Synthesis and Characterisation of ETS-10/Acetate-based Ionic Liquid/Chitosan Mixed Matrix Membranes for CO2/N2 Permeation
Abstract
:1. Introduction
| Name | Molecular Formula | Properties |
|---|---|---|
| 1-Ethyl-3-methylimidazolium acetate, [emim][Ac] (IL) | 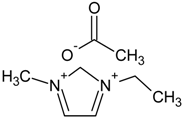 | Density = 1.03 g/cm3 [23] |
| Chitosan (CS) | 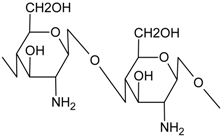 | Density = 0.942 g/cm3 [27]
ΔHm (J·g−1) = 334.4 [28] |
| ETS-10 |  | Density = 1.75 g/cm3 [29] |
2. Results and Discussion
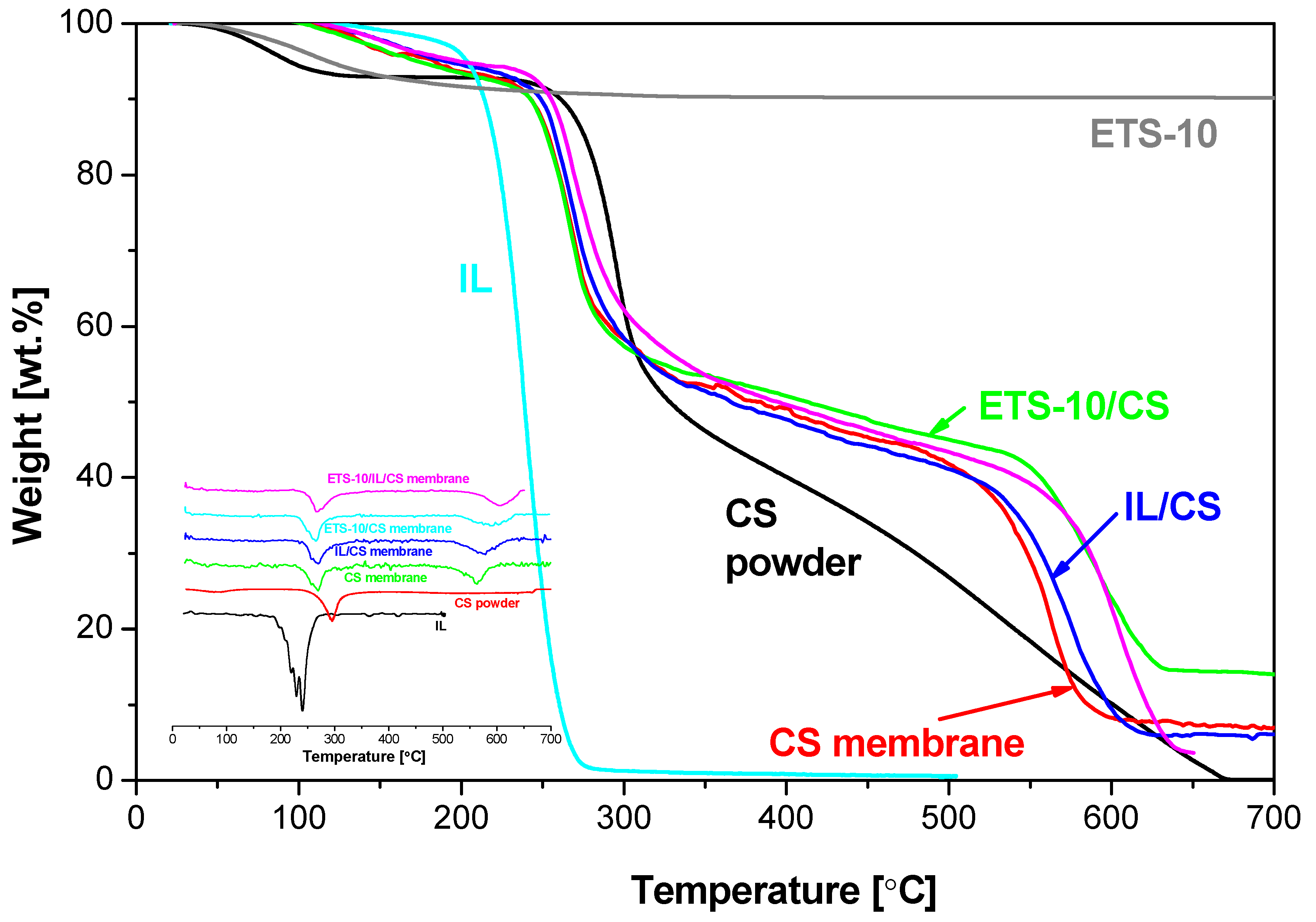
2.1. Thermal Properties

2.2. Mechanical Properties
| Membrane Material Composition | Thickness
(μm) | Filler Content
(wt %) | Tensile Strength
(MPa) | Elongation at Break (%) |
|---|---|---|---|---|
| CS | 121.9 ± 3.96 | 0 | 31.63 ± 7.41 | 18.52 ± 8.23 |
| IL/CS | 128.0 ± 3.57 | 5 | 16.09 ± 11.04 | 40.44 ± 12.45 |
| ETS-10/CS | 130.0 ± 4.50 | 5 | 24.30 ± 4.88 | 14.40 ± 9.38 |
| ETS-10/IL/CS | 168.0 ± 5.0 | 5 (each) | 19.93 ± 5.01 | 36.15 ± 3.03 |
2.3. Structural and Morphological Properties
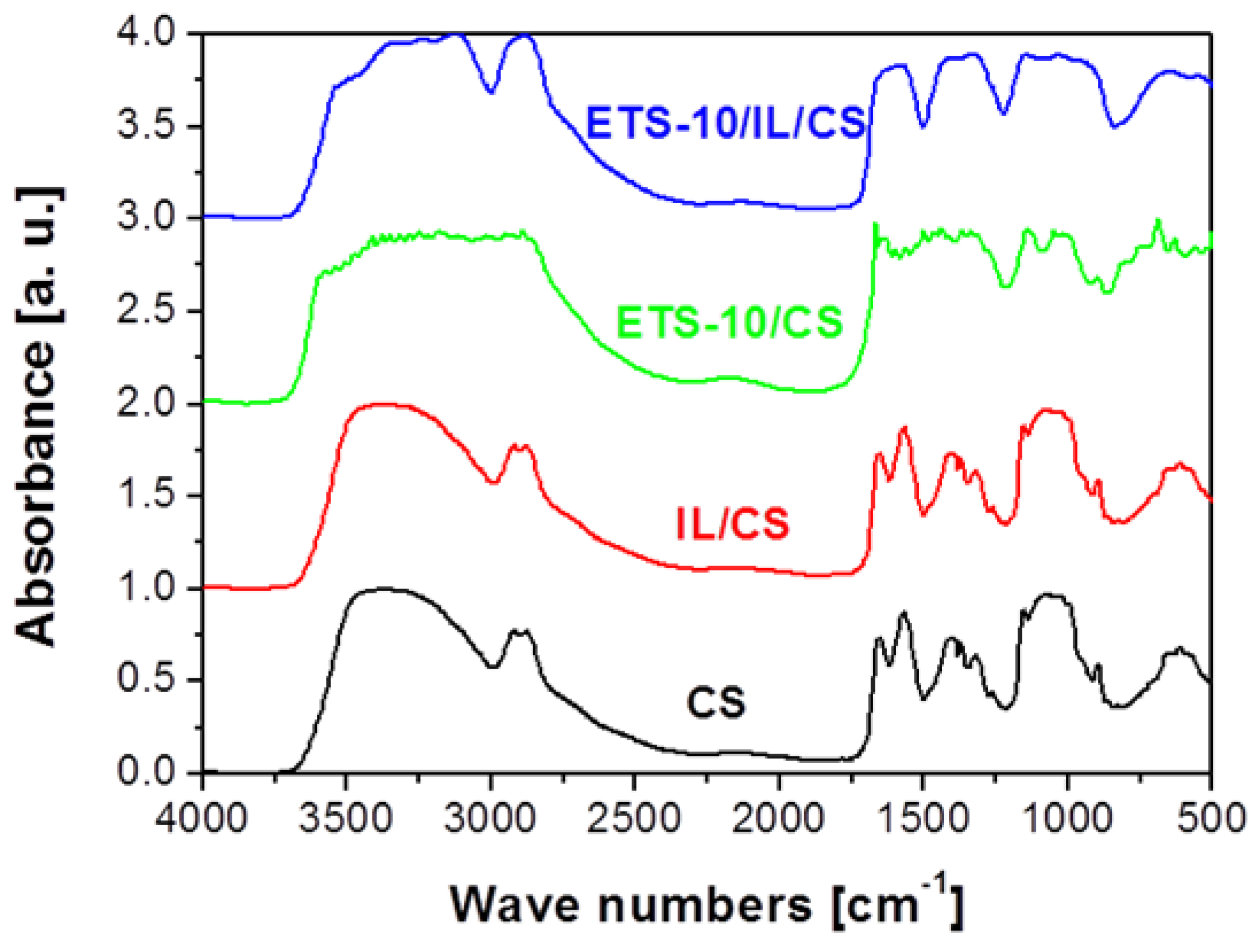
| Membrane materials | ρadd (g/cm3) | ρm (g/cm3) | ϕv (vol/vol) | ϕd (vol/vol) | χ (-) |
|---|---|---|---|---|---|
| CS | 0.942 | 0.727 ± 0.26 | 0.228 | 0.520 | 0.14 |
| IL/CS | 0.941 | 1.108 ± 0.48 | −0.165 | 0.032 | 0.18 |
| ETS-10/CS | 1.014 | 0.637 ± 0.14 | 0.372 | 0.028 | 0.28 |
| ETS-10/IL/CS | 1.442 | 0.714 ± 0.14 | 0.505 | 0.304 | 0.34 |

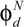 , is described as:
, is described as:



| Membrane material | S(CO2),
cm3 (STP)/cm3 cmHg | S(N2),
cm3 (STP)/cm3 cmHg | Solubility Selectivity (CO2/N2) |
|---|---|---|---|
| IL | 0.0114 | - a | - a |
| ETS-10 | 0.1019 | - a | - a |
| CS | 0.0767 | 0.0050 | 15.34 |
| IL/CS | 0.5065 | 0.0255 | 19.84 |
| ETS-10/CS | 0.0798 | 0.0029 | 27.58 |
| ETS-10/IL/CS | 0.0724 | 0.0019 | 38.48 |
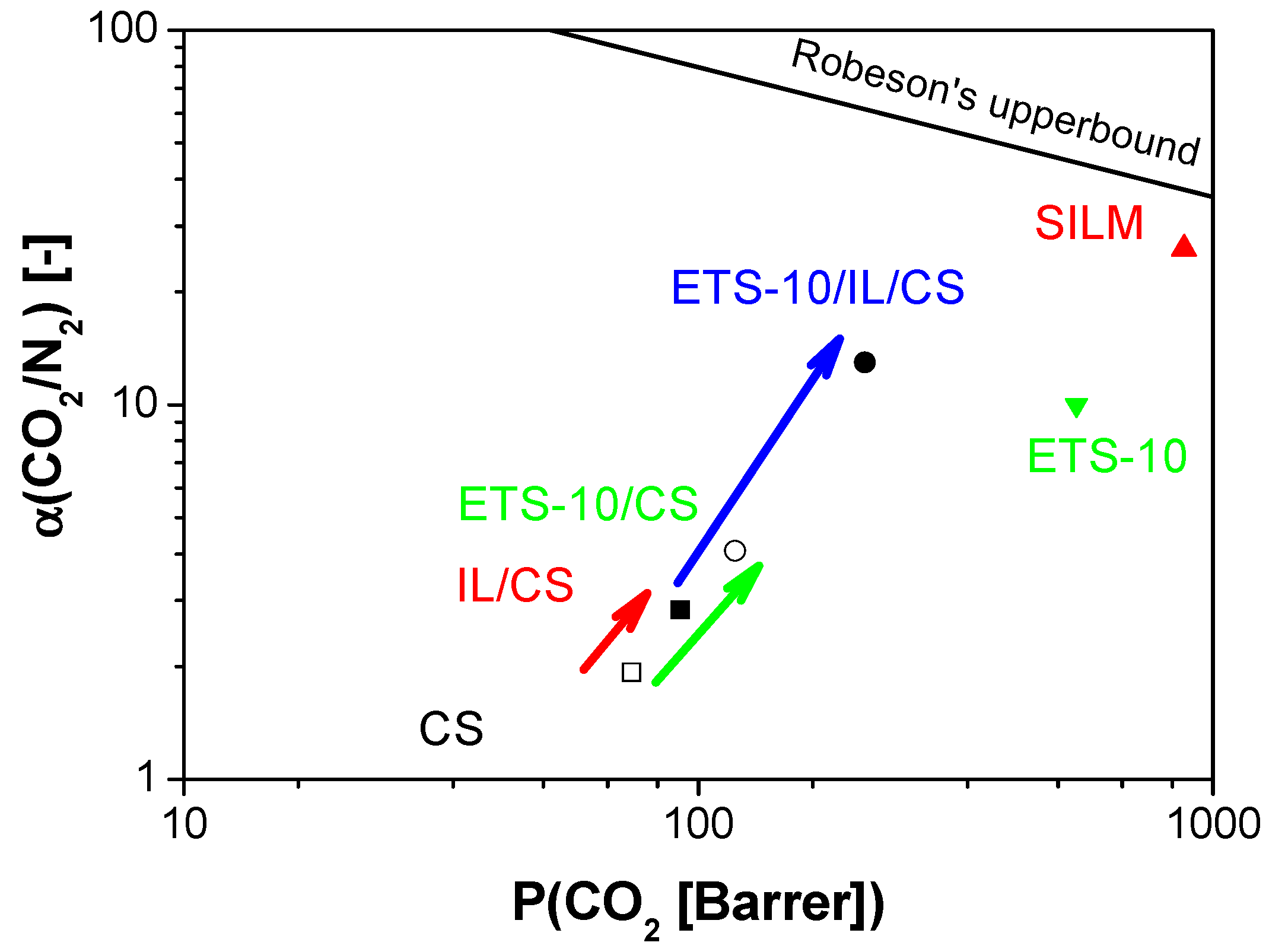
3. Experimental Section
3.1. Membrane Preparation
3.2. Characterization Methods
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Favre, E. Carbon dioxide recovery from post-combustion processes: Can gas permeation membranes compete with absorption? J. Membr. Sci. 2007, 294, 50–59. [Google Scholar] [CrossRef]
- Merkel, T.C.; Lin, H.; Wei, X.; Baker, R. Power plant post-combustion carbon dioxide capture: An opportunity for membranes. J. Membr. Sci. 2010, 359, 126–139. [Google Scholar] [CrossRef]
- Gascón, J.; Kapteijn, F.; Zornoza, B.; Sebastián, V.; Casado, C.; Coronas, J. Practical approach to zeolitic membranes and coatings: State of the art, opportunities, barriers and future perspectives. Chem. Mater. 2012, 24, 2829–2844. [Google Scholar] [CrossRef]
- Pera-Titus, M. Porous Inorganic Membranes for CO2 Capture: Present and Prospects. Chem. Rev. 2014, 114, 1413–1492. [Google Scholar] [CrossRef]
- Bernal, M.P.; Coronas, J.; Menendez, M.; Santamaría, J. On the effect of morphological features on the properties of MFI zeolite membranes. Micropor. Mesopor. Mater. 2003, 60, 99–110. [Google Scholar] [CrossRef]
- Jiang, L.Y.; Chung, T.S.; Kulprathipanja, S. Fabrication of mixed matrix hollow fibers with intimate polymer-zeolite interface for gas separation. AIChE J. 2006, 52, 2898–2908. [Google Scholar] [CrossRef]
- Hamad, F.; Khulbe, K.C.; Matsuura, T. Comparison of gas separation performance and morphology of homogeneous and composite PPO membranes. J. Membr. Sci. 2005, 256, 29–37. [Google Scholar]
- Robeson, L.M. The upper bound revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Freeman, B.D. Basis of permeability/selectivity trade-off relations in polymeric gas separation membranes. Macromolecules 1999, 32, 375–380. [Google Scholar] [CrossRef]
- Hudiono, Y.C.; Carlisle, T.K.; Bara, J.E.; Zhang, Y.; Gin, D.L.; Noble, R.D. A three-component mixed-matrix membrane with enhanced CO2 separation properties based on zeolites and ionic liquid materials. J. Membr. Sci. 2010, 350, 117–123. [Google Scholar] [CrossRef]
- Hao, L.; Li, P.; Yang, T.; Chung, T.-S. Room temperature ionic liquid/ZIF-8 mixed-matrix membranes for natural gas sweetening and post-combustion CO2 capture. J. Membr. Sci. 2013, 436, 221–231. [Google Scholar] [CrossRef]
- Liu, L.; Chakma, A.; Feng, X. Gas permeation through water-swollen hydrogel membranes. J. Membr. Sci. 2008, 310, 66–75. [Google Scholar] [CrossRef]
- Ito, A.; Sato, M.; Anma, T. Permeability of CO2 through chitosan membrane swollen by water vapor in feed gas. Angew. Makromol. Chem. 1997, 248, 85–94. [Google Scholar] [CrossRef]
- El-Azzami, L.A.; Grulke, E.A. Carbon dioxide separation from hydrogen and nitrogen by fixed facilitated transport in swollen chitosan membranes. J. Membr. Sci. 2008, 323, 225–234. [Google Scholar] [CrossRef]
- El-Azzami, L.A.; Grulke, E.A. Parametric study of CO fixed carrier facilitated transport through swollen chitosan membranes. Ind. Eng. Chem. Res. 2009, 48, 894–902. [Google Scholar] [CrossRef]
- Kai, T.; Kouketsu, T.; Duan, S.; Kazama, S.; Yamada, K. Development of commercial-sized dendrimer composite membrane modules for CO2 removal from flue gas. Sep. Purif. Technol. 2008, 63, 524–530. [Google Scholar] [CrossRef]
- Xiao, S.; Feng, X.; Huang, R.Y.M. Trimesoyl chloride crosslinked chitosan membranes for CO2/N2 separation and pervaporation dehydration of isopropanol. J. Membr. Sci. 2007, 306, 36–46. [Google Scholar] [CrossRef]
- Casado-Coterillo, C.; Andrés, F.; Téllez, C.; Coronas, J.; Irabien, A. Synthesis and characterization of ETS-10/chitosan nanocomposite materials for pervaporation. Sep. Sci. Technol. 2014. [Google Scholar] [CrossRef]
- Casado, C.; Amghouz, Z.; García, J.R.; Boulahya, K.; González-Calbet, J.M.; Téllez, C.; Coronas, J. Synthesis and characterization of microporous titanosilicate ETS-10 obtained with different Ti sources. Mater. Res. Bull. 2009, 44, 1225–1231. [Google Scholar] [CrossRef]
- Tiscornia, I.; Irusta, S.; Prádanos, P.; Téllez, C.; Coronas, J.; Santamaría, J. Preparation and characterization of titanosilicate Ag-ETS-10 for propylene and propane adsorption. J. Phys. Chem. C 2007, 111, 4702–4709. [Google Scholar] [CrossRef]
- Tiscornia, I.; Kumakiri, I.; Bredesen, R.; Téllez, C.; Coronas, J. Microporous titanosilicate ETS-10 membrane for high pressure CO2 separation. Sep. Purif. Technol. 2010, 73, 8–12. [Google Scholar] [CrossRef]
- Santos, E.; Albo, J.; Irabien, A. Acetate based supported ionic liquid membranes (SILMs) for CO2 separation: Influence of the temperature. J. Membr. Sci. 2014, 452, 277–283. [Google Scholar] [CrossRef]
- Blath, J.; Deubler, N.; Hirth, T.; Schiestel, T. Chemisorption of carbon dioxide in imidazolium based ionic liquids with carboxylic anions. Chem. Eng. J. 2012, 181–182, 152–158. [Google Scholar]
- Alvarez-Guerra, M.; Irabien, A. Design of ionic liquids: An ecotoxicity (Vibrio fischeri) discrimination approach. Green Chem. 2011, 13, 1507–1516. [Google Scholar] [CrossRef]
- Ding, Z.-D.; Chi, Z.; Gu, W.-X.; Gu, S.-M.; Liu, J.-H.; Wang, H.-J. Theoretical and experimental investigation on dissolution and regeneration of cellulose in ionic liquid. Carbohydr. Polym. 2012, 89, 7–16. [Google Scholar] [CrossRef]
- Xie, H.; Zhang, S.; Li, S. Chitin and chitosan dissolved in ionic liquids as reversible sorbents of CO2. Green Chem. 2006, 8, 630–633. [Google Scholar] [CrossRef]
- Mi, F.-L.; Shyu, S.-S.; Wu, Y.-B.; Lee, S.-T.; Shyong, J.-Y.; Huang, R.-N. Fabrication and characterization of a sponge-like asymmetric chitosan membrane as a wound dresssing. Biomaterials 2001, 22, 165–173. [Google Scholar] [CrossRef]
- Lee, S.B.; Ha, D.I.; Cho, S.K.; Kim, S.J.; Lee, Y.M. Temperature/pH-sensitive comb-type graft hydrogels composed of chitosan and poly(N-isopropylacrylamide). J. Appl. Polym. Sci. 2004, 92, 2612–2620. [Google Scholar] [CrossRef]
- Ji, Z.; Warzywoda, J., Jr. Competitive nucleation and growth in seeded batch crystallization of titanosilicate ETS-10 using Ti(SO4)2. Micropor. Mesopor. Mater. 2005, 81, 201–210. [Google Scholar] [CrossRef]
- Balau, L.; Lisa, G.; Popa, M.I.; Tura, V.; Melnig, V. Physico-chemical properties of chitosan films. Cent. Eur. J. Chem. 2004, 2, 638–647. [Google Scholar] [CrossRef]
- Zuo, G.; Wan, Y.; Wang, L.; Liu, C.; He, F.; Luo, H. Synthesis and characterization of laminated hydroxyapatite/chitosan nanocomposites. Mater. Lett. 2010, 64, 2126–2128. [Google Scholar] [CrossRef]
- Bernardo, P.; Jansen, J.C.; Bazzarelli, F.; Tasselli, F.; Fuoco, A. Gas transport properties of Pebax(R)/room temperature ionic liquid gel membranes. Sep. Purif. Technol. 2012, 97, 73–82. [Google Scholar] [CrossRef]
- Xu, D.; Loo, L.S.; Wang, K. Characterization and diffusion behavior of chitosan-POSS composite membranes. J. Appl. Polym. Sci. 2011, 122, 427–435. [Google Scholar] [CrossRef]
- Staroszczyk, H.; Sztuka, K.; Wolska, J.; Wojtasz-Pajak, A.; Kolodziejska, I. Interactions of fish gelatin and chitosan in uncrosslinked and crosslinked with EDC films: FTIR study. Spectrochim. Acta A 2014, 117, 707–712. [Google Scholar] [CrossRef]
- Shen, Y.; Lua, A.C. Preparation and characterization of mixed matrix membranes based on PVDF and three inorganic fillers (fumed nonporous silica, zeolite 4A and mesoporous MCM-41) for gas separation. Chem. Eng. J. 2012, 192, 201–210. [Google Scholar] [CrossRef]
- Jeazet, H.B.T.; Koschine, T.; Staudt, C.; Raetzke, K.; Janiak, C. Correlation of gas permeability in a metal-organic framework MIL-101(Cr)-polysulfone mixed-matrix membrane with free volume measurements by positron annihilation lifetime spectroscopy (PALS). Membranes 2013, 3, 331–353. [Google Scholar] [CrossRef]
- Rocha, J.; Anderson, M.W. Microporous titanosilicates and other novel mixed octahedral-tetrahedral framework oxides. Eur. J. Inorg. Chem. 2000, 2000, 801–818. [Google Scholar] [CrossRef]
- El-Azzami, L.A.; Grulke, E.A. Dual mode model for mixed gas permeation of CO2, H2, and N2 through a dry chitosan membrane. J. Polym. Sci. B 2007, 45, 2620–2631. [Google Scholar] [CrossRef]
- Mayoral, Á.; Coronas, J.; Casado, C.; Téllez, C.; Díaz, I. Atomic resolution analysis of microporous titanosilicate ETS-10 through aberration corrected STEM imaging. ChemCatChem 2013, 5, 2595–2598. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Casado-Coterillo, C.; Del Mar López-Guerrero, M.; Irabien, Á. Synthesis and Characterisation of ETS-10/Acetate-based Ionic Liquid/Chitosan Mixed Matrix Membranes for CO2/N2 Permeation. Membranes 2014, 4, 287-301. https://doi.org/10.3390/membranes4020287
Casado-Coterillo C, Del Mar López-Guerrero M, Irabien Á. Synthesis and Characterisation of ETS-10/Acetate-based Ionic Liquid/Chitosan Mixed Matrix Membranes for CO2/N2 Permeation. Membranes. 2014; 4(2):287-301. https://doi.org/10.3390/membranes4020287
Chicago/Turabian StyleCasado-Coterillo, Clara, María Del Mar López-Guerrero, and Ángel Irabien. 2014. "Synthesis and Characterisation of ETS-10/Acetate-based Ionic Liquid/Chitosan Mixed Matrix Membranes for CO2/N2 Permeation" Membranes 4, no. 2: 287-301. https://doi.org/10.3390/membranes4020287
APA StyleCasado-Coterillo, C., Del Mar López-Guerrero, M., & Irabien, Á. (2014). Synthesis and Characterisation of ETS-10/Acetate-based Ionic Liquid/Chitosan Mixed Matrix Membranes for CO2/N2 Permeation. Membranes, 4(2), 287-301. https://doi.org/10.3390/membranes4020287







