Safety, Fear and Neuromuscular Responses after a Resisted Knee Extension Performed to Failure in Patients with Severe Haemophilia
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedures
2.3. Data Analysis
- (1)
- Maximum repetition values. A moving root-mean-squared (RMS) smoothing filter was applied to the EMG signals, implemented with a 500ms window (250ms backward and 250ms forward) for each signal sample. The peaks of maximum and minimum values of the RMS corresponding to each of the contractions performed were obtained for all three muscles. The maximum RMS activation percentage (amplitude) was calculated by normalising the result of each condition and muscle to the highest RMS activation value reached by the participant during the whole experimental session (nRMS), including MVICs, so a true maximum value was obtained.
- (2)
- Median frequency (MF). The original EMG signal was segmented into individual contractions based on the maximum and minimum values of the RMS EMG. To establish the start and end of each contraction, we considered a minimum activation of 10% with respect to the difference between the maximum and minimum values of each contraction. The MF was obtained from the power spectrum of each of the contractions registered in the EMG by applying a Fourier transformation. The MF represents the frequency at which the power spectrum of a signal was divided into two equal halves. The final MF value was normalised to the maximum MF value in the series.
- (3)
- The wavelet index between wavelengths at different scales (WIRW51) [26]. This index was more adequate to assess changes in muscle output than the traditional MF and other approaches, even in the presence of additive Gaussian noise in the EMG signal [26,27]. The discrete wavelet transform (DWT) breaks a signal into successive approximations using a wavelet function ψ (t) and a scaling function ϕ (t). The WIRW51 index expresses the ratio of the signal amplitude changes occurring at two different scales. Its mathematical expression is as follows:where D5(n) and D1(n) are the signal approximations in scales five and one, obtained by DWT with the sym5 wavelet function. The value of the WIRW51 index was also normalised to the maximum value of the session for each participant. Finally, the three parameters analysed were normalised in intervals of 20% of the total number of repetitions until task failure was reached. Neuromuscular fatigue was determined by a significant decrease in MF and significant increase in nRMS [28] and WIRW51 [26,27] over time. Since these parameters do not necessarily change simultaneously, all of them were needed to consider the occurrence of neuromuscular fatigue.
2.4. Statistical Analysis
3. Results
3.1. Descriptive, Safety and Fear of Movement Data
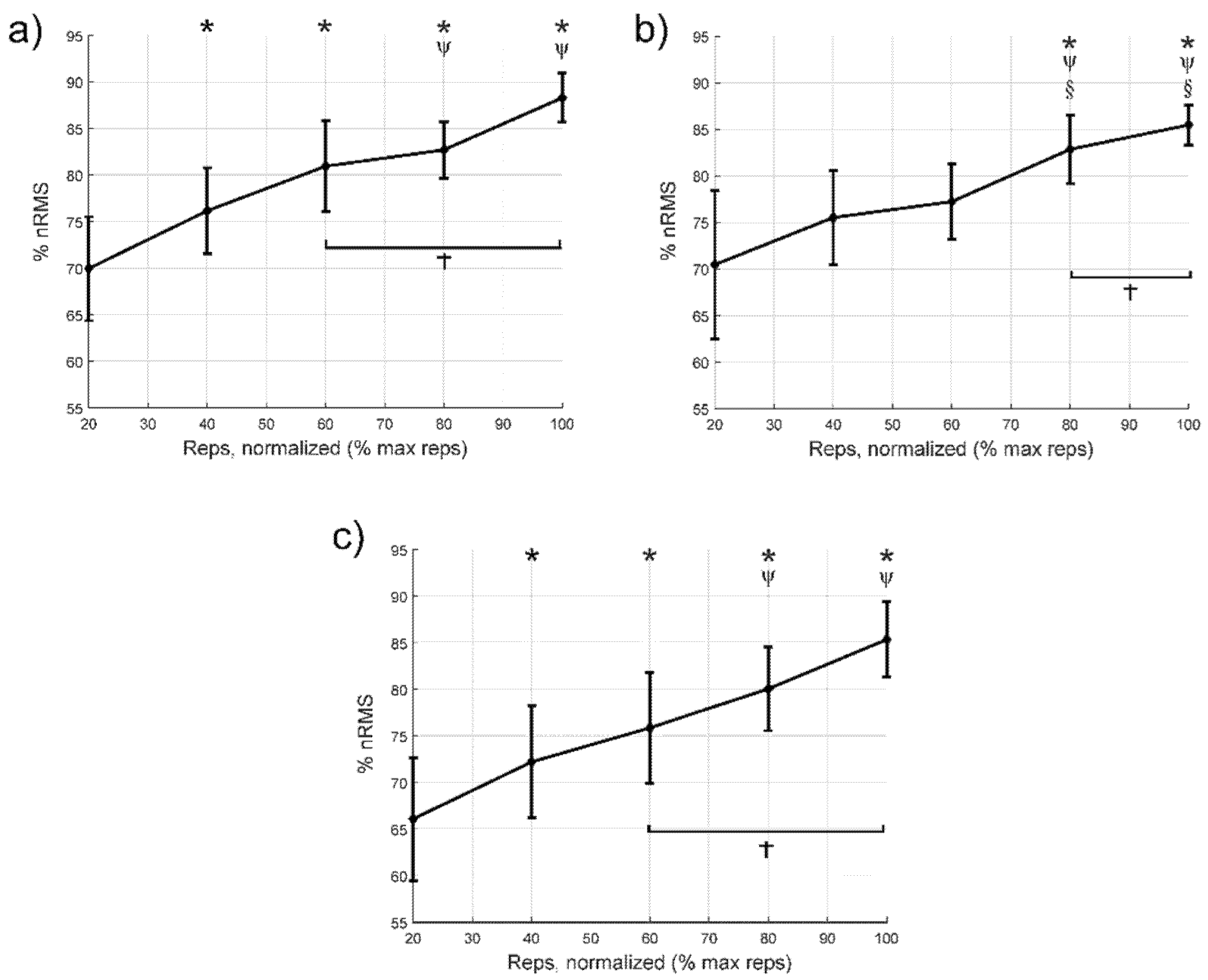
3.2. Normalised Values of Electromyographic Amplitude
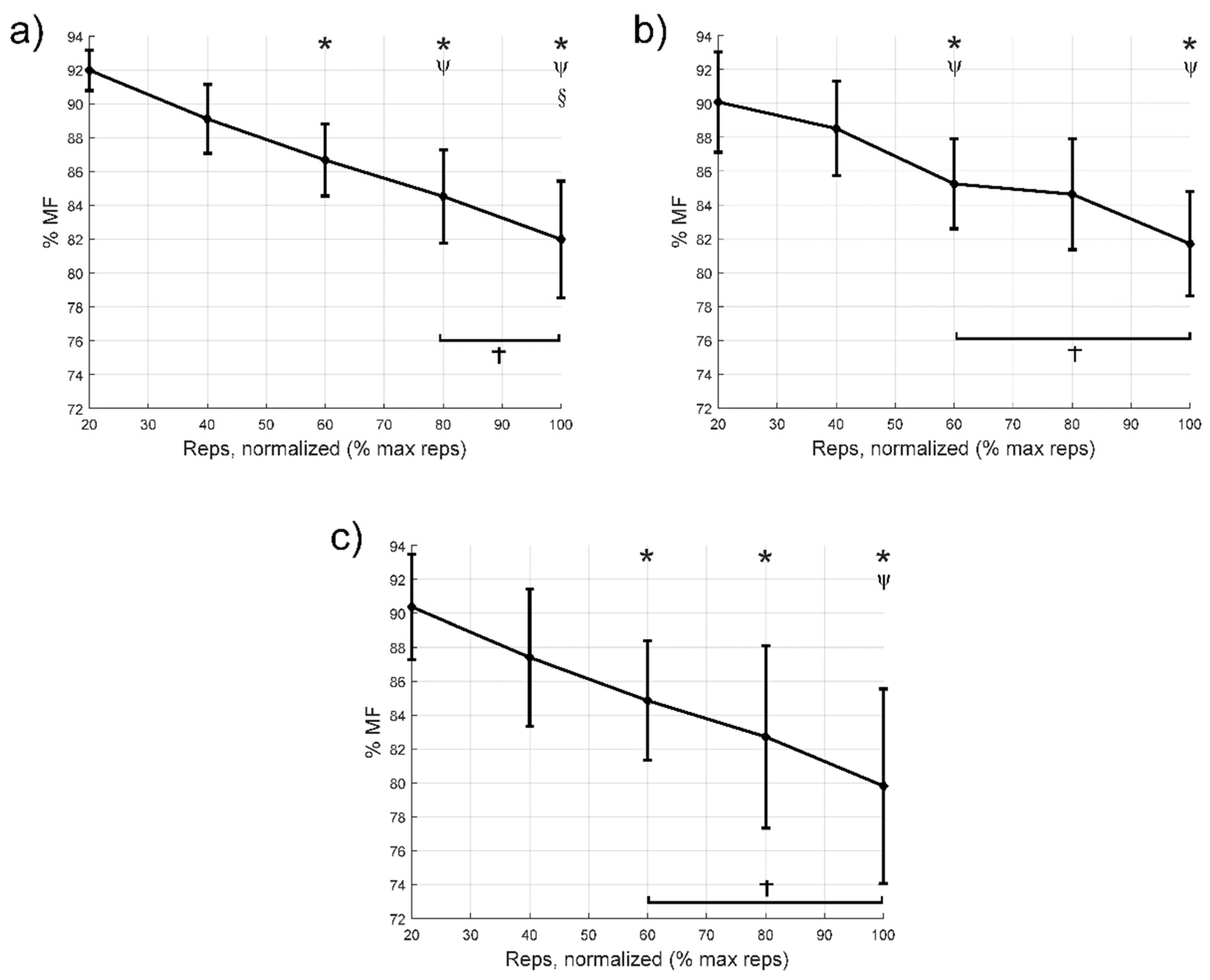
3.3. Median Frequency
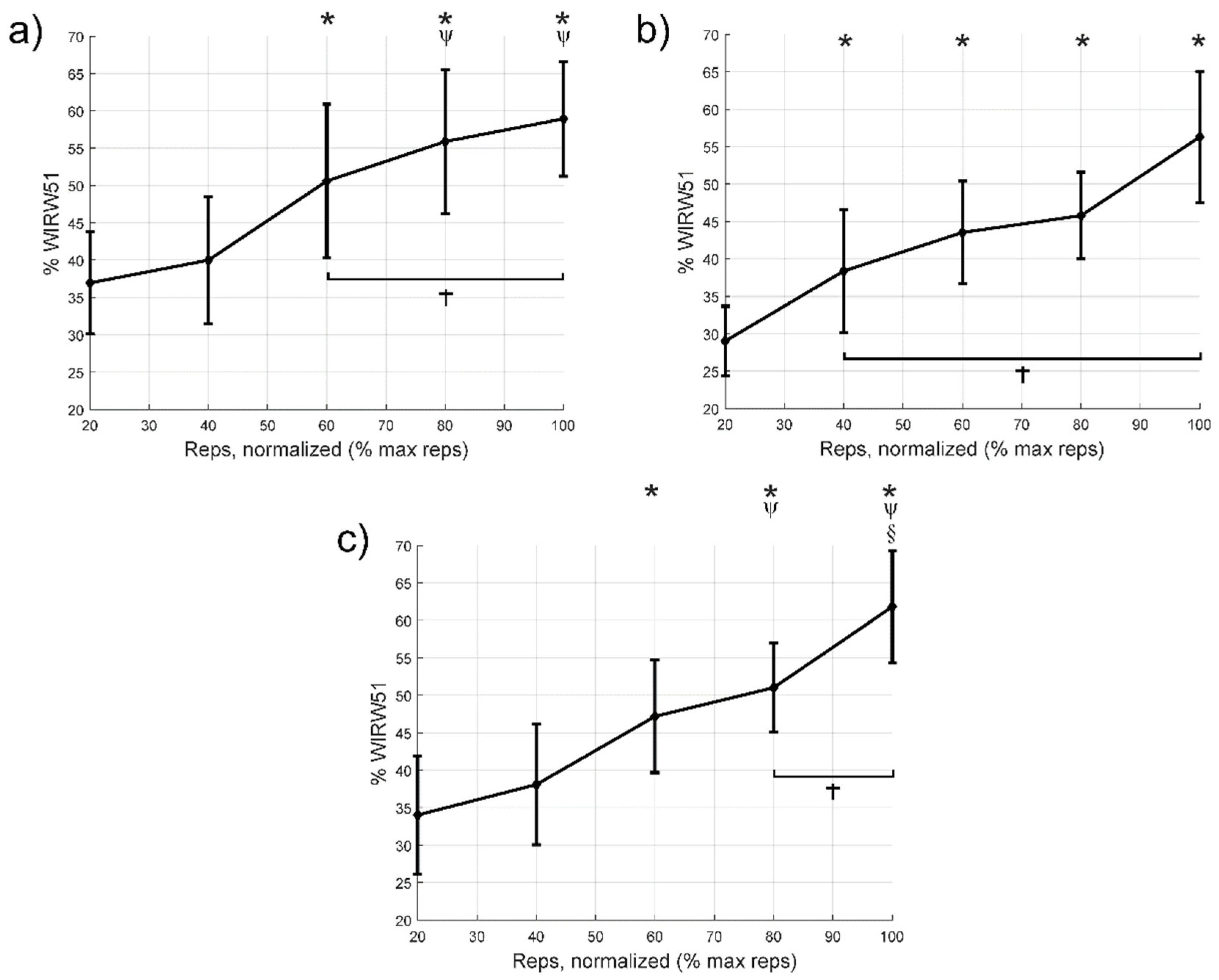
3.4. Wavelet Index between Wavelengths at Different Scales
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Srivastava, A.; Santagostino, E.; Dougall, A.; Kitchen, S.; Sutherland, M.; Pipe, S.W.; Carcao, M.; Mahlangu, J.; Ragni, M.V.; Windyga, J.; et al. WFH Guidelines for the Management of Hemophilia, 3rd ed.; Haemophilia: East Brunswick, NJ, USA, 2020. [Google Scholar]
- Iorio, A.; Stonebraker, J.S.; Chambost, H.; Makris, M.; Coffin, D.; Herr, C.; Germini, F. Establishing the Prevalence and Prevalence at Birth of Hemophilia in Males: A Meta-analytic Approach Using National Registries. Ann. Intern. Med. 2019, 171, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, G.S.; Valderramas, S.; Gomes, A.R.; Budib, M.B.; Wolff, Á.L.; Ramos, A.A.T. Physical exercise, pain and musculoskeletal function in patients with haemophilia: A systematic review. Haemophilia 2016, 22, e119–e129. [Google Scholar] [CrossRef] [PubMed]
- Van Vulpen, L.F.D.; Holstein, K.; Martinoli, C. Joint disease in haemophilia: Pathophysiology, pain and imaging. Haemophilia 2018, 24, 44–49. [Google Scholar] [CrossRef]
- Kurz, E.; Herbsleb, M.; Grassme, R.; Anders, C.; Hilberg, T. Trunk muscle activation characteristics in patients with severe haemophilia. Haemophilia 2016, 23, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Brewer, A.K.; Mauser-Bunschoten, E.P.; Key, N.S.; Kitchen, S.; Llinas, A.; Ludlam, C.A.; Mahlangu, J.N.; Mulder, K.; Poon, M.C.; et al. Guidelines for the management of hemophilia. Haemophilia 2012, 19, e1–e47. [Google Scholar] [CrossRef]
- Weyand, A.C.; Pipe, S.W. New therapies for hemophilia. Blood 2019, 133, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Strike, K.; Mulder, K.; Michael, R. Exercise for haemophilia. Cochrane Database Syst. Rev. 2016. [Google Scholar] [CrossRef]
- Souza, J.C.; Simoes, H.; Campbell, C.S.G.; Pontes, F.L.; Boullosa, D.; Prestes, J. Haemophilia and Exercise. Int. J. Sports Med. 2011, 33, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Tiktinsky, R.; Falk, B.; Heim, M.; Martinovitz, U. The effect of resistance training on the frequency of bleeding in haemophilia patients: A pilot study: Training and Bleeding Frequency in Haemophilia. Haemophilia 2002, 8, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Hilberg, T.; Herbsleb, M.; Puta, C.; Gabriel HH, W.; Schramm, W. Physical training increases isometric muscular strength and proprioceptive performance in haemophilic subjects: Proprioceptive Training in Haemophilics. Haemophilia 2003, 9, 86–93. [Google Scholar] [CrossRef]
- American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription, 10th ed.; Wolters Kluwer: Philadelphia, PA, USA, 2018. [Google Scholar]
- Lasevicius, T.; Ugrinowitsch, C.; Schoenfeld, B.J.; Roschel, H.; Tavares, L.D.; De Souza, E.O.; Laurentino, G.; Tricoli, V. Effects of different intensities of resistance training with equated volume load on muscle strength and hypertrophy. Eur. J. Sport Sci. 2018, 18, 772–780. [Google Scholar] [CrossRef]
- Morton, R.W.; Sonne, M.W.; Zuniga, A.F.; Mohammad, I.Y.; Jones, A.; McGlory, C.; Keir, P.J.; Potvin, J.R.; Phillips, S.M. Muscle fibre activation is unaffected by load and repetition duration when resistance exercise is performed to task failure. J. Physiol. 2019, 597, 4601–4613. [Google Scholar] [CrossRef]
- Henneman, E.; Mendell, L.M. Functional Organization of Motoneuron Pool and Its Inputs. In Comprehensive Physiology; Terjung, R., Ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2011; Available online: http://doi.wiley.com/10.1002/cphy.cp010211 (accessed on 5 May 2020).
- Nóbrega, S.R.; Ugrinowitsch, C.; Pintanel, L.; Barcelos, C.; Libardi, C.A. Effect of Resistance Training to Muscle Failure vs. Volitional Interruption at High- and Low-Intensities on Muscle Mass and Strength. J. Strength Cond. Res. 2018, 32, 162–169. [Google Scholar] [CrossRef]
- Schoenfeld, B.J.; Grgic, J. Does Training to Failure Maximize Muscle Hypertrophy? Strength Cond. J. 2019, 41, 108–113. [Google Scholar] [CrossRef]
- Sundstrup, E.; Jakobsen, M.D.; Andersen, C.H.; Zebis, M.K.; Mortensen, O.S.; Andersen, L.L. Muscle Activation Strategies During Strength Training With Heavy Loading vs. Repetitions to Failure. J. Strength Cond. Res. 2012, 26, 1897–1903. [Google Scholar] [CrossRef] [PubMed]
- Davies, T.; Orr, R.; Halaki, M.; Hackett, D. Effect of Training Leading to Repetition Failure on Muscular Strength: A Systematic Review and Meta-Analysis. Sports Med. 2016, 46, 487–502. [Google Scholar] [CrossRef] [PubMed]
- Hilliard, P.; Funk, S.; Zourikian, N.; Bergstrom, B.-M.; Bradley, C.S.; McLimont, M.; Manco-Johnson, M.; Petrini, P.; Berg, M.V.D.; Feldman, B.M. Hemophilia joint health score reliability study. Haemophilia 2006, 12, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Tkachuk, G.A.; Harris, C.A. Psychometric Properties of the Tampa Scale for Kinesiophobia-11 (TSK-11). J. Pain 2012, 13, 970–977. [Google Scholar] [CrossRef] [PubMed]
- Kurtze, N.; Rangul, V.; Hustvedt, B.-E.; Flanders, W.D. Reliability and validity of self-reported physical activity in the Nord-Trøndelag Health Study-HUNT 1. Scand. J. Public Health 2008, 36, 52–61. [Google Scholar] [CrossRef]
- Hermens, H.J.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of recommendations for SEMG sensors and sensor placement procedures. J. Electromyogr. Kinesiol. 2000, 10, 361–374. [Google Scholar] [CrossRef]
- Kendall, F.P.; McCreary, E.K.; Provance, P.G.; Rodgers, M.M.; Romani, W.A. Muscles: Testing and Testing and Function with Posture and Pain, 5th ed.; LWW: Baltimore, MD, USA, 2005. [Google Scholar]
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.M.; Nieman, D.C.; Swain, D.P. Quantity and Quality of Exercise for Developing and Maintaining Cardiorespir-atory, Musculoskeletal, and Neuromotor Fitness in Apparently Healthy Adults: Guidance for Prescribing Exercise. Med. Sci. Sports Exerc. 2011, 43, 1334–1359. [Google Scholar] [CrossRef]
- González-Izal, M.; Rodríguez-Carreño, I.; Mallor-Giménez, F.; Malanda, A.; Izquierdo, M. New Wavelet Indices to Assess Muscle Fatigue during Dy-namic Contractions. Int. J. Biomed. Biol. Eng. 2009, 3, 151–156. [Google Scholar]
- González-Izal, M.; Rodríguez-Carreño, I.; Malanda, A.; Mallor-Giménez, F.; Navarro-Amézqueta, I.; Gorostiaga, E.; Izquierdo, M. sEMG wavelet-based indices predicts muscle power loss during dynamic contractions. J. Electromyogr. Kinesiol. 2010, 20, 1097–1106. [Google Scholar] [CrossRef] [PubMed]
- Cifrek, M.; Medved, V.; Tonković, S.; Ostojić, S. Surface EMG based muscle fatigue evaluation in biomechanics. Clin. Biomech. 2009, 24, 327–340. [Google Scholar] [CrossRef]
- Edwards, R.H.T. Human Muscle Function and Fatigue. In Novartis Foundation Symposia; Porter, R., Whelan, J., Eds.; Wiley: Chichester, UK, 2008; pp. 1–18. Available online: http://doi.wiley.com/10.1002/9780470715420.ch1 (accessed on 5 May 2020).
- Newham, D.; Mills, K.R.; Quigley, B.M.; Edwards, R.H.T. Pain and Fatigue after Concentric and Eccentric Muscle Contractions. Clin. Sci. 1983, 64, 55–62. [Google Scholar] [CrossRef]
- Brody, L.R.; Pollock, M.T.; Roy, S.H.; De Luca, C.J.; Celli, B. pH-induced effects on median frequency and conduction velocity of the myoelectric signal. J. Appl. Physiol. 1991, 71, 1878–1885. [Google Scholar] [CrossRef] [PubMed]
- De Luca, C.J. Myoelectrical manifestations of localized muscular fatigue in humans. Crit. Rev. Biomed. Eng. 1984, 11, 251–279. [Google Scholar]
- Mau-Moeller, A.; Jacksteit, R.; Jackszis, M.; Feldhege, F.; Weippert, M.; Mittelmeier, W.; Bader, R.; Skripitz, R.; Behrens, M. Neuromuscular function of the quadriceps muscle during isometric maximal, submaximal and submaximal fatiguing voluntary contractions in knee osteoarthrosis patients. PLoS ONE 2017, 12, e0176976. [Google Scholar] [CrossRef]
- Pincivero, D.M.; Dixon, P.T.; Coelho, A.J. Knee extensor torque, work, and EMG during subjectively graded dynamic contrac-tions. Muscle Nerve 2003, 28, 54–61. [Google Scholar] [CrossRef]
- Mikkelsen, E.K.; Jakobsen, T.L.; Holsgaard-Larsen, A.; Andersen, L.L.; Bandholm, T. Strength Training to Contraction Failure Increases Voluntary Acti-vation of the Quadriceps Muscle Shortly After Total Knee Arthroplasty: A Cross-sectional Study. Am. J. Phys. Med. Rehabil. 2016, 95, 194–203. [Google Scholar] [CrossRef]
- Jenkins, N.D.M.; Housh, T.J.; Bergstrom, H.C.; Cochrane, K.C.; Hill, E.C.; Smith, C.M.; Johnson, G.O.; Schmidt, R.J.; Cramer, J.T. Muscle activation during three sets to failure at 80 vs. 30 % 1RM resistance exercise. Eur. J. Appl. Physiol. 2015, 115, 2335–2347. [Google Scholar] [CrossRef]
- Pincivero, D.M.; Gandhi, V.; Timmons, M.K.; Coelho, A.J. Quadriceps femoris electromyogram during concentric, isometric and eccen-tric phases of fatiguing dynamic knee extensions. J. Biomech. 2006, 39, 246–254. [Google Scholar] [CrossRef]
- Grabiner, M.D.; Koh, T.J.; Miller, G.F. Fatigue rates of vastus medialis oblique and vastus lateralis during static and dynamic knee extension. J. Orthop. Res. 1991, 9, 391–397. [Google Scholar] [CrossRef]
- Wickiewicz, T.L.; Roy, R.R.; Powell, P.L.; Edgerton, V.R. Muscle architecture of the human lower limb. Clin. Orthop. Relat. Res. 1983, 179, 275–283. [Google Scholar] [CrossRef]
- Willan, P.; Ransome, J.; Mahon, M. Variability in human quadriceps muscles: Quantitative study and review of clinical literature. Clin. Anat. 2002, 15, 116–128. [Google Scholar] [CrossRef]
- Farahmand, F.; Sejiavongse, W.; Amis, A.A. Quantitative study of the quadriceps muscles and trochlear groove geometry related to instability of the patellofemoral joint. J. Orthop. Res. 1998, 16, 136–143. [Google Scholar] [CrossRef]
- Johnson, M.; Polgar, J.; Weightman, D.; Appleton, D. Data on the distribution of fibre types in thirty-six human muscles: An autopsy study. J. Neurol. Sci. 1973, 18, 111–129. [Google Scholar] [CrossRef]
- Travnik, L.; Pernus, F.; Erzen, I. Histochemical and morphometric characteristics of the normal human vastus medialis lon-gus and vastus medialis obliquus muscles. J. Anat. 1995, 187, 403–411. [Google Scholar] [PubMed]
- De Luca, C.J.; Kline, J.C. Influence of proprioceptive feedback on the firing rate and recruitment of motoneurons. J. Neural. Eng. 2012, 9, 016007. [Google Scholar] [CrossRef] [PubMed]
- Vlaeyen, J.W.; Linton, S.J. Fear-avoidance and its consequences in chronic musculoskeletal pain: A state of the art. Pain 2000, 85, 317–332. [Google Scholar] [CrossRef]
- Hilberg, T.; Czepa, D.; Freialdenhoven, D.; Boettger, M. Joint pain in people with hemophilia depends on joint status. Pain 2011, 152, 2029–2035. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Valdes, E.; Negro, F.; Falla, D.; De Nunzio, A.M.; Farina, D. Surface electromyographic amplitude does not identify differences in neural drive to synergistic muscles. J. Appl. Physiol. 2018, 124, 1071–1079. [Google Scholar] [CrossRef] [PubMed]
| (n = 12) | Mean (SD) |
|---|---|
| Age (years) | 38.4 (10.1) |
| Height (cm) | 174.3 (9.0) |
| Body mass (kg) | 78.0 (11.3) |
| HJHS 1 dominant knee | 0.7 (1.1) |
| HJHS 1 non-dominant knee | 3.4 (6.0) |
| Total HJHS 1 | 25.6 (13.3) |
| FVIII dose (IU/Kg) 2 | 25.0 (10.3) |
| No of patients | |
| Knee pain intensity (0/1/2/7) 3 | 9/1/1/1 |
| Leisure-Time Physical Activity | |
| Frequency | n (%) |
| Never | 0 (0.0) |
| <1 time/week | 1 (8.3) |
| 1 time/week | 1 (8.3) |
| 2–3 times/week | 5 (41.7) |
| Almost daily | 5 (41.7) |
| Intensity | |
| Relatively easy | 7 (58.3) |
| Fairly strenuous | 4 (33.3) |
| Near the limit of exhaustion | 1 (8.3) |
| Duration | |
| <15 min | |
| 16–30 min | 1 (8.3) |
| 30–60 min | 5 (41.7) |
| >1 h | 6 (50.0) |
| Resistance training | |
| Yes | 8 (66.7) |
| No | 4 (33.3) |
| Frequency | |
| 1 time/week | 2 (25.0) |
| 2 times/week | 4 (50.0) |
| 3 times/week | 1 (12.5) |
| 4 times/week | 1 (12.5) |
| Years of experience | |
| 1 year | 2 (25.0) |
| 2 years | 2 (25.0) |
| ≥3 years | 4 (50.0) |
| Intensity | |
| Moderate (60–70%) | 7 (87.5) |
| Heavy (>80%) | 1 (12.5) |
| Item Number | Mean (SD) |
|---|---|
| 1 | 2.5 (1.1) |
| 2 | 2.9 (1.1) |
| 3 | 1.9 (0.9) |
| 4 | 2.5 (1.3) |
| 5 | 2.6 (1.4) |
| 6 | 2.6 (1.1) |
| 7 | 2.7 (1.0) |
| 8 | 3.4 (0.8) |
| 9 | 1.6 (0.9) |
| 10 | 2.7 (0.9) |
| 11 | 2.3 (1.3) |
| Total | 27.7 (7.0) |
| Patient | Selected Resistance Band | No of Repetitions | Pain (Post) | Fear of Exercise to Task Failure Pre (0–10) | Fear Improvement (Post) |
|---|---|---|---|---|---|
| 1 | Silver | 35 | 4 | 5 | Has improved something |
| 2 | Silver | 59 | 0 | 3 | Has improved a lot |
| 3 | Silver | 60 | 0 | 2 | Has not changed |
| 4 | Silver | 34 | 0 | 5 | Has improved something |
| 5 | Gold | 30 | 0 | 0 | Has not changed |
| 6 | Black | 31 | 0 | 2 | Has not changed |
| 7 | Gold | 14 | 0 | 0 | Has improved something |
| 8 | Silver | 65 | 2 | 5 | Has not changed |
| 9 | Gold | 64 | 0 | 3 | Has improved something |
| 10 | Gold | 27 | 0 | 5 | Has improved something |
| 11 | Gold | 36 | 0 | 6 | Has improved a lot |
| 12 | Silver | 82 | 0 | 1 | Has minimally improved |
| Vastus Medialis | Vastus Lateralis | Rectus Femoris | ||
|---|---|---|---|---|
| nRMS | ||||
| Cycle to failure (%) | 40 | −6.2 [−11.5:−0.9]; 0.019; 0.77 | −5.1 [−12.1:2.0]; 0.29; | −6:1 [−11.6:−0.7]; 0.024; 0.63 |
| 60 | −11.0 [−16.6:−5.4]; <0.001; 1.33 | −6.8 [−15.5:1.9]; 0.20; | −9.8 [−19.1:−0.5]; 0.035; 1.01 | |
| 80 | −12.8 [−19.9:−5.6]; <0.001; 1.80 | −12.4 [−22.1:−2.7]; 0.009; 1.28 | −14.0 [−21.5:−6.5]; <0.001; 1.61 | |
| 100 | −18.3 [−28.8:−7.9]; <0.001; 2.68 | −15.0 [−27.4:−2.7]; 0.014; 1.64 | −19.3 [−30.5:−8.1]; <0.001; 2.30 | |
| MF | ||||
| Cycle to failure (%) | 40 | 2.9 [−0.3:6.0]; 0.086; | 1.6 [−2.7:5.9]; 1; | 3.0 [−0.2:6.2]; 0.08; |
| 60 | 5.3 [1.3:9.3]; 0.007; 1.96 | 4.8 [1.0:8.7]; 0.011; 1.09 | 5.5 [1.7:9.3]; 0.003; 1.06 | |
| 80 | 7.5 [2.0:12.9]; 0.006; 2.24 | 5.5 [−0.8:11.7]; 0.11; | 7.7 [0.7:14.6]; 0.026; 1.11 | |
| 100 | 10.0 [3.8:16.3]; 0.002; 2.46 | 8.3 [2.0:14.8]; 0.008; 1.76 | 10.6 [2.5:18.7]; 0.008; 1.46 | |
| WIRW51 | ||||
| Cycle to failure (%) | 40 | −3.1 [−12.7:6.6]; 1; | −9.4 [−17.7:−1.0]; 0.024; 0.89 | −4.1 [−13.8:5.6]; 1; |
| 60 | −13.7 [−24.4:−2.9]; 0.010; 0.99 | −14.5 [−22.8:−6.2]; <0.001; 1.57 | −13.2 [−23.6:−2.7]; 0.011; 1.09 | |
| 80 | −19.0 [−28.2:−9.7]; <0.001; 1.44 | −16.8 [−28.3:−5.2]; 0.004; 2.02 | −17.0 [−25.9:−8.1]; <0.001; 1.54 | |
| 100 | −22.0 [−33.5:−10.5]; <0.001; 1.93 | −27.3 [−44.2:−10.4]; <0.001; 2.47 | −27.8 [−44.9:−10.7]; <0.001; 2.30 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calatayud, J.; Martín-Cuesta, J.; Carrasco, J.J.; Pérez-Alenda, S.; Cruz-Montecinos, C.; Andersen, L.L.; Querol-Giner, F.; Casaña, J. Safety, Fear and Neuromuscular Responses after a Resisted Knee Extension Performed to Failure in Patients with Severe Haemophilia. J. Clin. Med. 2021, 10, 2587. https://doi.org/10.3390/jcm10122587
Calatayud J, Martín-Cuesta J, Carrasco JJ, Pérez-Alenda S, Cruz-Montecinos C, Andersen LL, Querol-Giner F, Casaña J. Safety, Fear and Neuromuscular Responses after a Resisted Knee Extension Performed to Failure in Patients with Severe Haemophilia. Journal of Clinical Medicine. 2021; 10(12):2587. https://doi.org/10.3390/jcm10122587
Chicago/Turabian StyleCalatayud, Joaquín, Jonathan Martín-Cuesta, Juan J. Carrasco, Sofía Pérez-Alenda, Carlos Cruz-Montecinos, Lars L. Andersen, Felipe Querol-Giner, and José Casaña. 2021. "Safety, Fear and Neuromuscular Responses after a Resisted Knee Extension Performed to Failure in Patients with Severe Haemophilia" Journal of Clinical Medicine 10, no. 12: 2587. https://doi.org/10.3390/jcm10122587
APA StyleCalatayud, J., Martín-Cuesta, J., Carrasco, J. J., Pérez-Alenda, S., Cruz-Montecinos, C., Andersen, L. L., Querol-Giner, F., & Casaña, J. (2021). Safety, Fear and Neuromuscular Responses after a Resisted Knee Extension Performed to Failure in Patients with Severe Haemophilia. Journal of Clinical Medicine, 10(12), 2587. https://doi.org/10.3390/jcm10122587









