Abstract
Delayed graft function (DGF) following kidney transplantation is associated with increased risk of graft failure, but biomarkers to predict DGF are scarce. We evaluated serum uromodulin (sUMOD), a potential marker for tubular integrity with immunomodulatory capacities, in kidney transplant recipients and its association with DGF. We included 239 kidney transplant recipients and measured sUMOD pretransplant and on postoperative Day 1 (POD1) as independent variables. The primary outcome was DGF, defined as need for dialysis within one week after transplantation. In total, 64 patients (27%) experienced DGF. In multivariable logistic regression analysis adjusting for recipient, donor and transplant associated risk factors each 10 ng/mL higher pretransplant sUMOD was associated with 47% lower odds for DGF (odds ratio (OR) 0.53, 95% confidence interval (95%-CI) 0.30–0.82). When categorizing pretransplant sUMOD into quartiles, the quartile with the lowest values had 4.4-fold higher odds for DGF compared to the highest quartile (OR 4.41, 95%-CI 1.54–13.93). Adding pretransplant sUMOD to a model containing established risk factors for DGF in multivariable receiver-operating-characteristics (ROC) curve analysis, the area-under-the-curve improved from 0.786 [95%-CI 0.723–0.848] to 0.813 [95%-CI 0.755–0.871, p = 0.05]. SUMOD on POD1 was not associated with DGF. In conclusion, higher pretransplant sUMOD was independently associated with lower odds for DGF, potentially serving as a non-invasive marker to stratify patients according to their risk for developing DGF early in the setting of kidney transplantation.
1. Introduction
Delayed graft function (DGF), commonly defined as need for dialysis within the first week after kidney transplantation, affects around 25–50% of patients, and is associated with a higher risk for acute rejection episodes and reduced long-term graft survival [1,2,3,4]. DGF presents histologically mainly as severe ischemia-reperfusion injury (IRI) with inflammatory tubular damage [5]. IRI triggers a long-term inflammation leading to interstitial fibrosis and tubular atrophy and reduces overall graft survival [6,7,8]. Therefore, understanding and potentially targeting the pathophysiology of IRI might improve long-term kidney graft survival [9]. However, measures to ameliorate IRI and markers predicting DGF before transplantation are scarce and still have limited diagnostic value [10].
Uromodulin (also known as Tamm-Horsfall protein), is a kidney derived glycoprotein with a molecular weight of around 100 kDa, exclusively expressed by epithelial cells of the thick ascending limb of the loop of Henle and the distal tubule [11,12,13]. The molecule is secreted both into the urine as well as the interstitium and circulation [14,15,16]. Thereby, interstitial uromodulin largely corresponds to serum concentrations in different forms of kidney disease [16]. Uromodulin is encoded by the UMOD gene, and mice lacking the UMOD gene showed more inflammation and tubular injury compared to wild type following renal IRI. In addition, they also demonstrate a greater necrotic and inflammatory phenotype of cell death rather than apoptotic, suggesting that interstitial uromodulin may have immunomodulating and anti-inflammatory capacities [17,18,19]. Uromodulin deficiency is also associated with delayed and in part incomplete kidney recovery following renal IRI in mice [14]. These data suggest that higher serum uromodulin (sUMOD) in the acute phase of kidney transplantation may be protective against IRI. Furthermore, higher sUMOD post-transplant is associated with lower risk for long-term kidney transplant failure [20,21]. However, the role of sUMOD in the early setting of transplantation and IRI remains to be investigated.
Here, we propose that recipient’s sUMOD plays an important role in the recovery from IRI after kidney transplantation, and thus sUMOD might be of predictive value for the incidence of DGF after kidney transplantation. In this study we evaluated recipient’s sUMOD pretransplant and on postoperative Day 1 (POD1) as a marker for prediction/early detection of DGF in kidney transplant recipients.
2. Materials and Methods
2.1. Study Participants and Study Design
In this single-center prospective observational cohort study, we recruited 239 patients undergoing kidney or combined kidney-pancreas transplantation following deceased or living donation in our tertiary care hospital. All patients who were able to provide informed consent were included in the study. Local institutional review boards of the Technical University of Munich, Germany approved the study methods. The study adheres to the declaration of Helsinki and the declaration of Istanbul.
2.2. Exposure
Serum samples for measuring sUMOD in the recipients were obtained 24 h prior to kidney transplantation in living organ donations and up to 5 h pretransplant in deceased donations, again on the first postoperative day (POD1) and subsequent time points later. Since all patients were hospitalized during the sample collection and no patient withdrew from the study, no patients were lost to follow-up for the primary endpoint (see below).
The samples were stored at −80 °C until they were thawed. sUMOD analyses were performed in singlicate using a commercial enzyme-linked immunosorbent assay (ELISA, Euroimmun, Medizinische Labordiagnostika AG, Lübeck, Germany) based on the manufacturer’s instructions. This assay is based on a colorimetric sandwich immunoassay using a polyclonal antibody against human uromodulin as the capture antibody and a biotinylated polyclonal antibody against human uromodulin as the detection antibody. Quality characteristics of the ELISA are as follows: intra-assay coefficient of variation 1.8–3.2%, inter-assay coefficient of variation 6.6–7.8%, mean linearity recovery 97%, and lower limit of detection 2.0 ng/mL.
2.3. Outcomes
The primary outcome was DGF, defined as the need for more than one dialysis within one week after transplantation as has been defined in prior clinical studies [22,23]. For example, one dialysis due to potassium lowering was not considered as DGF. Notably, in our tertiary center we avoid pretransplant dialysis to reduce cold ischemia time whenever reasonable, which leads to postoperative dialysis for hyperkalemia in some cases.
2.4. Statistical Analysis
We describe the population using mean (± standard deviation) for continuous variables and number with percentages for binary and categorical variables.
Multivariable logistic regression models were used to evaluate the association of sUMOD pretransplant and on POD1 as independent variables and DGF as the dependent variable. We applied a series of nested models: (i) unadjusted; (ii) adjusted for age, body-mass index (BMI) and dialysis vintage; (iii) Model 1 plus serum creatinine on POD1 (“Model 2”; we adjusted serum creatinine on POD1 as it appears to be an important variable for the decision to apply kidney replacement therapy postoperatively); (iv) Model 2 plus cold ischemia time (CIT), living vs. deceased donor transplantation, and expanded criteria donors (ECD) vs. standard criteria donors (“Model 3”). ECD are donors that are either older than 60 years, or 50–59 years old and meet at least two of the following criteria: cerebrovascular death, history of hypertension, and/or last serum creatinine greater than 1.5 mg/dL [24]. Due to the number of endpoints, we limited the analysis to these co-variables. Of note, we use the ECD classification for the adjustment because it covers donor age, donor serum creatinine and the donor cardiovascular cause of death. All variables were selected based on their clinical relevance for the outcomes of interest and are known risk factors for the primary endpoint DGF [2,5,25]. We performed multivariable receiver-operating-characteristic (ROC) curve analyses to evaluate the diagnostic value of preoperative sUMOD in addition to established risk factors (recipient age and BMI, dialysis vintage, CIT, deceased vs. living donation, ECD, “Model A”) for the prediction of DGF (“Model B”). All analyses were conducted using R, version 3.5.1 (R Core Team (2018), Vienna, Austria).
3. Results
3.1. Population Characteristics
The mean age of the cohort was 51 ± 14 years, 31.4% were female, 90 (37.7%) received an organ from a living donor, 79 (33.1%) had cardiovascular disease at baseline. Mean serum creatinine concentration was 6.0 ± 2.3 mg/dL on POD1. Demographics of the entire cohort and stratified by preoperative sUMOD quartiles are shown in Table 1.

Table 1.
Overall baseline characteristics (n = 239) and baseline characteristics of participants stratified by quartiles distributed according to pretransplant serum uromodulin (sUMOD).
A total of 64 (26.8%) renal allograft recipients experienced DGF. Patients who experienced DGF were older, more often male, had a higher BMI and a greater prevalence of cardiovascular disease (Table 2). The time on dialysis before transplantation (dialysis vintage) was significantly longer in recipients who developed DGF (2208 ± 1456 days vs. 1321 ± 1331, p < 0.001). The mean serum creatinine on POD1 was significantly higher in patients with DGF (7.1 ± 2.4 mg/dL vs. 5.6 ± 2.1 mg/dL, p < 0.001). Referring to donor characteristics, kidney transplants with subsequent DGF were derived from donors who were more often male, had a higher BMI, a higher prevalence of diabetes and had a significantly higher serum creatinine before donation. Furthermore, cold and warm ischemia time were significantly longer for donor kidneys who developed DGF. Further information on DGF vs. non-DGF patients can be found in Table 2.

Table 2.
Baseline characteristics of participants stratified by status regarding delayed graft function (DGF).
3.2. Course of sUMOD during the Transplant Process and Short Term Follow Up
The mean sUMOD levels in the total cohort was 14.9 ± 23.8 ng/mL preoperatively, 52.3 ± 50.2 ng/mL on POD1 and remained stable after this up to 31–120 days after transplantation (Figure 1). Patients with DGF had significantly lower pretransplant sUMOD levels compared to patients without DGF (5.9 ± 6.4 ng/mL vs. 18.3 ± 26.8 ng/mL, p < 0.001). There was no significant difference in sUMOD levels on POD1 between patients with and without DGF (54.0 ± 50.4 ng/mL vs. 51.7 ± 50.3 ng/mL, p = 0.888; Table 1). However, while sUMOD levels decreased again in patients with DGF in the postoperative period, we did see a further increase in patients without DGF (Figure 1). In contrast, serum creatinine levels were higher in the DGF subgroup pretransplant and remained higher over the whole postoperative period compared to the non-DGF subgroup (Figure 1).
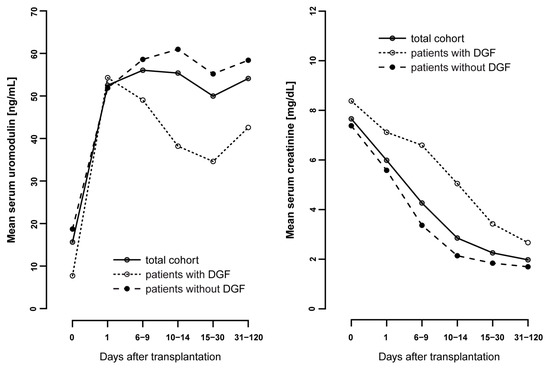
Figure 1.
Mean serum uromodulin values [ng/mL] from pretransplant to follow-up (up to 120 days after transplantation) compared to the mean serum creatinine [mg/dL] in the total cohort and in patients with and without delayed graft function (DGF).
3.3. Pretransplant sUMOD and DGF
In univariable analysis, each 10 ng/mL higher preoperative sUMOD was associated with 49% lower odds for DGF (OR 0.51, 95%-CI 0.32–0.73, Table 3). This association remained statistically significant after multivariable adjustment (OR 0.53, 95%-CI 0.30–0.82). When categorized into quartiles, the quartile with the lowest preoperative sUMOD levels had 4.4-fold higher odds for DGF compared to the highest quartile in multivariable analysis (OR 4.41, 95%-CI 1.54–13.93, Table 3).

Table 3.
Associations of serum uromodulin (sUMOD) pretransplant and on postoperative Day 1 with delayed graft function (DGF) in the kidney transplant.
In order to rule out potential confounding through preemptive transplantation we performed a sensitivity analysis in which we adjusted our multivariable logistic regression Model 1 for preemptive transplantation (categorical variable yes vs. no) instead of dialysis vintage. We did not add preemptive transplantation as another covariable in order to avoid overfitting of the model. In this additional analysis with pretransplant sUMOD as a continuous variable, we identified a similar OR for the association of sUMOD with DGF (OR 0.50 (95%-CI 0.28–0.79) per 10 ng/mL higher sUMOD).
sUMOD on POD1 was not significantly associated with DGF, neither as a continuous nor a categorical variable (Table 3).
3.4. ROC-Analysis to Evaluate Preoperative sUMOD as a Predictor for DGF
In multivariable ROC curve analysis, Model A (including risk factors for DGF without preoperative sUMOD) worked moderately well to predict DGF (area under the curve (AUC) 0.786 [95%-CI 0.723–0.848], Figure 2). Model B (i.e., adding sUMOD to Model A) increased the predictive accuracy at borderline statistical significance (AUC 0.813 [95%-CI 0.755–0.871], p = 0.05) as presented in Figure 2.
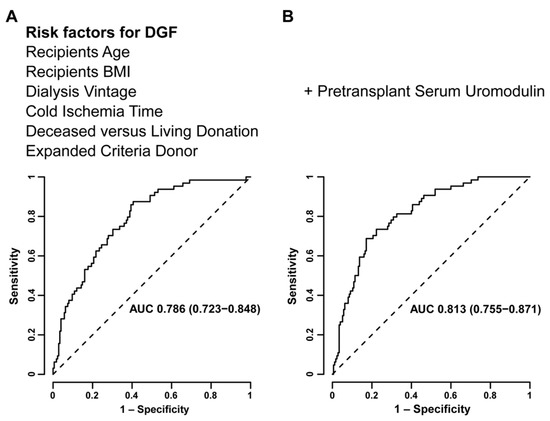
Figure 2.
Multivariable receiver-operating-characteristic (ROC) curve analysis evaluating models including established risk factors (recipient age and body-mass-index (BMI), dialysis vintage, cold ischemia time, deceased vs. living donation, expanded criteria donors) for the prediction of delayed graft function (DGF) without (A) and with preoperative serum uromodulin (B).
4. Discussion
In the current study, we demonstrate that a higher pretransplant sUMOD in kidney transplant recipients is independently associated with a lower risk for DGF. Furthermore, preoperative sUMOD was of additional predictive value when added to a model of established risk factors for DGF. Surprisingly, we detected no association between sUMOD on POD1 and DGF.
We further mapped the course of sUMOD before, during and in the early phase after transplantation (up to 120 days following kidney transplantation) with and without the occurrence of DGF. We demonstrated that over the longer course after transplantation patients without DGF maintained higher sUMOD levels, while in patients with DGF we detected a subsequent decline in sUMOD in the postoperative period. The subsequent decrease in sUMOD is consistent with a recent study showing decrease in circulating uromodulin following AKI in a cohort of liver transplant patients undergoing surgery [26], reflecting tubular mass and function in the longer-term, non-acute setting.
sUMOD has been positively associated with reduced risk for kidney failure, cardiovascular events and mortality in geriatric and chronic kidney disease populations [27,28]. In kidney transplant setting, higher sUMOD in the first year after transplantation has also been associated with better long-term allograft survival in kidney transplant recipients [20,21]. Further, decreased concentrations of sUMOD can be observed in the early course of tubulointerstitial injury in the kidney transplant [29]. This is in line with our observations, that a higher pretransplant sUMOD is associated with lower risk for DGF due to IRI and subsequently higher sUMOD levels over the longer-term course following renal transplantation. None of the previous studies performed uromodulin measurements just before and after kidney transplantation.
Higher pretransplant sUMOD could represent the anti-inflammatory capacity of the recipient towards the following inflammation due to IRI. Interstitial or sUMOD has been shown to downregulate proinflammatory signaling in the kidney, reflecting its immunomodulatory and reno-protective capacity [30]. Recently, it was demonstrated that uromodulin inhibits the generation of reactive oxygen species both in the kidney and systemically [26]. In line with this, UMOD deficient mice experiencing IRI are at higher risk for acute kidney injury with higher interstitial inflammation and cell infiltration [17,19]. Furthermore, UMOD deficient mice showed delayed and incomplete recovery from acute kidney injury after IRI, which is explained by a lack of upregulation of uromodulin expression after IRI [14].
Although it is challenging to directly extrapolate results from murine models of IRI to human transplantation, results from these models support our observations, that a higher sUMOD in the recipient just before transplantation is associated with lower risk for DGF [31]. SUMOD is hypothesized to be a molecule with abilities in modulating inflammation against an evolving IRI, which in turn is thought to be one of the main mechanisms predisposing to DGF [32]. The findings that higher levels of preoperative sUMOD are associated with less risk of DGF leads to the hypothesis that there is a “high uromodulin” state before transplantation may be beneficial. However, given the observational nature of this data, we cannot conclude on whether sUMOD has a causal role to play in the development of DGF. Despite we detected significant differences in sUMOD levels between patients with and without DGF, absolute differences appear to be small compared to differences in sUMOD levels between healthy individuals and patients at different CKD stages [16]. Therefore, it remains to be validated that the differences we detected between DGF and non-DGF translate into physiologically relevant differences in uromodulin activity.
It is interesting that sUMOD increases initially in patients with or without DGF, which might reflect the release of “donor” sUMOD from the transplanted kidney. Patients with DGF have a subsequent profound and persistent decrease in sUMOD. The fact that we do not see an association between sUMOD on POD1 and delayed graft function could reflect the dynamic pathophysiological process occurring during this early time period in the transplanted kidney, which may be critical to the subsequent course of injury or recovery. The initial increase could represent general reactive reno-protection-intended induction of uromodulin production in the setting of renal IRI, which is related to its immune-modulatory capacities in the interstitium [14,17].
While sUMOD on POD1 might be influenced by acute inflammation and hypoxic stress, long-term sUMOD should reflect tubular function/mass [20,21]. However, as the primary aim of our study was to evaluate sUMOD as a predictive marker or a marker for early detection of DGF, sUMOD pre-transplant and on POD1 was the focus of our statistical analysis.
One strength of our study is the use of both pre- as well as the post-transplant period. While we did not directly adjust for residual kidney function in the multivariable approach, we propose that with adjusting for dialysis vintage and kidney transplantation after living donation we also captured residual kidney function to some extent, as it is well known that residual kidney function decreases along with the time spent of dialysis. In general, due to its molecular mass of 95 kDa sUMOD is highly unlikely to be removed by both hemo- and peritoneal dialysis.
A major limitation in the present study is that patients without DGF received kidneys from “healthier” donors with shorter ischemia time (see Table 2), that are less vulnerable to tubular injury. Although, we tried to account for this difference by adjusting for a number of covariables, which are supposed to be relevant risk factors for DGF (i.e., ECD, deceased vs. living donation, CIT) [2,5,25] there remains the potential residual confounding. Furthermore, DGF due to renal IRI is a common problem after deceased donation [2], but the proportion of patients after living donation in the present cohort is relatively high at almost 38%. We included transplant patients both after deceased as well as after living donation due to the otherwise small number of patients in a single center analysis. Further, we adjusted for living donation in statistical analysis as mentioned above. However, even after adjustment for deceased vs. living donation as well as dialysis vintage with expected shorter dialysis time before transplantation after living donation due to the large proportion of preemptive transplantations, recipients pretransplant sUMOD was independently associated with lower risk for DGF following transplantation. Finally, we lack data on single nucleotide polymorphisms (SNPs) that are known to influence uromodulin concentrations [33,34], and therefore, cannot comment on how these SNPs may affect our findings.
5. Conclusions
In conclusion, lower pretransplant sUMOD is independently associated with DGF after kidney transplantation and might therefore function as an early non-invasive marker to identify patients at increased risk for DGF following IRI and subsequent complicated course after kidney transplantation.
Author Contributions
D.S. designed the study. S.K., D.S. and C.H.-L. performed analysis of the study and wrote the manuscript. S.K., H.S., V.A., L.R. and D.S. were involved in collecting the blood samples. S.K., C.H.-L., H.S., F.H., C.T., Q.B. and C.S. collected data. P.S.G. and D.S. critically discussed statistical analysis. U.H., T.M.E.-A., P.S.G. and J.S. oversaw the study and critically discussed the manuscript. All co-authors have contributed substantially to the final version of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
There are no funding sources.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the ethics committee of the Technical University of Munich (No: 246/14).
Informed Consent Statement
Informed consent to participate and to publish was obtained from all individual participants included in the study.
Data Availability Statement
The dataset generated during the current study is available from corresponding author upon reasonable request.
Acknowledgments
The authors are grateful to the study participants and to the residents on the transplant ward for drawing blood from study participants.
Conflicts of Interest
The authors declare that they have no conflict of interest.
List of Abbreviations
| AUC | Area under the curve |
| BMI | Body-mass index |
| CIT | Cold ischemia time |
| ECD | Expanded criteria donor |
| eGFR | Estimated glomerular filtration rate |
| ELISA | Enzyme-linked immunosorbent assay |
| DGF | Delayed graft function |
| IRI | Ischemia-reperfusion injury |
| 95%-CI | 95% confidence interval |
| OR | Odds ratio |
| POD1 | Postoperative Day 1 |
| ROC | Receiver-operating-characteristics |
| sUMOD | Serum uromodulin. |
References
- Perico, N.; Cattaneo, D.; Sayegh, M.H.; Remuzzi, G. Delayed graft function in kidney transplantation. Lancet 2004, 364, 1814–1827. [Google Scholar] [CrossRef]
- Siedlecki, A.; Irish, W.; Brennan, D.C. Delayed graft function in the kidney transplant. Am. J. Transpl. 2011, 112, 2279–2296. [Google Scholar] [CrossRef]
- Yarlagadda, S.G.; Coca, S.G.; Garg, A.X.; Doshi, M.; Poggio, E.; Marcus, R.J.; Parikh, C.R. Marked variation in the definition and diagnosis of delayed graft function: A systematic review. Nephrol. Dial. Transpl. 2008, 23, 2995–3003. [Google Scholar] [CrossRef]
- Bahl, D.; Haddad, Z.; Datoo, A.; Qazi, Y.A. Delayed graft function in kidney transplantation. Curr. Opin. Organ Transpl. 2019, 24, 82–86. [Google Scholar] [CrossRef]
- Schroppel, B.; Legendre, C. Delayed kidney graft function: From mechanism to translation. Kidney Int. 2014, 86, 251–258. [Google Scholar] [CrossRef]
- Linkermann, A.; Stockwell, B.R.; Krautwald, S.; Anders, H.J. Regulated cell death and inflammation: An auto-amplification loop causes organ failure. Nat. Rev. Immunol. 2014, 14, 759–767. [Google Scholar] [CrossRef]
- Chawla, L.S.; Eggers, P.W.; Star, R.A.; Kimmel, P.L. Acute kidney injury and chronic kidney disease as interconnected syndromes. N. Engl. J. Med. 2014, 371, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Venkatachalam, M.A.; Weinberg, J.M.; Kriz, W.; Bidani, A.K. Failed Tubule Recovery, AKI-CKD Transition, and Kidney Disease Progression. J. Am. Soc. Nephrol. 2015, 26, 1765–1776. [Google Scholar] [CrossRef] [PubMed]
- Chapman, J.R. Progress in Transplantation: Will It Be Achieved in Big Steps or by Marginal Gains? Am. J. Kidney Dis. 2017, 69, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Lohkamp, L.N.; Ollinger, R.; Chatzigeorgiou, A.; Illigens, B.M.; Siepmann, T. Intraoperative biomarkers in renal transplantation. Nephrology 2016, 21, 188–199. [Google Scholar] [CrossRef] [PubMed]
- Pennica, D.; Kohr, W.J.; Kuang, W.J.; Glaister, D.; Aggarwal, B.B.; Chen, E.Y.; Goeddel, D.V. Identification of human uromodulin as the Tamm-Horsfall urinary glycoprotein. Science 1987, 236, 83–88. [Google Scholar] [CrossRef]
- Zhu, X.; Cheng, J.; Gao, J.; Lepor, H.; Zhang, Z.T.; Pak, J.; Wu, X.R. Isolation of mouse THP gene promoter and demonstration of its kidney-specific activity in transgenic mice. Am. J. Physiol. Ren. Physiol. 2002, 282, F608–F617. [Google Scholar] [CrossRef][Green Version]
- Serafini-Cessi, F.; Malagolini, N.; Cavallone, D. Tamm-Horsfall glycoprotein: Biology and clinical relevance. Am. J. Kidney Dis. 2003, 42, 658–676. [Google Scholar] [CrossRef]
- El-Achkar, T.M.; McCracken, R.; Liu, Y.; Heitmeier, M.R.; Bourgeois, S.; Ryerse, J.; Wu, X.R. Tamm-Horsfall protein translocates to the basolateral domain of thick ascending limbs, interstitium, and circulation during recovery from acute kidney injury. Am. J. Physiol. Ren. Physiol. 2013, 304, F1066–F1075. [Google Scholar] [CrossRef] [PubMed]
- El-Achkar, T.M.; Wu, X.R. Uromodulin in kidney injury: An instigator, bystander, or protector? Am. J. Kidney Dis 2012, 59, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Scherberich, J.E.; Gruber, R.; Nockher, W.A.; Christensen, E.I.; Schmitt, H.; Herbst, V.; Block, M.; Kaden, J.; Schlumberger, W. Serum uromodulin-a marker of kidney function and renal parenchymal integrity. Nephrol. Dial. Transpl. 2018, 33, 284–295. [Google Scholar] [CrossRef] [PubMed]
- El-Achkar, T.M.; Wu, X.R.; Rauchman, M.; McCracken, R.; Kiefer, S.; Dagher, P.C. Tamm-Horsfall protein protects the kidney from ischemic injury by decreasing inflammation and altering TLR4 expression. Am. J. Physiol. Ren. Physiol. 2008, 295, F534–F544. [Google Scholar] [CrossRef] [PubMed]
- Micanovic, R.; Khan, S.; Janosevic, D.; Lee, M.E.; Hato, T.; Srour, E.F.; Winfree, S.; Ghosh, J.; Tong, Y.; Rice, S.E.; et al. Tamm-Horsfall Protein Regulates Mononuclear Phagocytes in the Kidney. J. Am. Soc. Nephrol. 2018, 29, 841–856. [Google Scholar] [CrossRef] [PubMed]
- El-Achkar, T.M.; McCracken, R.; Rauchman, M.; Heitmeier, M.R.; Al-Aly, Z.; Dagher, P.C.; Wu, X.R. Tamm-Horsfall protein-deficient thick ascending limbs promote injury to neighboring S3 segments in an MIP-2-dependent mechanism. Am. J. Physiol. Ren. Physiol. 2011, 300, F999–F1007. [Google Scholar] [CrossRef] [PubMed]
- Steubl, D.; Block, M.; Herbst, V.; Schlumberger, W.; Nockher, A.; Angermann, S.; Schmaderer, C.; Heemann, U.; Renders, L.; Scherberich, J. Serum uromodulin predicts graft failure in renal transplant recipients. Biomarkers 2017, 22, 171–177. [Google Scholar] [CrossRef]
- Bostom, A.; Steubl, D.; Garimella, P.S.; Franceschini, N.; Roberts, M.B.; Pasch, A.; Ix, J.H.; Tuttle, K.R.; Ivanova, A.; Shireman, T.; et al. Serum Uromodulin: A Biomarker of Long-Term Kidney Allograft Failure. Am. J. Nephrol. 2018, 47, 275–282. [Google Scholar] [CrossRef]
- Hall, I.E.; Reese, P.P.; Doshi, M.D.; Weng, F.L.; Schroppel, B.; Asch, W.S.; Ficek, J.; Thiessen-Philbrook, H.; Parikh, C.R. Delayed Graft Function Phenotypes and 12-Month Kidney Transplant Outcomes. Transplantation 2017, 101, 1913–1923. [Google Scholar] [CrossRef]
- Wu, W.K.; Famure, O.; Li, Y.; Kim, S.J. Delayed graft function and the risk of acute rejection in the modern era of kidney transplantation. Kidney Int. 2015, 88, 851–858. [Google Scholar] [CrossRef]
- Querard, A.H.; Le Borgne, F.; Dion, A.; Giral, M.; Mourad, G.; Garrigue, V.; Rostaing, L.; Kamar, N.; Loupy, A.; Legendre, C.; et al. Propensity score-based comparison of the graft failure risk between kidney transplant recipients of standard and expanded criteria donor grafts: Toward increasing the pool of marginal donors. Am. J. Transpl. 2018, 18, 1151–1157. [Google Scholar] [CrossRef]
- Sharif, A.; Borrows, R. Delayed graft function after kidney transplantation: The clinical perspective. Am. J. Kidney Dis. 2013, 62, 150–158. [Google Scholar] [CrossRef]
- LaFavers, K.A.; Macedo, E.; Garimella, P.S.; Lima, C.; Khan, S.; Myslinski, J.; McClintick, J.; Witzmann, F.A.; Winfree, S.; Phillips, C.L.; et al. Circulating uromodulin inhibits systemic oxidative stress by inactivating the TRPM2 channel. Sci. Transl. Med. 2019, 11, eaaw3639. [Google Scholar] [CrossRef] [PubMed]
- Steubl, D.; Buzkova, P.; Garimella, P.S.; Ix, J.H.; Devarajan, P.; Bennett, M.R.; Chaves, P.H.M.; Shlipak, M.G.; Bansal, N.; Sarnak, M.J. Association of Serum Uromodulin with ESKD and Kidney Function Decline in the Elderly: The Cardiovascular Health Study. Am. J. Kidney Dis. 2019, 74, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Steubl, D.; Buzkova, P.; Garimella, P.S.; Ix, J.H.; Devarajan, P.; Bennett, M.R.; Chaves, P.H.M.; Shlipak, M.G.; Bansal, N.; Sarnak, M.J. Association of serum uromodulin with mortality and cardiovascular disease in the elderly-the Cardiovascular Health Study. Nephrol. Dial. Transpl. 2020, 35, 1399–1405. [Google Scholar] [CrossRef]
- Borstnar, S.; Veceric-Haler, Z.; Bostjancic, E.; Pipan Tkalec, Z.; Kovac, D.; Lindic, J.; Kojc, N. Uromodulin and microRNAs in Kidney Transplantation-Association with Kidney Graft Function. Int. J. Mol. Sci. 2020, 21, 5592. [Google Scholar] [CrossRef] [PubMed]
- Micanovic, R.; Chitteti, B.R.; Dagher, P.C.; Srour, E.F.; Khan, S.; Hato, T.; Lyle, A.; Tong, Y.; Wu, X.R.; El-Achkar, T.M. Tamm-Horsfall Protein Regulates Granulopoiesis and Systemic Neutrophil Homeostasis. J. Am. Soc. Nephrol. 2015, 26, 2172–2182. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-C.; Chapman, W.C.; Hanto, D.W. Ischemia-Reperfusion injury in kidney transplantation. Front. Biosci. 2015, 7, 117–134. [Google Scholar]
- Grenda, R. Delayed graft function and its management in children. Pediatric Nephrol. 2017, 32, 1157–1167. [Google Scholar] [CrossRef]
- Kottgen, A.; Hwang, S.J.; Larson, M.G.; Van Eyk, J.E.; Fu, Q.; Benjamin, E.J.; Dehghan, A.; Glazer, N.L.; Kao, W.H.; Harris, T.B.; et al. Uromodulin levels associate with a common UMOD variant and risk for incident CKD. J. Am. Soc. Nephrol. 2010, 21, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Delgado, G.E.; Kleber, M.E.; Scharnagl, H.; Kramer, B.K.; Marz, W.; Scherberich, J.E. Serum Uromodulin and Mortality Risk in Patients Undergoing Coronary Angiography. J. Am. Soc. Nephrol. 2017, 28, 2201–2210. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).