus management in preterm neonates? Acta Paediatr. Int. J. Paediatr. 2012, 101, 213–218. [Google Scholar] [CrossRef]
- Ramakrishnan, S.; Heung, Y.M.; Round, J.; Morris, T.P.; Collinson, P.; Williams, A.F. Early N-terminal pro-brain natriuretic peptide measurements predict clinically significant ductus arteriosus in preterm infants. Acta Paediatr. Int. J. Paediatr. 2009, 98, 1254–1259. [Google Scholar] [CrossRef] [PubMed]
- Dhuper, S.; Kim, R.; Weichbrod, L.; Mahdi, E.; Shah, N.; Kona, S.; Sokal, M.; Buddhe, S. NT-proBNP levels improve the ability of predicting a hemodynamically significant patent ductus arteriosus in very low-birth-weight infants. J. Clin. Neonatol. 2012, 1, Sa82. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | hsPDA | Non-hsPDA | p-Value |
|---|---|---|---|
| weight (g) | 976 ± 287 | 1049 ± 261 | 0.078 |
| gestation (weeks) | 27.2 ± 2.62 | 27.9 ± 2.47 | 0.072 |
| male gender | 24 (52.2) | 29 (54.7) | 0.879 |
| vaginal delivery | 8 (17.4) | 18 (33.9) | 0.071 |
| antenatal steroids | 19 (41.3) | 26 (49.0) | 0.493 |
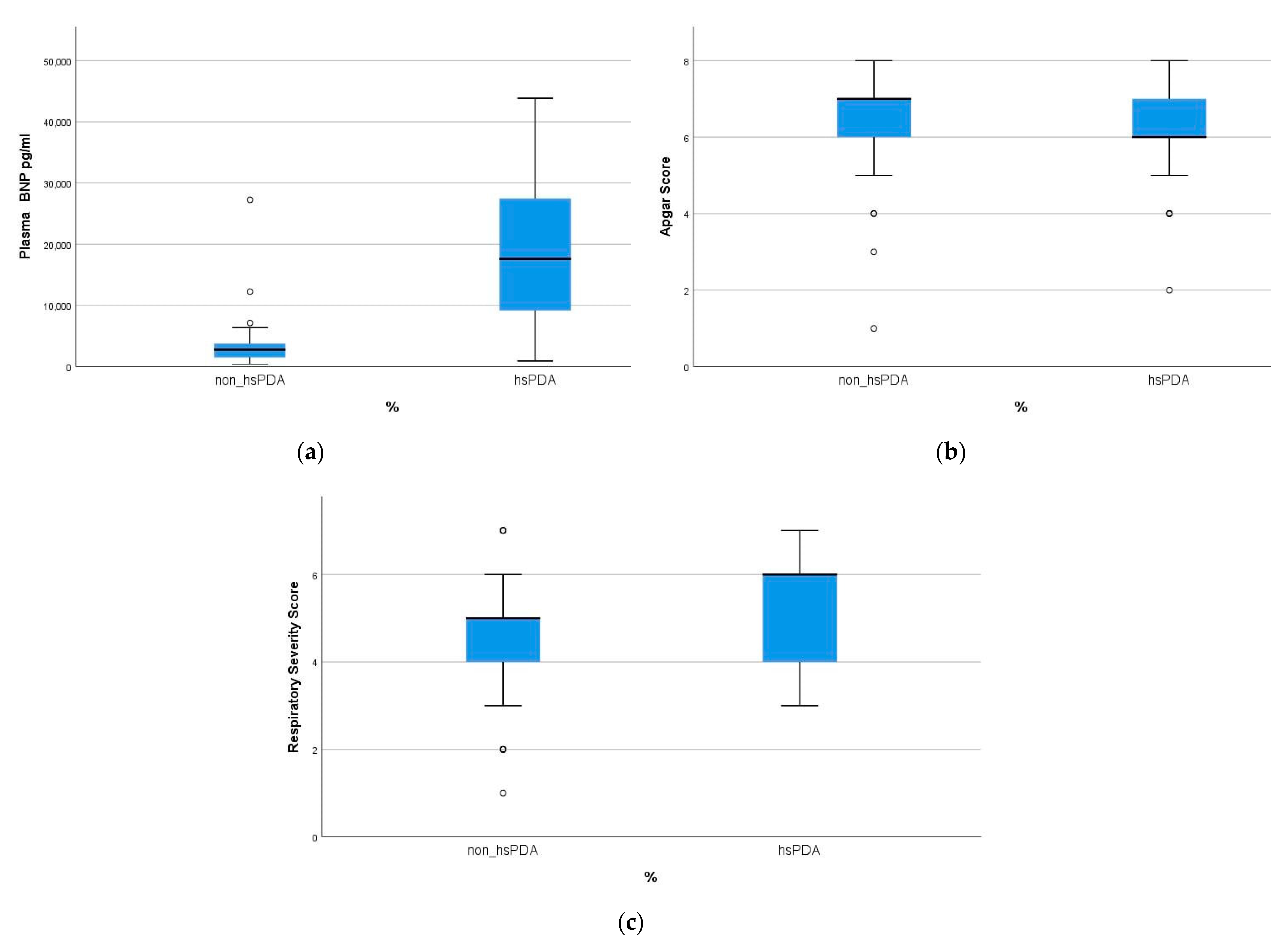
| Apgar score (≤6) | 12 (26.1) | 5 (9.4) | 0.021 |
| Silverman–Anderson score (≥5) | 33 (71.7) | 2 (49.1) | 0.022 |
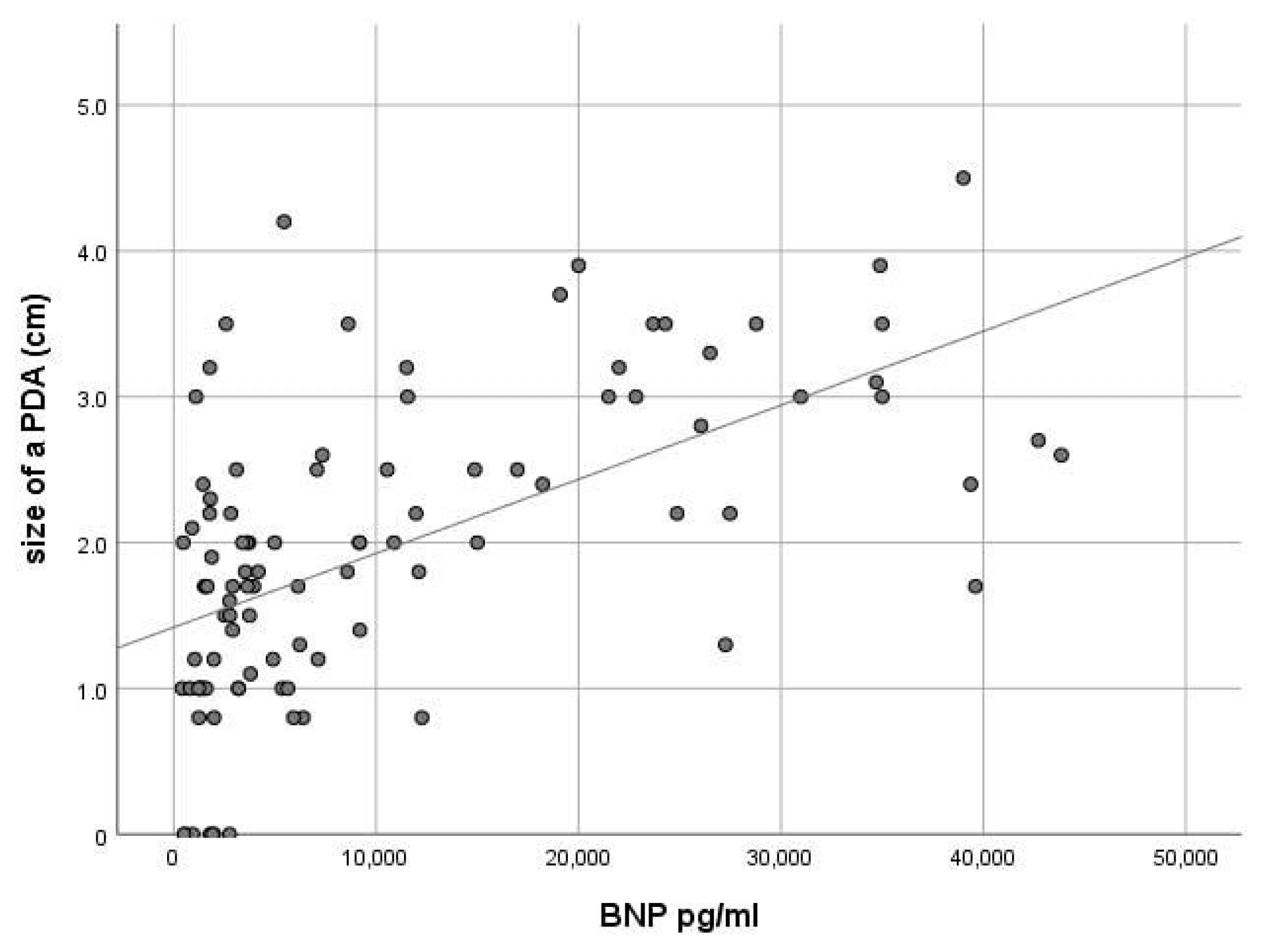
| NT-pro-BNP level (pg/mL) | 18.967 ± 12.518 | 3448 ± 3926 | 0.001 |
| Parameters | hsPDA | Non-hsPDA |
|---|---|---|
| DA (mm) | 2.78 ± 0.72 | 1.26 ± 0.66 |
| DA ≥ 1.5 mm | 45 (97.8) | 22 (41.5) |
| LA/Ao ≥ 1.4 mm | 34 (73.9) | 2 (3.8) |
| Retrograde descending aortic flow ≥ 50% of antegrade blood flow | 35 (76.1) | 4 (7.5) |
| Actual Classification | Predicted Group Membership | Total | |
|---|---|---|---|
| Non-hsPDA | hsPDA | ||
| non-hsPDA | 47 (88.7) | 6 (11.3) | 53 |
| hsPDA | 8 (17.4) | 38 (82.6) | 46 |
| Total | 55 | 44 | 99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Permyakova, A.V.; Porodikov, A.; Kuchumov, A.G.; Biyanov, A.; Arutunyan, V.; Furman, E.G.; Sinelnkov, Y.S. Discriminant Analysis of Main Prognostic Factors Associated with Hemodynamically Significant PDA: Apgar Score, Silverman–Anderson Score, and NT-Pro-BNP Level. J. Clin. Med. 2021, 10, 3729. https://doi.org/10.3390/jcm10163729
Permyakova AV, Porodikov A, Kuchumov AG, Biyanov A, Arutunyan V, Furman EG, Sinelnkov YS. Discriminant Analysis of Main Prognostic Factors Associated with Hemodynamically Significant PDA: Apgar Score, Silverman–Anderson Score, and NT-Pro-BNP Level. Journal of Clinical Medicine. 2021; 10(16):3729. https://doi.org/10.3390/jcm10163729
Chicago/Turabian StylePermyakova, Anna V., Artem Porodikov, Alex G. Kuchumov, Alexey Biyanov, Vagram Arutunyan, Evgeniy G. Furman, and Yuriy S. Sinelnkov. 2021. "Discriminant Analysis of Main Prognostic Factors Associated with Hemodynamically Significant PDA: Apgar Score, Silverman–Anderson Score, and NT-Pro-BNP Level" Journal of Clinical Medicine 10, no. 16: 3729. https://doi.org/10.3390/jcm10163729
APA StylePermyakova, A. V., Porodikov, A., Kuchumov, A. G., Biyanov, A., Arutunyan, V., Furman, E. G., & Sinelnkov, Y. S. (2021). Discriminant Analysis of Main Prognostic Factors Associated with Hemodynamically Significant PDA: Apgar Score, Silverman–Anderson Score, and NT-Pro-BNP Level. Journal of Clinical Medicine, 10(16), 3729. https://doi.org/10.3390/jcm10163729









