The Role of Physiotherapy in the New Treatment Landscape for Haemophilia
Abstract
:1. Introduction
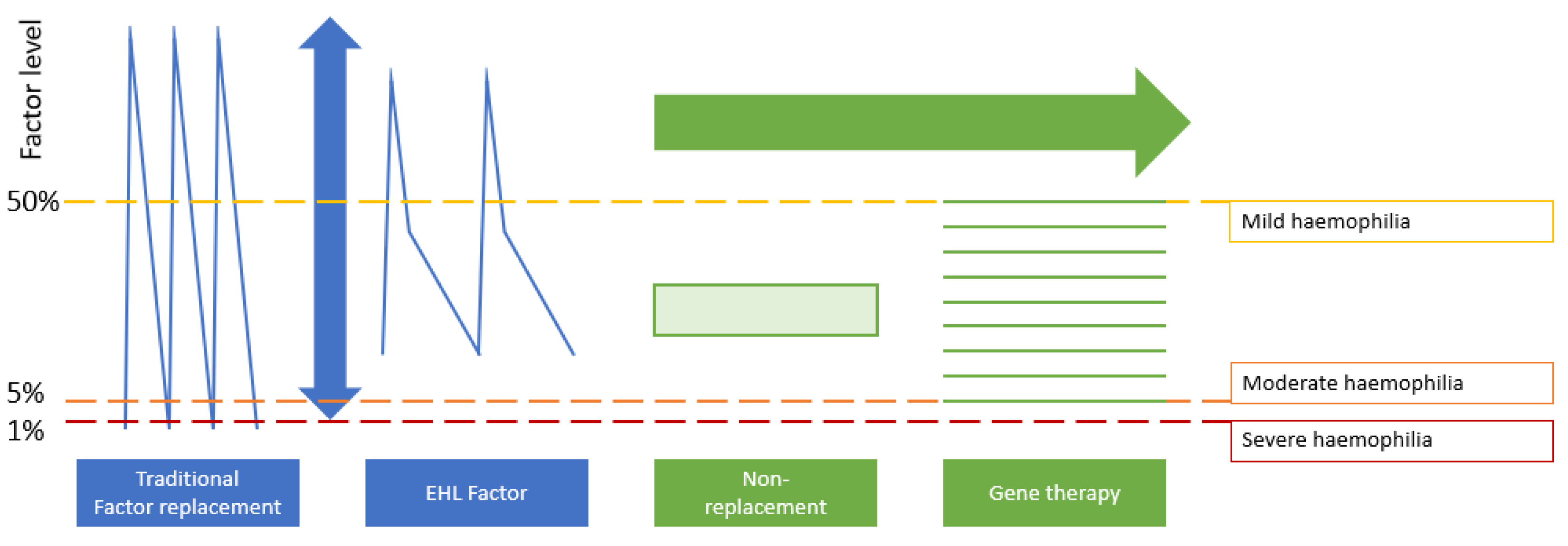
2. Pharmacologic Innovation: Impact on the Musculoskeletal System
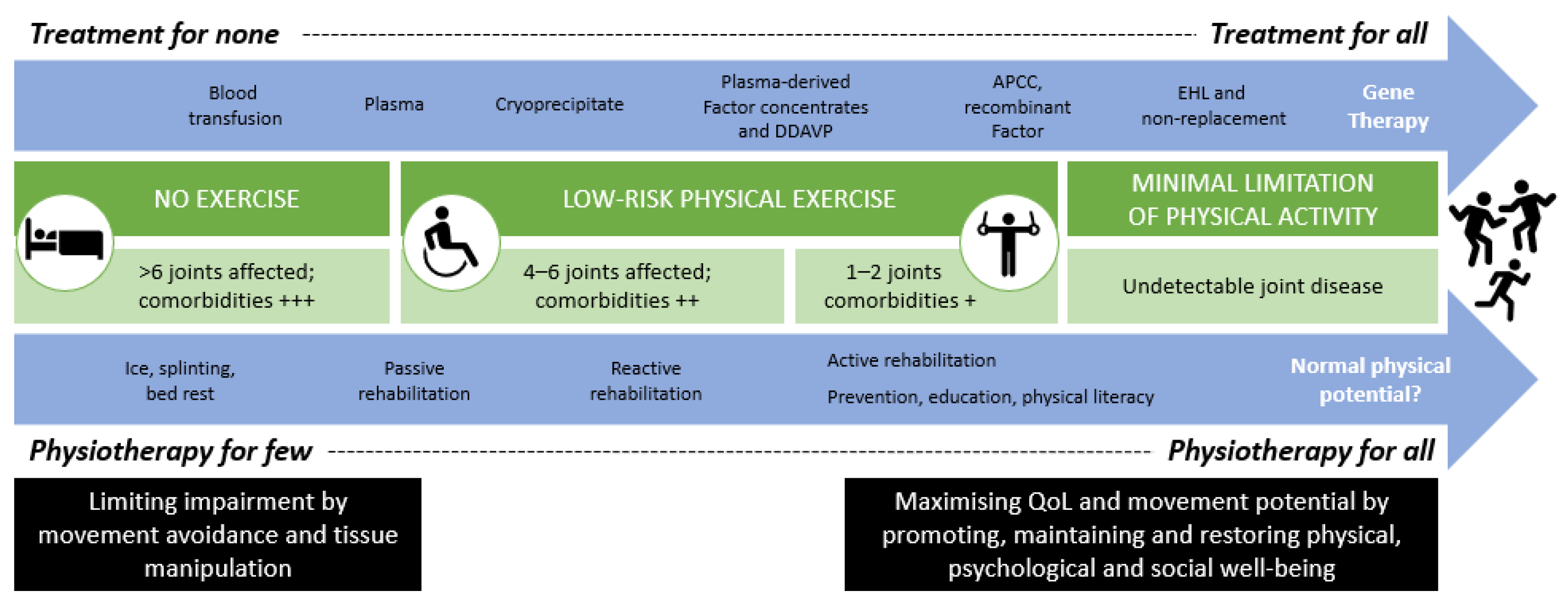
3. Comprehensive Care: The Evolving Role of the Physiotherapist
4. Physiotherapy: Core and Specific Roles in PWH Management
4.1. Providing Education
4.2. Monitoring Joint Health and Physical Functioning
4.3. Treatment and Prevention
5. Future Considerations
6. Discussion: Impact of Treatment Innovation on Physiotherapy in Haemophilia
- The physiotherapist is recognised as an essential member of the multidisciplinary team for the care of PWH.
- Physiotherapy will remain an important element of care even for people with little joint damage and low risks.
- The physiotherapist should be in contact with local practitioners who can provide day-to-day care outside the treatment centre.
- Experienced physiotherapists from expert haemophilia treatment centres should provide support, education, and resources for other providers.
- The physiotherapist will continue to play a key role in educating children and adults with haemophilia on how to identify and treat bleeds and how to undertake physical activity safely.
- Advanced therapies may widen the generational gap in the musculoskeletal com-plications seen in PWH; services will need to adapt to reflect that.
- Competencies will evolve, and physiotherapists should work with colleagues in haematology to develop more sensitive tools for the detection of early joint chang-es.
- Treatment interactions will also evolve; physiotherapists should seek ways to de-liver joint and injury assessments via telehealth platforms.
- Physiotherapy is critical to promote a harmonious haemostatic and physical state in PWH transitioned to new therapies.
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Atilla, B.; Güney-Deniz, H. Musculoskeletal treatment in haemophilia. EFORT Open Rev. 2019, 4, 230–239. [Google Scholar] [CrossRef]
- Heijnen, L.; Dirat, G.; Chen, L.; Tulaar, A.B.M.; Moysisyan, L.; Nassar, N.M.M.; Batistella, L.R. The role of the physiatrist in the haemophilia comprehensive care team in different parts of the world. Haemophilia 2008, 14, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Dunn, A.L. Pathophysiology, diagnosis and prevention of arthropathy in patients with haemophilia. Haemophilia 2011, 17, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Pulles, A.E.; Mastbergen, S.C.; Schutgens, R.; Lafeber, F.P.; van Vulpen, L. Pathophysiology of hemophilic arthropathy and potential targets for therapy. Pharmacol. Res. 2017, 115, 192–199. [Google Scholar] [CrossRef]
- Blamey, G.; Forsyth, A.; Zourikian, N.; Short, L.; Jankovic, N.; De Kleijn, P.; Flannery, T. Comprehensive elements of a physiotherapy exercise programme in haemophil-ia—a global perspective. Haemophilia 2010, 16 (Suppl. 5), 136–145. [Google Scholar] [CrossRef]
- Stephensen, D.; Bladen, M.; McLaughlin, P. Recent advances in musculoskeletal physiotherapy for haemophilia. Ther. Adv. Hematol. 2018, 9, 227–237. [Google Scholar] [CrossRef] [Green Version]
- Fromme, A.; Dreeskamp, K.; Pollmann, H.; Thorwesten, L.; Mooren, F.C.; Völker, K. Participation in sports and physical activity of haemophilia patients. Haemophilia 2007, 13, 323–327. [Google Scholar] [CrossRef]
- Kuijlaars, I.A.R.; Timmer, M.A.; De Kleijn, P.; Pisters, M.F.; Fischer, K. Monitoring joint health in haemophilia: Factors associated with deterioration. Haemophilia 2017, 23, 934–940. [Google Scholar] [CrossRef] [PubMed]
- Lobet, S.; Hermans, C.; Lambert, C. Optimal management of hemophilic arthropathy and hematomas. J. Blood Med. 2014, 5, 207–218. [Google Scholar] [CrossRef] [Green Version]
- McLaughlin, P.; Hermans, C.; Asghar, S.; Burke, T.; Nissen, F.; Aizenas, M.; Meier, O.; Dhillon, H.; O’Hara, J. Problem Joints and Their Clinical and Humanistic Burden in Children and Adults with Moderate and Severe Hemophilia a: CHESS Paediatrics and CHESS II. Available online: https://ash.confex.com/ash/2020/webprogram/Paper140306.html (accessed on 25 June 2021).
- Lobet, S.; Hermans, C.; Stephensen, D. The emerging clinical and scientific role of the physiotherapist in haemophilia care. Haemophilia 2020, 26, 560–562. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, P.; Aspdahl, M.; Matlary, R.E.D.; Grinda, N.; Katzerova, M.; O’Mahony, B.; Stephensen, D.; Lobet, S. Comprehensive care on paper only? The challenge for physiotherapy provision in day to day haemophilia practice. Haemophilia 2021, 27. [Google Scholar] [CrossRef] [PubMed]
- Daluiso-King, G.; Hebron, C. Is the biopsychosocial model in musculoskeletal physiotherapy adequate? An evolutionary concept analysis. Physiother. Theory Pr. 2020, 1–17. [Google Scholar] [CrossRef]
- Srivastava, A.; Santagostino, E.; Dougall, A.; Kitchen, S.; Sutherland, M.; Pipe, S.W.; Carcao, M.; Mahlangu, J.; Ragni, M.V.; Windyga, J.; et al. WFH Guidelines for the Management of Hemophilia, 3rd edition. Haemophilia 2020, 26, 1–158. [Google Scholar] [CrossRef] [PubMed]
- Scott, M.J.; Xiang, H.; Hart, D.P.; Palmer, B.; Collins, P.W.; Stephensen, D.; Sima, C.S.; Hay, C.R.M. Treatment regimens and outcomes in severe and moderate haemophilia A in the UK: The THUNDER study. Haemophilia 2019, 25, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Oladapo, A.O.; Epstein, J.D.; Williams, E.; Ito, D.; Gringeri, A.; Valentino, L.A. Health-related quality of life assessment in haemophilia patients on prophylaxis therapy: A systematic review of results from prospective clinical trials. Haemophilia 2015, 21, e344–e358. [Google Scholar] [CrossRef] [PubMed]
- Noone, D.; O’Mahony, B.; van Dijk, J.P.; Prihodova, L. A survey of the outcome of prophylaxis, on-demand treatment or com-bined treatment in 18–35-year old men with severe haemophilia in six countries. Haemophilia 2013, 19, 44–50. [Google Scholar] [CrossRef] [Green Version]
- Warren, B.B.; Thornhill, D.; Stein, J.; Fadell, M.; Ingram, J.D.; Funk, S.; Norton, K.L.; Lane, H.D.; Bennett, C.M.; Dunn, A.; et al. Young adult outcomes of childhood prophylaxis for severe hemophilia A: Results of the Joint Outcome Continuation Study. Blood Adv. 2020, 4, 2451–2459. [Google Scholar] [CrossRef]
- Oldenberg, J. Optimal treatment strategies for hemophilia: Achievements and limitations of current prophylactic regimens. Blood 2015, 125, 2038–2044. [Google Scholar] [CrossRef]
- O’Hara, S.; Castro, F.A.; Black, J.; Chaplin, S.; Ruiz, L.; Hampton, R.J.; Sima, C.S.; O’Hara, J. Disease burden and remaining unmet need in patients with haemophilia A treated with primary prophylaxis. Haemophilia 2021, 27, 113–119. [Google Scholar] [CrossRef]
- Forsyth, A.; Witkop, M.; Lambing, A.; Garrido, C.; Dunn, S.; Cooper, D.L.; Nugent, D. Associations of quality of life, pain, and self-reported arthritis with age, employment, bleed rate, and utilization of hemophilia treatment center and health care provider services: Results in adults with hemophilia in the HERO study. Patient Prefer. Adherence 2015, 9, 1549–1560. [Google Scholar] [CrossRef] [Green Version]
- Oldenburg, J.; Kulkarni, R.; Srivastava, A.; Mahlangu, J.N.; Blanchette, V.S.; Tsao, E.; Winding, B.; Dumont, J.; Jain, N. Improved joint health in subjects with severe haemophilia A treated prophy-lactically with recombinant factor VIII Fc fusion protein. Haemophilia 2018, 24, 77–84. [Google Scholar] [CrossRef]
- Santagostino, E.; Kenet, G.; Fischer, K.; Biss, T.; Ahuja, S.; Steele, M.; Martínez, M.; Male, C.; Van Geet, C.; Mondelaers, V.; et al. PROTECT VIII Kids: BAY 94-9027 (PEGylated Recombinant Factor VIII) safety and efficacy in previously treated children with severe haemophilia A. Haemophilia 2020, 26, e55–e65. [Google Scholar] [CrossRef]
- Negrier, C.; Young, G.; Karim, F.A.; Collins, P.W.; Hanabusa, H.; Colberg, T.; Goldman, B.; Walsh, C.E. Recombinant long-acting glycoPEGylated factor IX (nonacog beta pegol) in haemo-philia B: Assessment of target joints in multinational phase 3 clinical trials. Haemophilia 2016, 22, 507–513. [Google Scholar] [CrossRef]
- Hartmann, J.; Croteau, S.E. 2017 Clinical trials update: Innovations in hemophilia therapy. Am. J. Hematol. 2016, 91, 1252–1260. [Google Scholar] [CrossRef] [Green Version]
- Marietta, M.; Luppi, M. Non-replacement therapy for haemophilia treatment: Fetching the east by the west. Blood Transfus. Trasfus. Del Sangue 2018, 16, 408–409. [Google Scholar]
- Arruda, V.R.; Doshi, B.S.; Samelson-Jones, B.J. Emerging therapies for hemophilia: Controversies and unanswered questions. F1000Research 2018, 7, 489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arruda, V.R.; Doshi, B.S.; Samelson-Jones, B.J. Novel approaches to hemophilia therapy: Successes and challenges. Blood 2017, 130, 2251–2256. [Google Scholar] [CrossRef] [Green Version]
- Anderson, A.; Forsyth, A. Playing It Safe: Bleeding Disorders, Sport and Exercise. National Hemophilia Foundation (NHF). 2017. Available online: https://www.hemophilia.org/sites/default/files/document/files/Playing-It-Safe_0.pdf (accessed on 27 October 2020).
- Dargaud, Y.; Delavenne, X.; Hart, D.; Meunier, S.; Mismetti, P. Individualized PK-based prophylaxis in severe haemophilia. Haemophilia 2018, 24, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Broderick, C.R.; Herbert, R.D.; Latimer, J.; Barnes, C.; Curtin, J.A.; Mathieu, E.; Monagle, P.; Simon, A. Brown Association between physical activity and risk of bleeding in children with he-mophilia. JAMA 2012, 308, 1452–1459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, A.P.; Burke, T.; Asghar, S.; Noone, D.; Pedra, G.; O’Hara, J. Understanding minimum and ideal factor levels for participation in physical activities by people with haemophilia: An expert elicitation exercise. Haemophilia 2020, 26, 711–717. [Google Scholar] [CrossRef]
- Wells, A.; Stephensen, D. The role of the physiotherapist in the management of people with haemophilia: Defining the new normal. Br. J. Hosp. Med. 2020, 81, 1–8. [Google Scholar] [CrossRef]
- Nijdam, A.; Foppen, W.; De Kleijn, P.; Mauser-Bunschoten, E.P.; Roosendaal, G.; Van Galen, K.P.M.; Schutgens, R.E.G.; Van Der Schouw, Y.T.; Fischer, K. Discontinuing early prophylaxis in severe haemophilia leads to deterioration of joint status despite low bleeding rates. Thromb. Haemost. 2016, 115, 931–938. [Google Scholar] [CrossRef] [PubMed]
- Eichler, H.; Albisetti Pedroni, M.; Halimeh, S.; Halimeh, S.; Königs, C.; Langer, F.; Miesbach, W.; Oldenburg, J.; Scholz, U.; Streif, W.; et al. Leitlinie der Gesellschaft für Thrombose- und Hämostaseforschung (GTH) zur Struktur- und Prozessqualität von Hämophilie-Zentren. Hamostaseologie 2019, 39, 311–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stephensen, D.; De Kleijn, P.; Matlary, R.E.D.; Katzerova, M.; McLaughlin, P.; Ryan, A.; Lobet, S. The EAHAD Physiotherapists Committee; EAHAD Physiotherapists Committee Scope of practice of haemophilia physiotherapists: A European survey. Haemophilia 2019, 25, 514–520. [Google Scholar] [CrossRef] [Green Version]
- World Congress of Physical Therapy. Description of Physical Therapy: Policy Statement. Available online: https://world.physio/sites/default/files/2020-07/PS-2019-Description-of-physical-therapy.pdf (accessed on 25 January 2021).
- World Health Organization. International Classification of Functioning, Disability and Health: ICF; WHO: Geneva, Switzerland, 2001. [Google Scholar]
- De la Corte-Rodriguez, H.; Rodriguez-Merchan, E.C. The ICF (International Classification of Functioning, Disability and Health) developed by the WHO for measuring function in hemophilia. Expert Rev. Hematol. 2016, 9, 661–668. [Google Scholar] [CrossRef]
- Ignas, D.M.; Doria, A.S.; Von Drygalski, A.; Blanchette, V.S.; Chang, E.Y.; Dover, S.; Fischer, K.; Gibikote, S.; Keshava, S.N.; Querol, F.; et al. Use of ultrasound for assessment of musculoskeletal disease in persons with haemophilia: Results of an International Prophylaxis Study Group global survey. Haemophilia 2020, 26, 685–693. [Google Scholar] [CrossRef]
- Zhou, J.Y.; Rappazzo, K.C.; Volland, L.; Barnes, R.F.W.; Brackman, M.; Steiner, B.; Kruse-Jarres, R.; Quon, D.V.; Bailey, C.; Chang, E.Y.; et al. Pocket handheld ultrasound for evaluation of the bleeding haemophilic joint: A novel and reliable way to recognize joint effusions. Haemophilia 2018, 24, e77–e80. [Google Scholar] [CrossRef] [PubMed]
- Oldenburg, J.; Zimmermann, R.; Katsarou, O.; Zanon, E.; Kellermann, E.; Lundin, B.; Ellinghaus, P. Potential biomarkers of haemophilic arthropathy: Correlations with com-patible additive magnetic resonance imaging scores. Haemophilia 2016, 22, 760–764. [Google Scholar] [CrossRef]
- Noone, M.D.; Nissen, F.; Xu, T.; Burke, T.; Asghar, S.; Dhillon, H.; Aizenas, M.; Meier, O.; O’Hara, M.J.; Khair, K. An Insight into the Impact of Hemophilia a on Daily Life According to Disease Severity: A Preliminary Analysis of the CHESS II Study. Blood 2020, 136, 1–3. [Google Scholar] [CrossRef]
- George, L.A.; Sullivan, S.K.; Giermasz, A.; Rasko, J.E.; Samelson-Jones, B.J.; Ducore, J.; Cuker, A.; Sullivan, L.M.; Majumdar, S.; Teitel, J.; et al. Hemophilia B Gene Therapy with a High-Specific-Activity Factor IX Variant. N. Engl. J. Med. 2017, 377, 2215–2227. [Google Scholar] [CrossRef]
- Yıldız, M.; Özdemir, N.; Önal, H.; Koç, B.; Tipici, B.E.; Zülfikar, B. Evaluation of Unfavorable Cardiovascular and Metabolic Risk Factors in Children and Young Adults with Haemophilia. J. Clin. Res. Pediatr. Endocrinol. 2019, 11, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Limjoco, J.; Thornburg, C.D. Risk factors for cardiovascular disease in children and young adults with haemophilia. Haemophilia 2018, 24, 747–754. [Google Scholar] [CrossRef]
- Eaton, S.; Roberts, S.; Turner, B. Delivering person centred care in long term conditions. BMJ 2015, 350, h181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Timmer, M.A.; Kloek, C.J.; de Kleijn, P.; Kuijlaars, I.A.; Schutgens, R.E.; Veenhof, C.; Pisters, M.F. A Blended Physiotherapy Intervention for Persons with Hemophilic Arthropathy: Development Study. J. Med. Internet Res. 2020, 22, e16631. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lobet, S.; Timmer, M.; Königs, C.; Stephensen, D.; McLaughlin, P.; Duport, G.; Hermans, C.; Mancuso, M.E. The Role of Physiotherapy in the New Treatment Landscape for Haemophilia. J. Clin. Med. 2021, 10, 2822. https://doi.org/10.3390/jcm10132822
Lobet S, Timmer M, Königs C, Stephensen D, McLaughlin P, Duport G, Hermans C, Mancuso ME. The Role of Physiotherapy in the New Treatment Landscape for Haemophilia. Journal of Clinical Medicine. 2021; 10(13):2822. https://doi.org/10.3390/jcm10132822
Chicago/Turabian StyleLobet, Sébastien, Merel Timmer, Christoph Königs, David Stephensen, Paul McLaughlin, Gaetan Duport, Cédric Hermans, and Maria Elisa Mancuso. 2021. "The Role of Physiotherapy in the New Treatment Landscape for Haemophilia" Journal of Clinical Medicine 10, no. 13: 2822. https://doi.org/10.3390/jcm10132822
APA StyleLobet, S., Timmer, M., Königs, C., Stephensen, D., McLaughlin, P., Duport, G., Hermans, C., & Mancuso, M. E. (2021). The Role of Physiotherapy in the New Treatment Landscape for Haemophilia. Journal of Clinical Medicine, 10(13), 2822. https://doi.org/10.3390/jcm10132822






