Treatment of Two-Phase Olive Mill Wastewater and Recovery of Phenolic Compounds Using Membrane Technology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Olive Mill Wastewater
2.2. Extraction Experiments
2.3. Membrane Units
2.4. Total Phenolic Content Measurement
2.5. Total Carbohydrates Measurement
2.6. Pilot Scale Experiments
3. Results and Discussion
3.1. Parametric Investigation of the Extraction Procedure
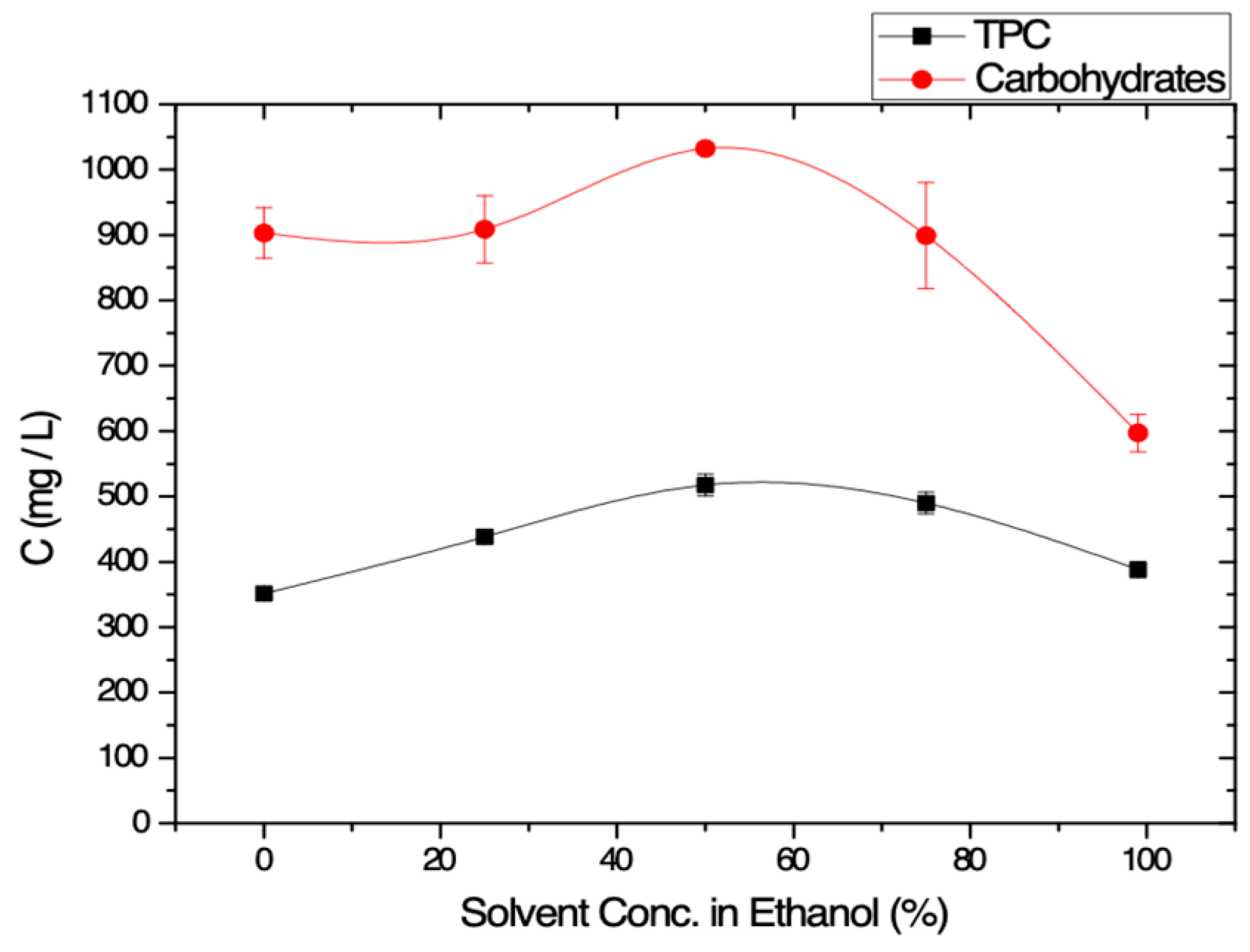
3.1.1. Effect of the Solvent
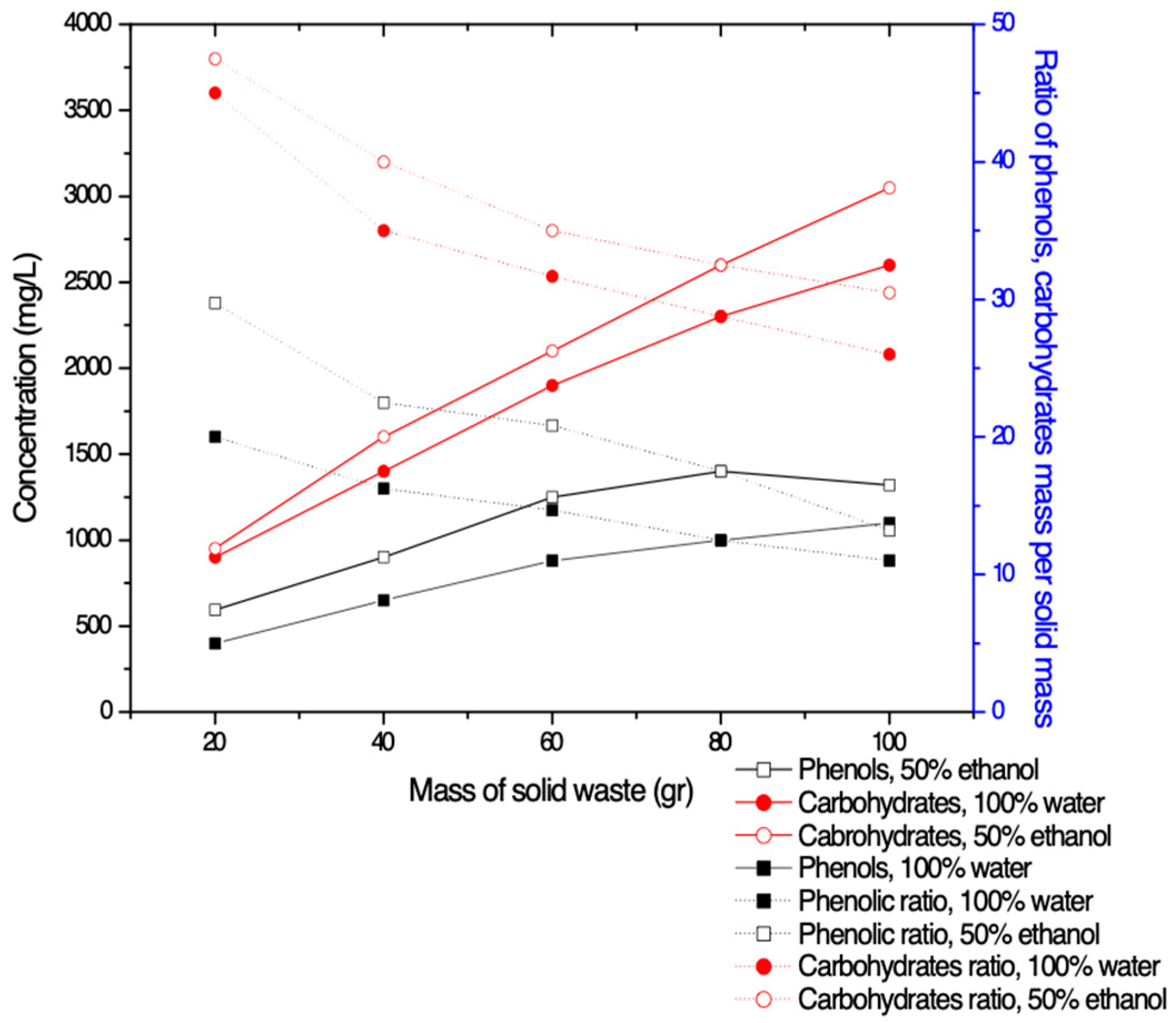
3.1.2. Maximum Quantity of Solids Per Solvent
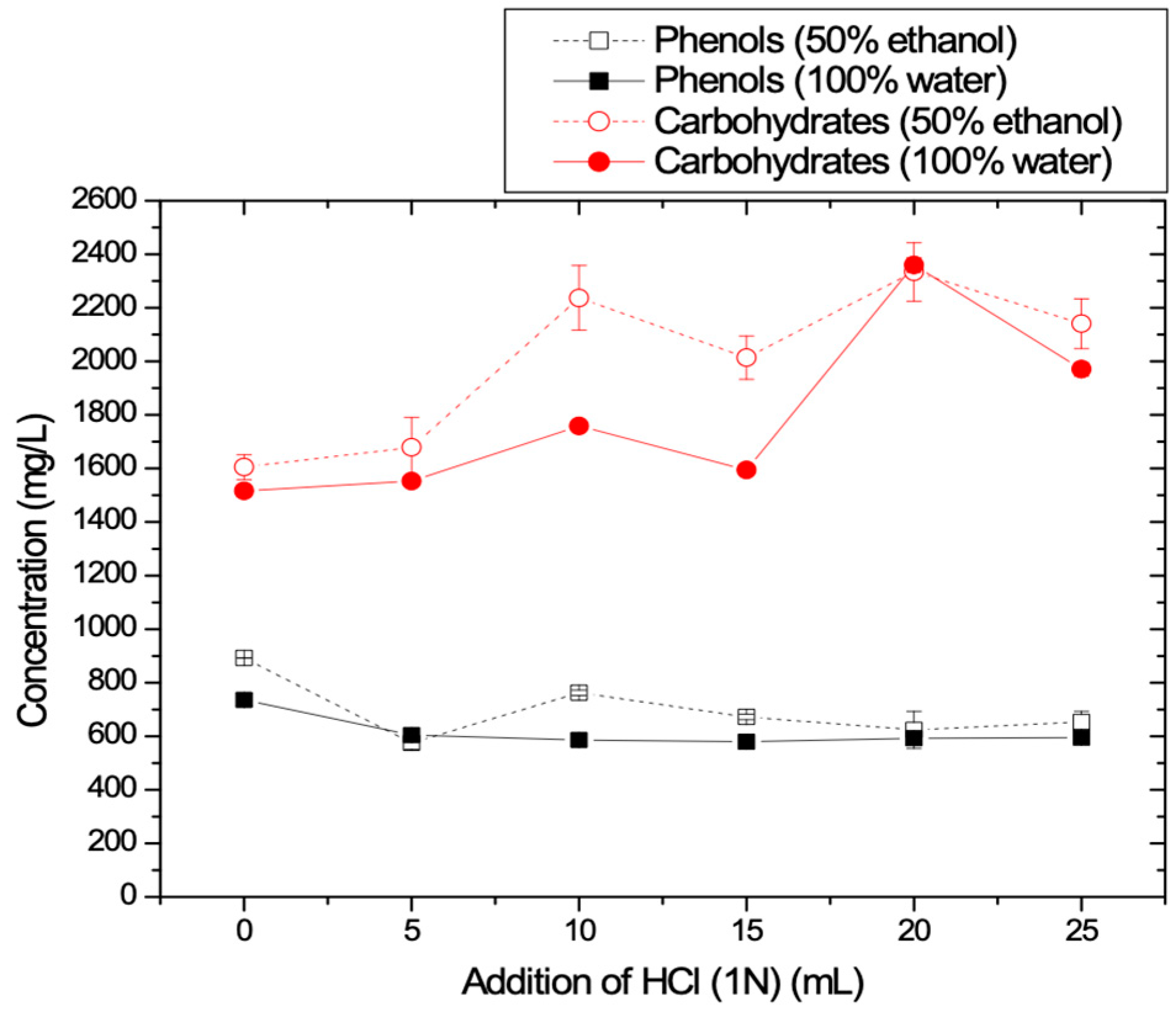
3.1.3. Effect of HCl
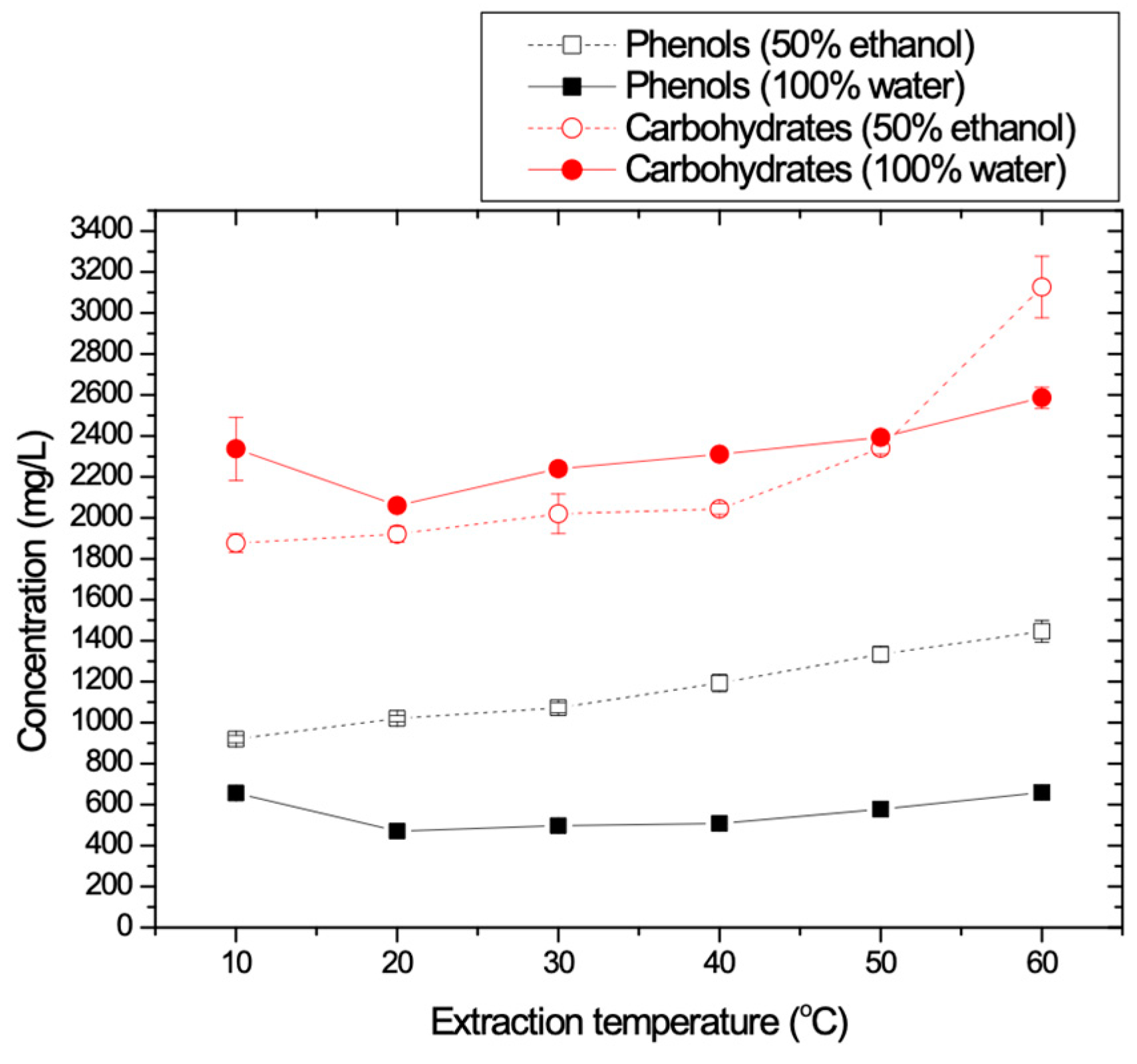
3.1.4. Temperature Effect
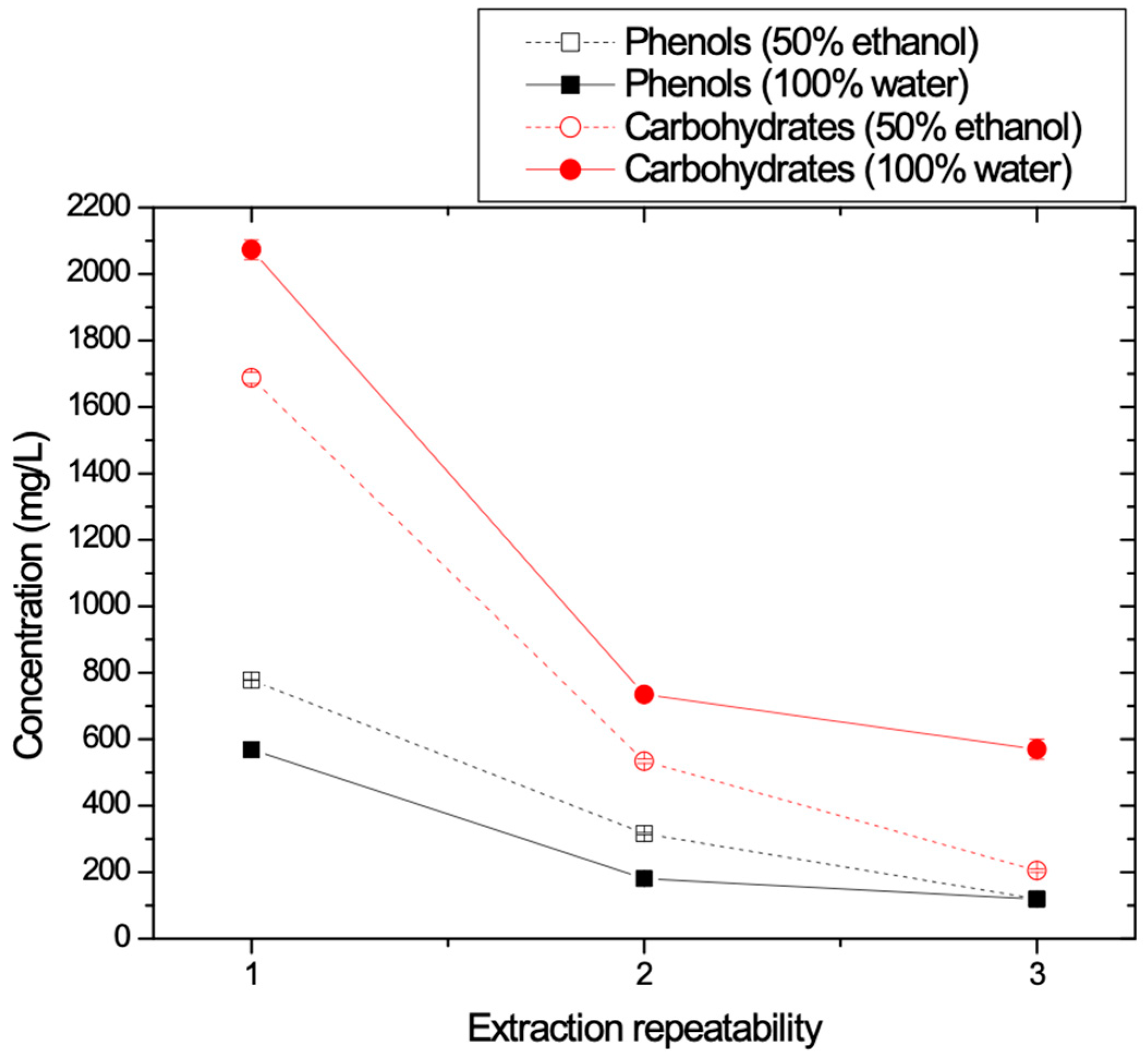
3.1.5. Optimization of the Maximum Recovery of Phenolics from the Solid Waste
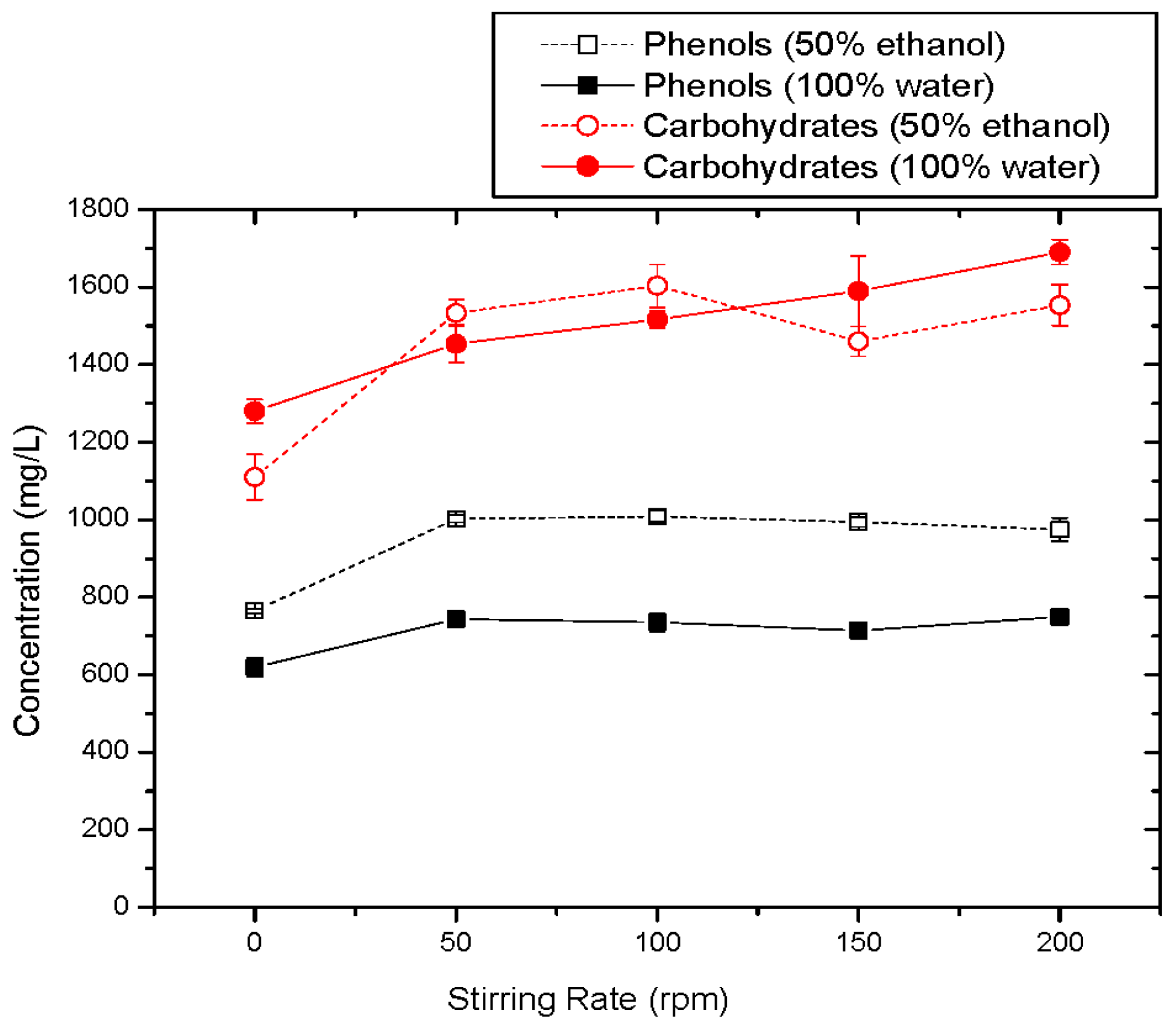
3.1.6. Effect of Stirring Rate
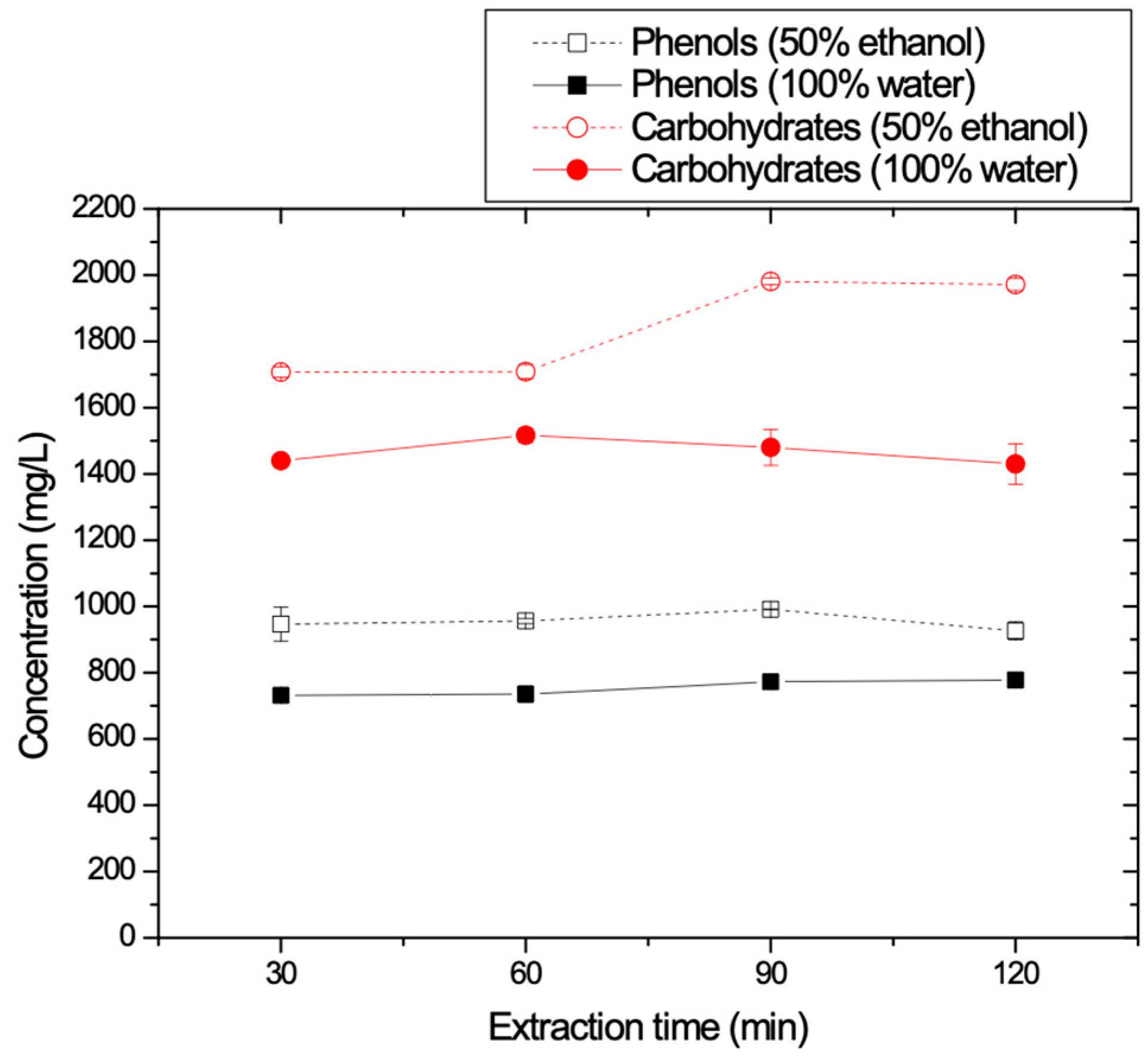
3.1.7. Effect of the Extraction Time
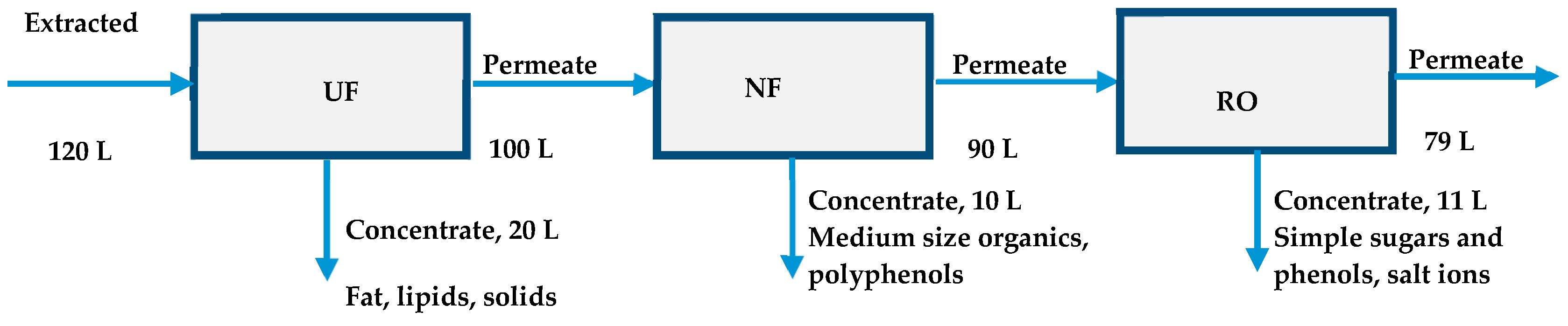
3.2. Pilot scale Experiments
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Caporaso, N.; Formisano, D.; Genovese, A. Use of phenolic compounds from olive mill wastewater as valuable ingredients for functional foods. Crit. Rev. Food Sci. Nutr. 2017, 29, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Bonetti, A.; Venturini, S.; Ena, A.; Faraloni, C. Innovative method for recovery and valorization of hydroxytyrosol from olive mill wastewaters. Water Sci. Technol. 2016, 74, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Turano, E.; Curcio, S.; De Paola, M.G.; Calabrò, V.; Iorio, G. An integrated centrifugation–ultrafiltration system in the treatment of olive mill wastewater. J. Membr. Sci. 2002, 206, 519–531. [Google Scholar] [CrossRef]
- Roig, A.; Cayuela, M.L.; Sanchez-Monedero, M.A. An overview on olive mill wastes and their valorisation methods. Waste Manag. 2006, 26, 960–969. [Google Scholar] [CrossRef] [PubMed]
- Paraskeva, P.; Diamantopoulos, E. Technologies for olive mill wastewater (OMW) treatment. J. Chem. Technol. Biotechnol. 2006, 81, 1475–1485. [Google Scholar] [CrossRef]
- Chowdhuri, A.K.; Md, M.B.; Akratos, C.; Vayenas, D.V.; Pavlou, S. Olive mill waste composting. Int. Biodeterior. Biodegrad. 2013, 85, 108–119. [Google Scholar]
- Ochando-Pulido, J.M.; Martinez-Ferez, A. Optimization of the fouling behavior of a reverse osmosis membrane for purification of olive-oil washing wastewater. Process Saf. Environ. Prot. 2018, 114, 323–333. [Google Scholar] [CrossRef]
- Ochando-Pulido, J.M.; González-Hernández, R.; Martinez-Ferez, A. On the effect of the operating parameters for two-phase olive-oil washing wastewater combined phenolic compounds recovery and reclamation by novel ion exchange resins. Sep. Purif. Technol. 2018, 195, 50–59. [Google Scholar] [CrossRef]
- Vlyssides, A.G.; Lamprou, G.K.; Vlyssides, A. Industrial Case Studies on the Detoxification of OMWW Using Fenton Oxidation Process Followed by Biological Processes for Energy and Compost Production; Recent Advances for Sustainable Management; Galanakis, C.M., Ed.; Elsevier: New York, NY, USA, 2017. [Google Scholar]
- Boari, G.; Brunetti, A.; Passino, R.; Rozzi, A. Anaerobic digestion of olive oil mill wastewaters. Agric. Wastes 1984, 10, 161–175. [Google Scholar] [CrossRef]
- Tziotzios, G.; Michailakis, S.; Vayenas, D.V. Aerobic biological treatment of olive mill wastewater by olive pulp bacteria. Int. Biodeterior. Biodegrad. 2007, 60, 209–214. [Google Scholar] [CrossRef]
- Michailides, M.; Panagopoulos, P.; Akratos, C.S.; Tekerlekopoulou, A.G.; Vayenas, D.V. A full-scale system for aerobic biological treatment of olive mill wastewater. J. Chem. Technol. Biotechnol. 2011, 86, 888–892. [Google Scholar] [CrossRef]
- Zagklis, D.P.; Arvaniti, E.C.; Papadakis, V.G.; Paraskeva, C.A. Sustainability analysis and benchmarking of olive mill wastewater treatment methods. J. Chem. Technol. Biotechnol. 2013, 88, 742–750. [Google Scholar] [CrossRef]
- Stoller, M.; Chianese, A. Technical optimization of a batch olive wash wastewater treatment membrane plant. Desalination 2006, 200, 734–736. [Google Scholar] [CrossRef]
- Paraskeva, C.A.; Papadakis, V.G.; Tsarouchi, E.; Kanellopoulou, D.G.; Koutsoukos, P.G. Membrane processing for olive mill wastewater fractionation. Desalination 2007, 213, 218–229. [Google Scholar] [CrossRef]
- Stoller, M.; Bravi, M.; Chianese, A. Threshold flux measurements of a nanofiltration membrane module by critical flux data conversion. Desalination 2013, 315, 142–148. [Google Scholar] [CrossRef]
- Akdemir, E.O.; Ozer, A. Investigation of two ultrafiltration membranes for treatment of olive oil mill wastewater. Desalination 2009, 249, 660–666. [Google Scholar] [CrossRef]
- Coskun, T.; Debik, E.; Demir, N.M. Treatment of olive mill wastewaters by nanofiltration and reverse osmosis membranes. Desalination 2010, 259, 65–70. [Google Scholar] [CrossRef]
- Di Lecce, G.; Cassano, A.; Bendini, A.; Conidi, C.; Giorno, L.; Gallina, T. Characterization of olive mill wastewater fractions treatment by integrated membrane process. J. Sci. Food Agric. 2014, 94, 2935–2942. [Google Scholar] [CrossRef]
- Ochando-Pulido, J.M.; Martinez-Ferez, A. On the Recent Use of Membrane Technology for Olive Mill Wastewater. Purif. Membr. 2015, 5, 513–531. [Google Scholar] [CrossRef]
- Paraskeva, C.A.; Papadakis, V.G.; Kanellopoulou, D.G.; Koutsoukos, P.G.; Angelopoulos, K.C. Membrane Filtration of Olive Mill Wastewater and Exploitation of Its Fractions. Water Environ. Res. 2007, 79, 421–429. [Google Scholar] [CrossRef]
- Stamatelatou, K.; Kopsahelis, A.; Blika, P.S.; Paraskeva, C.A.; Lyberatos, G. Anaerobic digestion of olive mill wastewater in a periodic anaerobic baffled reactor (PABR) followed by further effluent purification via membrane separation technologies. J. Chem. Technol. Biotechnol. 2009, 84, 909–917. [Google Scholar] [CrossRef]
- Ioannou-Ttofa, L.; Michael-Kordatou, I.; Fattas, S.C.; Eusebio, A.; Ribeiro, B.; Rusan, M.; Amer, A.R.; Zuraiqi, S.; Waismand, M.; Linder, C.; et al. Treatment efficiency and economic feasibility of biological oxidation, membrane filtration and separation processes, and advanced oxidation for the purification and valorization of olive mill wastewater. Water Res. 2017, 114, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zagklis, D.P.; Paraskeva, C.A. Membrane filtration of agro-industrial wastewaters and isolation of organic compounds with high added values. Water Sci. Technol. 2014, 69, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Kontos, S.S.; Iakovides, I.C.; Pantziaros, A.G.; Paraskeva, C.A. Available Treatment Methods for the Development of a Sustainable Solution for the Pollution Caused in Air, Soil and Water by the Olive Mill Wastewaters. JSM Environ. Sci. Ecol. 2015, 3, 1016. [Google Scholar]
- Kontos, S.S.; Koutsoukos, P.G.; Paraskeva, C.A. Application of combined physicochemical techniques for the efficient treatment of olive mill wastewaters. Desalin. Water Treat. 2016, 57, 17051–17060. [Google Scholar] [CrossRef]
- Zagklis, D.P.; Paraskeva, C.A. Isolation of organic compounds with high added values from agroindustrial solid wastes. J. Environ. Manag. 2017, 216, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Petrotos, K.B.; Kokkora, M.I.; Papaioannou, C.; Gkoutsidis, P.E. Olive mill wastewater concentration by two-stage reverse osmosis in tubular configuration, in a scheme combining open and tight membranes. Desalin. Water Treat. 2016, 57, 20621–20630. [Google Scholar] [CrossRef]
- Zagklis, D.P.; Vavouraki, A.I.; Kornaros, M.E.; Paraskeva, C.A. Purification of olive mill wastewater phenols through membrane filtration and resin adsorption/desorption. J. Hazard. Mater. 2015, 285, 69–76. [Google Scholar] [CrossRef]
- Grafias, P.; Xekoukoulotakis, N.P.; Mantzavinos, D.; Diamantopoulos, E. Pilot treatment of olive pomace leachate by vertical-flow constructed wetland and electrochemical oxidation: An efficient hybrid process. Water Res. 2010, 44, 2773–2780. [Google Scholar] [CrossRef]
- Mavros, M.; Xekoukoulotakis, P.N.; Mantzavinos, D.; Diamantopoulos, E. Complete Treatment of olive pomace leachate by coagulation, activated carbon adsorption and electrochemical oxidation. Water Res. 2008, 42, 2883–2888. [Google Scholar] [CrossRef]
- Fernandez-Bolanos, J.; Rodriguez, G.; Rodriguez, R.; Heredia, A.; Guillen, R.; Jimenez, A. Production in large quantities of highly purifies hydroxytyrosol from liquid-solid waste of two-phase olive oil processing or ‘Alperujo’. J. Agric. Food Chem. 2002, 50, 6804–6811. [Google Scholar] [CrossRef] [PubMed]
- Araujo, M.; Pimentel, F.B.; Alves, R.C.; Beatriz, M.; Oliveira, P.P. Phenolic compound from olive mill wastes: Health effects, analytical approach and application as food antioxidants. Trends Food Sci. Technol. 2015, 45, 200–211. [Google Scholar] [CrossRef]
- Obied, H.K.; Allen, M.S.; Bedgood, D.R., Jr.; Prenzler, P.D.; Robards, K. Investigation of Australian olive mill waste for recovery of biophenols. J. Agric. Food Chem. 2005, 53, 9911–9920. [Google Scholar] [CrossRef] [PubMed]
- Obied, H.K.; Bedgood, D.R., Jr.; Prenzler, P.D.; Robards, K. Bioscreening of Australian olive mill waste extracts: Biophenol content, antioxidant, antimi-Crobial and molluscicidal activities. Food Chem. Toxicol. 2007, 45, 1238–1248. [Google Scholar] [CrossRef] [PubMed]
- Perez-Serradilla, J.A.; Japon-Lujan, R.; Luque de Castro, M.D. Static-dynamic sequential superheated liquid extraction of phenols and fatty acids from alperujo. Anal. Bioanal. Chem. 2008, 392, 1241–1248. [Google Scholar] [CrossRef] [PubMed]
- Cioffi, G.; Pesca, M.S.; De Caprariis, P.; Braca, A.; Severino, L.; De Tommasi, N. Phenolic compounds in olive oil and olive pomace from Cilento (Cam-pania, Italy) and their antioxidant activity. Food Chem. 2010, 121, 105–111. [Google Scholar] [CrossRef]
- Alu’datt, M.H.; Alli, I.; Ereifej, K.; Alhamad, M.; Al-Tawaha, A.R.; Rababah, T. Optimisation, characterisation and quantification of phenolic compounds in οlive cake. Food Chem. 2010, 123, 117–122. [Google Scholar] [CrossRef]
- Japon-Lujan, R.; Luque de Castro, M.D. Static-dynamic superheated liquid extraction of hydroxytyrosol and other biophenols from alperujo (a semisolid residue of the olive oil industry). J. Agric. Food Chem. 2007, 55, 3629–3634. [Google Scholar] [CrossRef] [PubMed]
- Lafka, T.-I.; Lazou, A.E.; Sinanoglou, V.J.; Lazos, E.S. Phenolic and anti-oxidant potential of olive oil mill wastes. Food Chem. 2011, 125, 92–98. [Google Scholar] [CrossRef]
- Sabatini, Ν. Recent patents in olive oil industry: New technologies for the recovery of phenols compounds from olive oil, olive oil waste byproducts and wastewaters. Recent Pat. Food Nutr. Agric. 2010, 2, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Agalias, A.; Magiatis, P.; Skaltsounis, A.L.; Mikros, E.; Tsarbopoulos, A.; Gikas, E.; Spanos, I.; Manios, T. A new process for the management of olive mill waste water and recovery of natural antioxidants. J. Agric. Food Chem. 2007, 55, 2671–2676. [Google Scholar] [CrossRef] [PubMed]
- Pizzichini, M.; Russo, C. Process for Recovering the Component of Olive Mill Wastewater with Membrane Technologies. WIPO Patent WO2005123603 A1, 29 December 2005. [Google Scholar]
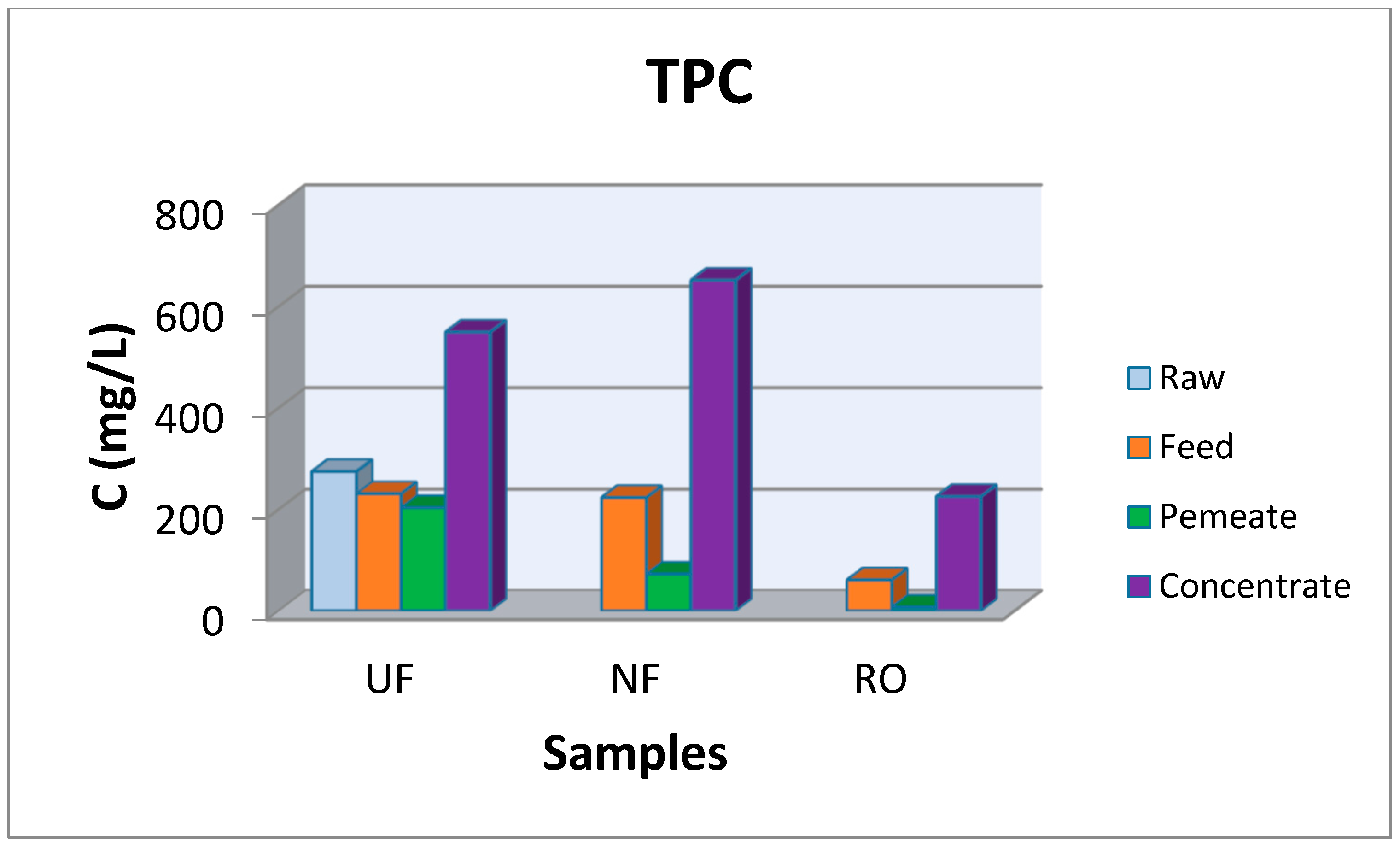
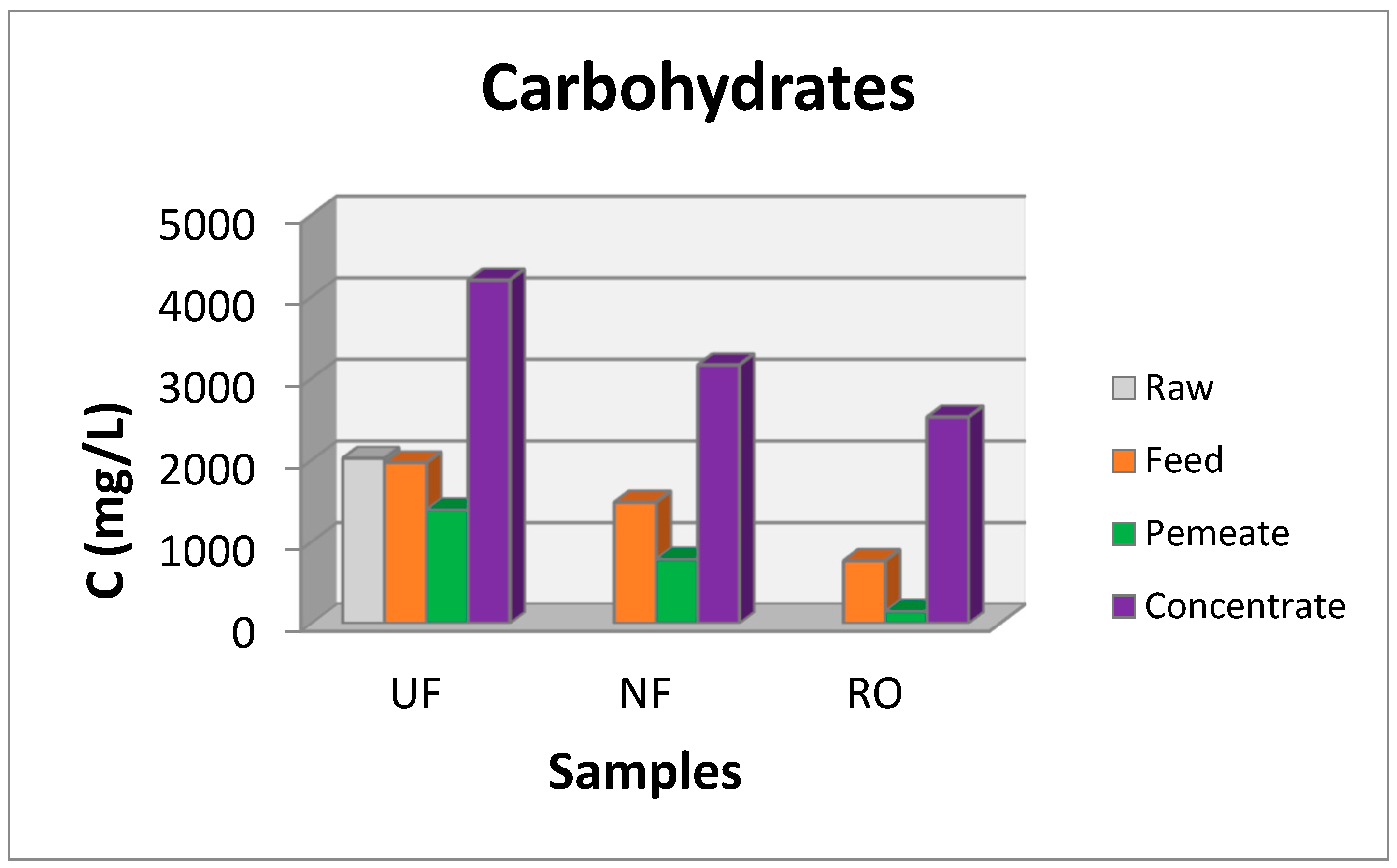

| Parameter | Maximum TPC Concentration (mg/L) | Maximum Carbohydrate Concentration (mg/L) | ||
|---|---|---|---|---|
| Solvent Type Conditions | 100% H2O | 50% E (95%), 50%W | 100% H2O | 50% E (95%), 50% W |
| Temperature, 20 °C | 471.0 | 1020.3 | 2060.0 | 1373.3 |
| Rate, 100 rpm | 735.5 | 1008.3 | 1516.7 | 1603.3 |
| Duration, 60 min | 735.5 | 956.3 | 1516.7 | 1708.3 |
| HCl, Ml | 678.0 | 892.5 | 1436.0 | 1605.3 |
| Average Concentration | 655.0 | 969.3 | 1632.3 | 1572.6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sygouni, V.; Pantziaros, A.G.; Iakovides, I.C.; Sfetsa, E.; Bogdou, P.I.; Christoforou, E.A.; Paraskeva, C.A. Treatment of Two-Phase Olive Mill Wastewater and Recovery of Phenolic Compounds Using Membrane Technology. Membranes 2019, 9, 27. https://doi.org/10.3390/membranes9020027
Sygouni V, Pantziaros AG, Iakovides IC, Sfetsa E, Bogdou PI, Christoforou EA, Paraskeva CA. Treatment of Two-Phase Olive Mill Wastewater and Recovery of Phenolic Compounds Using Membrane Technology. Membranes. 2019; 9(2):27. https://doi.org/10.3390/membranes9020027
Chicago/Turabian StyleSygouni, Varvara, Alexis G. Pantziaros, Iakovos C. Iakovides, Evangelia Sfetsa, Polychronia I. Bogdou, Emilia A. Christoforou, and Christakis A. Paraskeva. 2019. "Treatment of Two-Phase Olive Mill Wastewater and Recovery of Phenolic Compounds Using Membrane Technology" Membranes 9, no. 2: 27. https://doi.org/10.3390/membranes9020027
APA StyleSygouni, V., Pantziaros, A. G., Iakovides, I. C., Sfetsa, E., Bogdou, P. I., Christoforou, E. A., & Paraskeva, C. A. (2019). Treatment of Two-Phase Olive Mill Wastewater and Recovery of Phenolic Compounds Using Membrane Technology. Membranes, 9(2), 27. https://doi.org/10.3390/membranes9020027






