Cutaneous and Vascular Deposits of 18F-NaF by PET/CT in the Follow-Up of Patients with Pseudoxanthoma Elasticum
Abstract
1. Introduction
2. Materials and Methods
3. Results
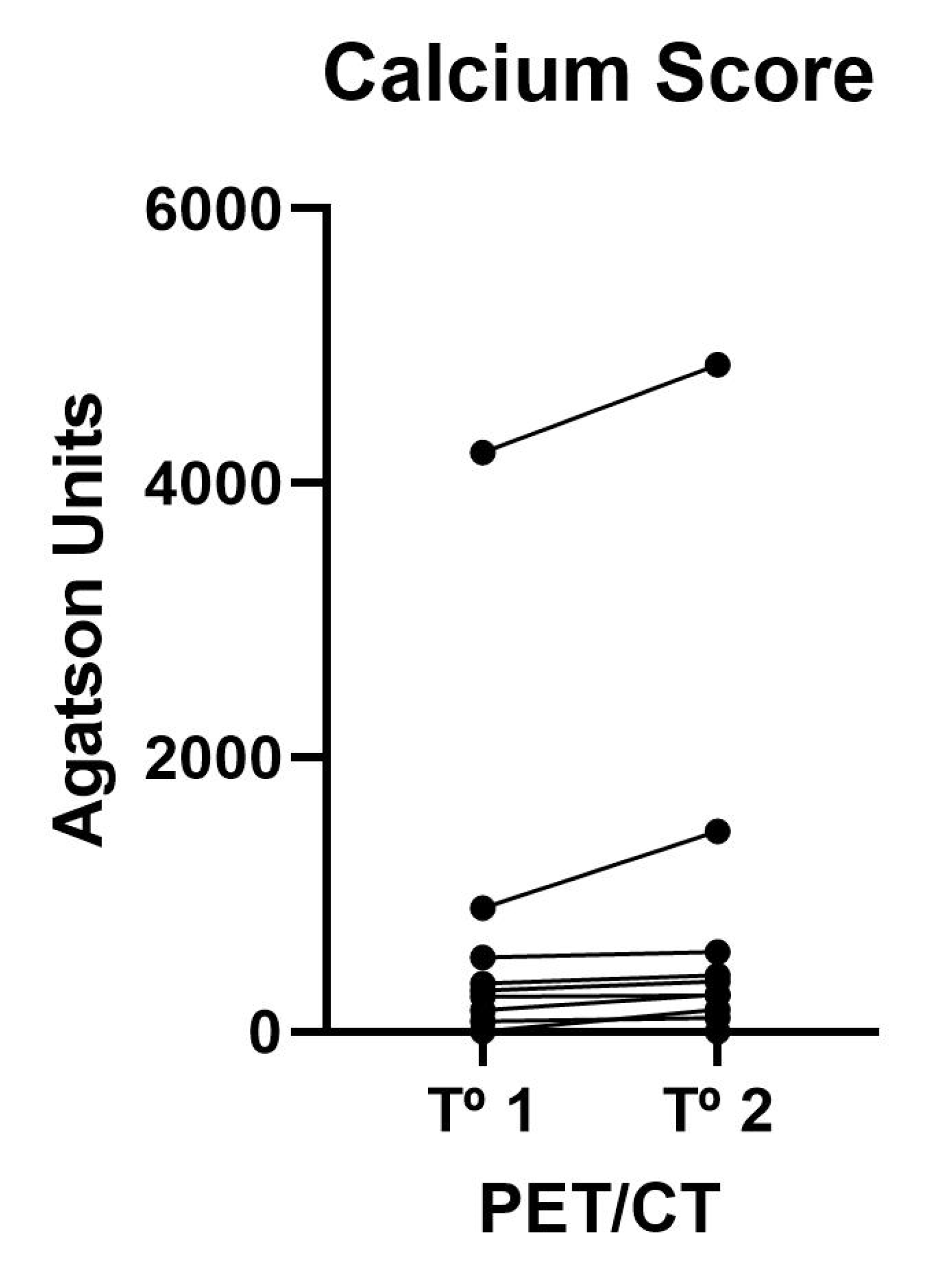
3.1. Vascular Calcium Parameters on CT
3.2. Arterial Wall Uptake of 18F-NaF
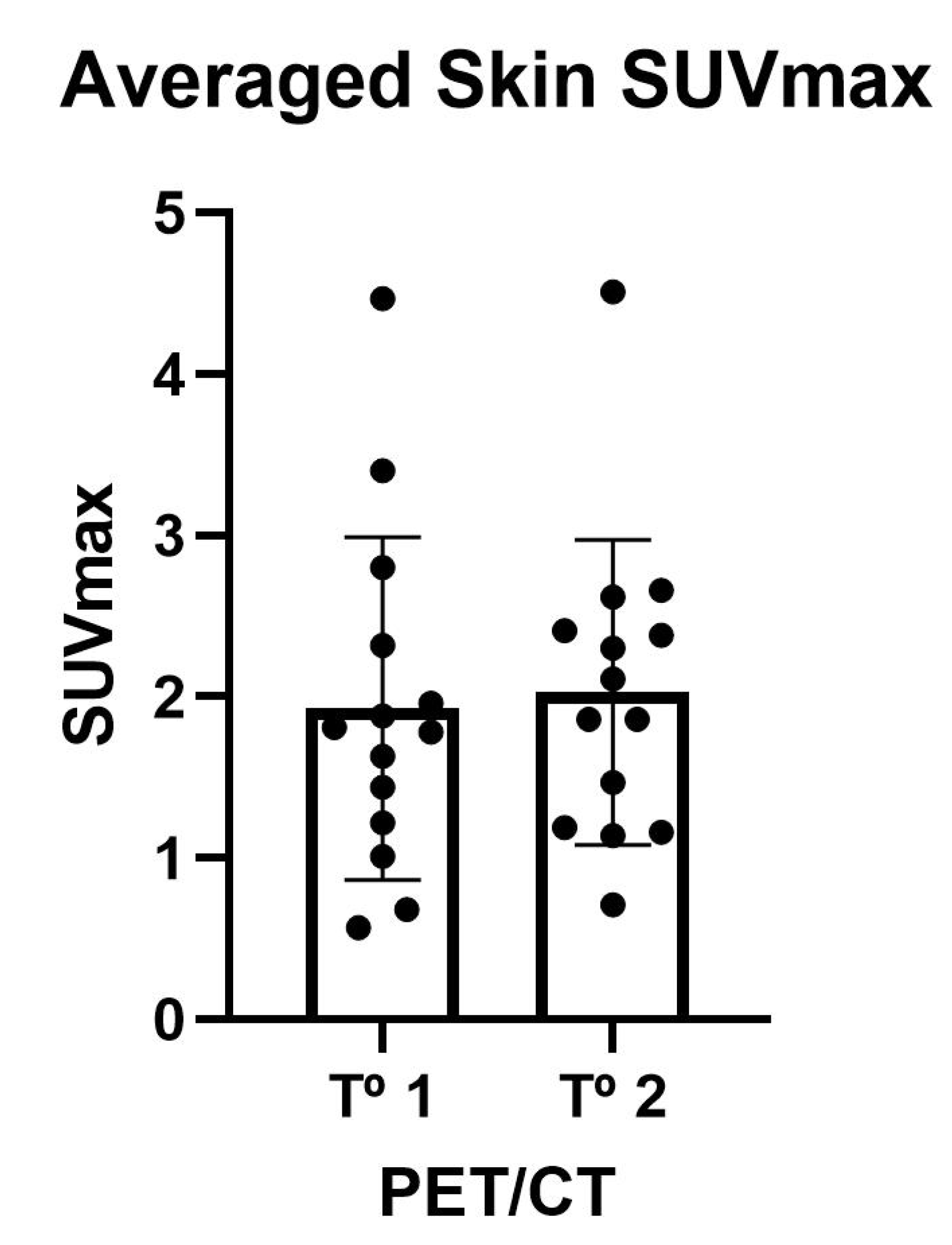
3.3. Skin Uptake of 18F-NaF
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nitschke, Y.; Rutsch, F. Inherited Arterial Calcification Syndromes: Etiologies and Treatment Concepts. Curr. Osteoporos. Rep. 2017, 15, 255–270. [Google Scholar] [CrossRef] [PubMed]
- Jansen, R.S.; Duijst, S.; Mahakena, S.; Sommer, D.; Szeri, F.; Váradi, A.; Plomp, A.; Bergen, A.A.; Oude Elferink, R.P.J.; Borst, P.; et al. ABCC6-mediated ATP secretion by the liver is the main source of the mineralization inhibitor inorganic pyrophosphate in the systemic circulation - Brief report. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1985–1989. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Tévar, A.M.; García-Fernández, M.; Murcia-Casas, B.; Rioja-Villodres, J.; Carrillo, J.L.; Camacho, M.; Van Gils, M.; Sánchez-Chaparro, M.A.; Vanakker, O.; Valdivielso, P. Plasma inorganic pyrophosphate and alkaline phosphatase in patients with pseudoxanthoma elasticum. Ann. Transl. Med. 2019, 7, 798. [Google Scholar] [CrossRef] [PubMed]
- Quaglino, D.; Boraldi, F.; Annovi, G.; Ronchetti, I. The Multifaceted Complexity of Genetic Diseases: A Lesson from Pseudoxanthoma Elasticum. In Advances in the Study of Genetic Disorders; InTechOpen: Rijeka, Croatia, 2021; ISBN 978-953-307-305-7. [Google Scholar]
- Uitto, J.; Li, Q.; van de Wetering, K.; Váradi, A.; Terry, S.F. Insights into Pathomechanisms and Treatment Development in Heritable Ectopic Mineralization Disorders: Summary of the PXE International Biennial Research Symposium—2016. J. Investig. Dermatol. 2017, 137, 790–795. [Google Scholar] [CrossRef] [PubMed]
- Morillo, M.J.; Mora, J.; Soler, A.; García-Campos, J.M.; García-Fernández, I.; Sánchez, P.; Valdivieso, P. Imágenes funduscópicas con autofluorescencia en pacientes con pseudoxantoma elástico. Arch. Soc. Esp. Oftalmol. 2011, 86, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Irkle, A.; Vesey, A.T.; Lewis, D.Y.; Skepper, J.N.; Bird, J.L.E.; Dweck, M.R.; Joshi, F.R.; Gallagher, F.A.; Warburton, E.A.; Bennett, M.R.; et al. Identifying active vascular microcalcification by 18F-sodium fluoride positron emission tomography. Nat. Commun. 2015, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Cardo, A.; Lillo, E.; Murcia-Casas, B.; Carrillo-Linares, J.L.; García-Argüello, F.; Sánchez-Sánchez, P.; Rodriguez-Morata, A.; Baquero Aranda, I.; Sánchez-Chaparro, M.Á.; García-Fernández, M.; et al. Skin and Arterial Wall Deposits of 18F-NaF and Severity of Disease in Patients with Pseudoxanthoma Elasticum. J. Clin. Med. 2020, 9, 1393. [Google Scholar] [CrossRef] [PubMed]
- Omarjee, L.; Mention, P.-J.; Janin, A.; Kauffenstein, G.; Pabic, E.L.; Meilhac, O.; Blanchard, S.; Navasiolava, N.; Leftheriotis, G.; Couturier, O.; et al. Assessment of inflammation and calcification in pseudoxanthoma elasticum arteries and skin with 18F-FluroDeoxyGlucose and 18F-Sodium fluoride positron emission tomography/computed tomography imaging: The GOCAPXE trial. J. Clin. Med. 2020, 9, 3448. [Google Scholar] [CrossRef] [PubMed]
- Kranenburg, G.; de Jong, P.A.; Bartstra, J.W.; Lagerweij, S.J.; Lam, M.G.; Ossewaarde-van Norel, J.; Risseeuw, S.; van Leeuwen, R.; Imhof, S.M.; Verhaar, H.J.; et al. Etidronate for prevention of ectopic mineralization in patients with pseudoxanthoma elasticum. J. Am. Coll. Cardiol. 2018, 71, 1117–1126. [Google Scholar] [CrossRef] [PubMed]
- Bartstra, J.W.; de Jong, P.A.; Kranenburg, G.; Wolterink, J.M.; Isgum, I.; Wijsman, A.; Wolf, B.; den Harder, A.M.; Th, M.; Mali, W.P. Etidronate halts systemic arterial calcification in pseudoxanthoma elasticum. Atherosclerosis 2019. [Google Scholar] [CrossRef]
- Plomp, A.S.; Toonstra, J.; Bergen, A.A.B.; van Dijk, M.R.; Jong, P.T.V.M. de Proposal for updating the pseudoxanthoma elasticum classification system and a review of the clinical findings. Am. J. Med. Genet. Part A 2010, 152A, 1049–1058. [Google Scholar] [CrossRef] [PubMed]
- European Pharmacopoeia Commission; EDQM. Sodium fluoride (18F) injection. In European Pharmacopoeia; EDQM: Strasbourg, France, 2014; pp. 1079–1080. [Google Scholar]
- Rudd, J.H.F.; Myers, K.S.; Bansilal, S.; Machac, J.; Pinto, C.A.; Tong, C.; Rafique, A.; Hargeaves, R.; Farkouh, M.; Fuster, V.; et al. Atherosclerosis inflammation imaging with 18F-FDG PET: Carotid, iliac, and femoral uptake reproducibility, quantification methods, and recommendations. J. Nucl. Med. 2008, 49, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Agatston, A.S.; Janowitz, W.R.; Hildner, F.J.; Zusmer, N.R.; Viamonte, M.; Detrano, R. Quantification of coronary artery calcium using ultrafast computed tomography. J. Am. Coll. Cardiol. 1990, 15, 827–832. [Google Scholar] [CrossRef]
- Risseeuw, S.; Van Leeuwen, R.; Imhof, S.M.; De Jong, P.A.; Mali, W.P.T.H.M.; Spiering, W.; Ossewaarde-van Norel, J. The effect of etidronate on choroidal neovascular activity in patients with pseudoxanthoma elasticum. PLoS ONE 2020, 15. [Google Scholar] [CrossRef] [PubMed]
- Jiménez Villodres, M.; García Gutiérrez, G.; García Frías, P.; Rioja Villodres, J.; Martín Velázquez, M.; Sánchez Chaparro, M.A.; Pérez López, C.; Valdivielso, P. Fractional excretion of phosphorus and vascular calcification in stage 3 chronic kidney disease. J. Investig. Med. 2019, 67. [Google Scholar] [CrossRef]
- Li, Q.; Huang, J.; Pinkerton, A.B.; Millan, J.L.; van Zelst, B.D.; Levine, M.A.; Sundberg, J.P.; Uitto, J. Inhibition of Tissue-Nonspecific Alkaline Phosphatase Attenuates Ectopic Mineralization in the Abcc6–/– Mouse Model of PXE but Not in the Enpp1 Mutant Mouse Models of GACI. J. Investig. Dermatol. 2019, 139, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Opdebeeck, B.; Neven, E.; Millán, J.L.; Pinkerton, A.B.; D’Haese, P.C.; Verhulst, A. Pharmacological TNAP inhibition efficiently inhibits arterial media calcification in a warfarin rat model but deserves careful consideration of potential physiological bone formation/mineralization impairment. Bone 2020, 137. [Google Scholar] [CrossRef] [PubMed]
- Pinkerton, A.B.; Sergienko, E.; Bravo, Y.; Dahl, R.; Ma, C.-T.; Sun, Q.; Jackson, M.R.; Cosford, N.D.P.; Millán, J.L. Discovery of 5-((5-chloro-2-methoxyphenyl)sulfonamido)nicotinamide (SBI-425), a potent and orally bioavailable tissue-nonspecific alkaline phosphatase (TNAP) inhibitor. Bioorg. Med. Chem. Lett. 2018, 28, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Van Gils, M.; Nollet, L.; Verly, E.; Deianova, N.; Vanakker, O.M. Cellular signaling in pseudoxanthoma elasticum: An update. Cell. Signal. 2019, 55, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Kauffenstein, G.; Yegutkin, G.G.; Khiati, S.; Pomozi, V.; Le Saux, O.; Leftheriotis, G.; Lenaers, G.; Henrion, D.; Martin, L. Alteration of Extracellular Nucleotide Metabolism in Pseudoxanthoma Elasticum. J. Investig. Dermatol. 2018, 138, 1862–1870. [Google Scholar] [CrossRef] [PubMed]

| First | Second | p Value | |
|---|---|---|---|
| Calcium Score | 123 (5–672) | 173 (4–518) | 0.008 |
| Calcium Volume | 169 (7–465) | 297 (3–495) | 0.023 |
| Calcium Mass | 32 (1–95) | 57 (1–108) | 0.009 |
| First | Second | p Value | |
|---|---|---|---|
| SUVMax | 0.88 (0.72–0.93) | 0.86 (0.76–0.97) | NS |
| SUVMean | 0.67 (0.51–0.74) | 0.66 (0.58–0.76) | NS |
| TBRmax | 2.58 (2.01–2.80) | 1.94 (1.72–2.25) | 0.04 |
| TBRmean | 2.10 (1.52–2.34) | 1.71 (1.52–1.85) | 0.04 |
| First | Second | p Value | |
|---|---|---|---|
| SUVMax | 1.79 (1.17–2.44) | 1.98 (1.18–2.45) | NS |
| SUVMean | 1.25 (0.90–1.69) | 1.12 (0.70–1.40) | NS |
| LSMax | 0.90 (0.64–1.06) | 0.84 (0.70–0.95) | NS |
| LSMean | 0.59 (0.44–0.70) | 0.62 (0.54–0.67) | NS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lillo, E.; Gutierrez-Cardo, A.; Murcia-Casas, B.; Carrillo-Linares, J.L.; Garcia-Argüello, F.; Chicharo de Freitas, R.; Baquero-Aranda, I.; Valdivielso, P.; García-Fernández, M.; Sánchez-Chaparro, M.Á. Cutaneous and Vascular Deposits of 18F-NaF by PET/CT in the Follow-Up of Patients with Pseudoxanthoma Elasticum. J. Clin. Med. 2021, 10, 2588. https://doi.org/10.3390/jcm10122588
Lillo E, Gutierrez-Cardo A, Murcia-Casas B, Carrillo-Linares JL, Garcia-Argüello F, Chicharo de Freitas R, Baquero-Aranda I, Valdivielso P, García-Fernández M, Sánchez-Chaparro MÁ. Cutaneous and Vascular Deposits of 18F-NaF by PET/CT in the Follow-Up of Patients with Pseudoxanthoma Elasticum. Journal of Clinical Medicine. 2021; 10(12):2588. https://doi.org/10.3390/jcm10122588
Chicago/Turabian StyleLillo, Eugenia, Antonio Gutierrez-Cardo, Belén Murcia-Casas, Juan Luis Carrillo-Linares, Francisco Garcia-Argüello, Reinaldo Chicharo de Freitas, Isabel Baquero-Aranda, Pedro Valdivielso, María García-Fernández, and Miguel Ángel Sánchez-Chaparro. 2021. "Cutaneous and Vascular Deposits of 18F-NaF by PET/CT in the Follow-Up of Patients with Pseudoxanthoma Elasticum" Journal of Clinical Medicine 10, no. 12: 2588. https://doi.org/10.3390/jcm10122588
APA StyleLillo, E., Gutierrez-Cardo, A., Murcia-Casas, B., Carrillo-Linares, J. L., Garcia-Argüello, F., Chicharo de Freitas, R., Baquero-Aranda, I., Valdivielso, P., García-Fernández, M., & Sánchez-Chaparro, M. Á. (2021). Cutaneous and Vascular Deposits of 18F-NaF by PET/CT in the Follow-Up of Patients with Pseudoxanthoma Elasticum. Journal of Clinical Medicine, 10(12), 2588. https://doi.org/10.3390/jcm10122588







