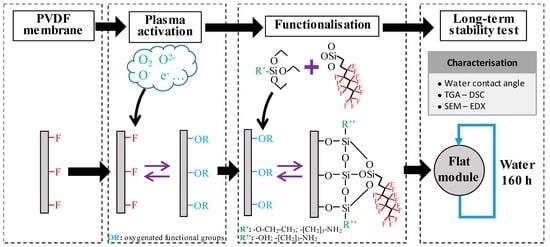
Stability of Superhydrophobicity and Structure of PVDF Membranes Treated by Vacuum Oxygen Plasma and Organofluorosilanisation
Abstract
1. Introduction
2. Materials and Methods
2.1. Membrane Surface Functionalisation Procedure
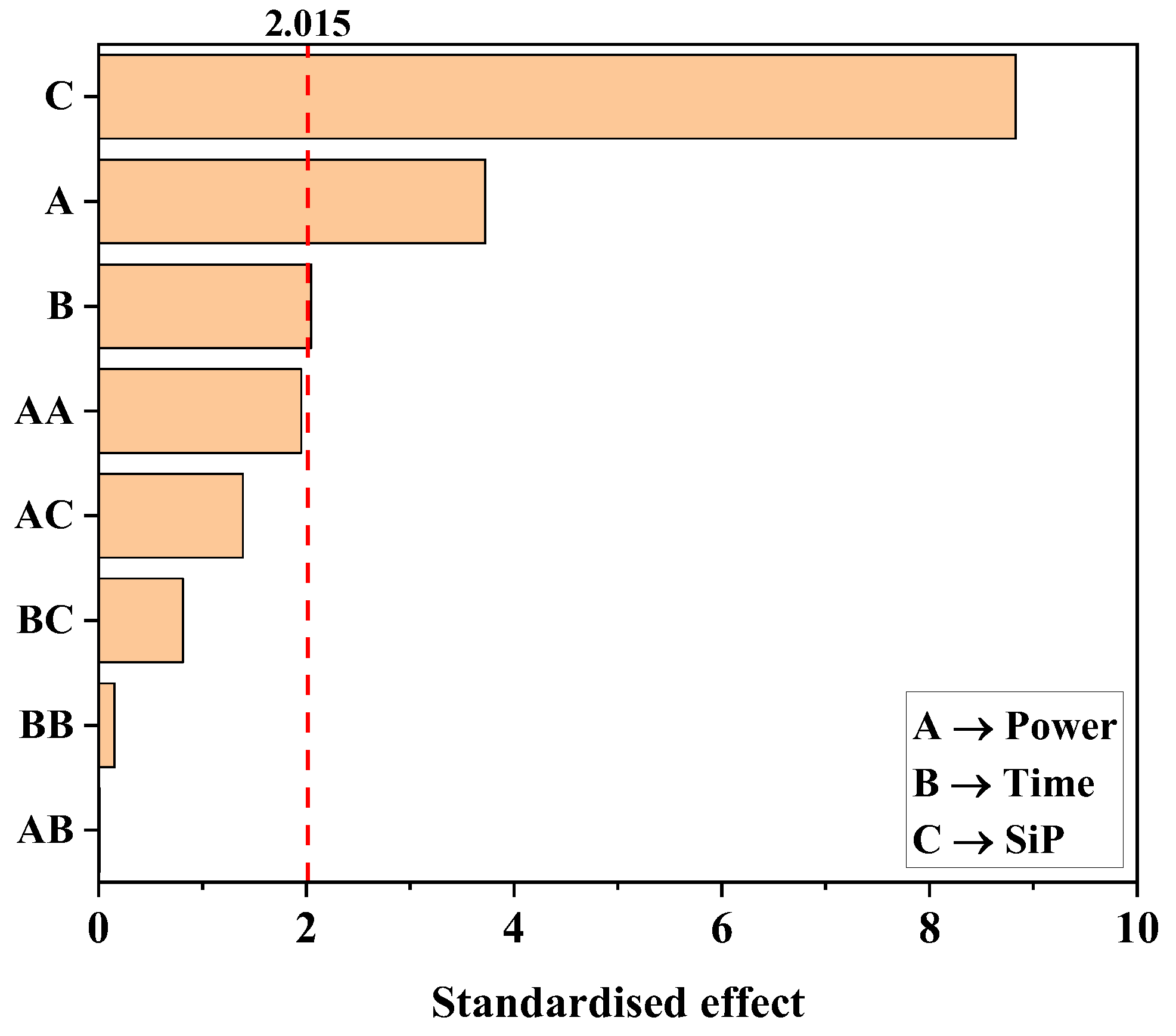
2.2. Design of Experiments and Statistical Analysis
2.3. Evaluation of Membrane Stability
2.3.1. Long-Term Stability Tests
2.3.2. Thermal Analysis
2.4. Contact Angle Measurements
2.5. Membrane Morphology and Chemical Composition
3. Results and Discussion
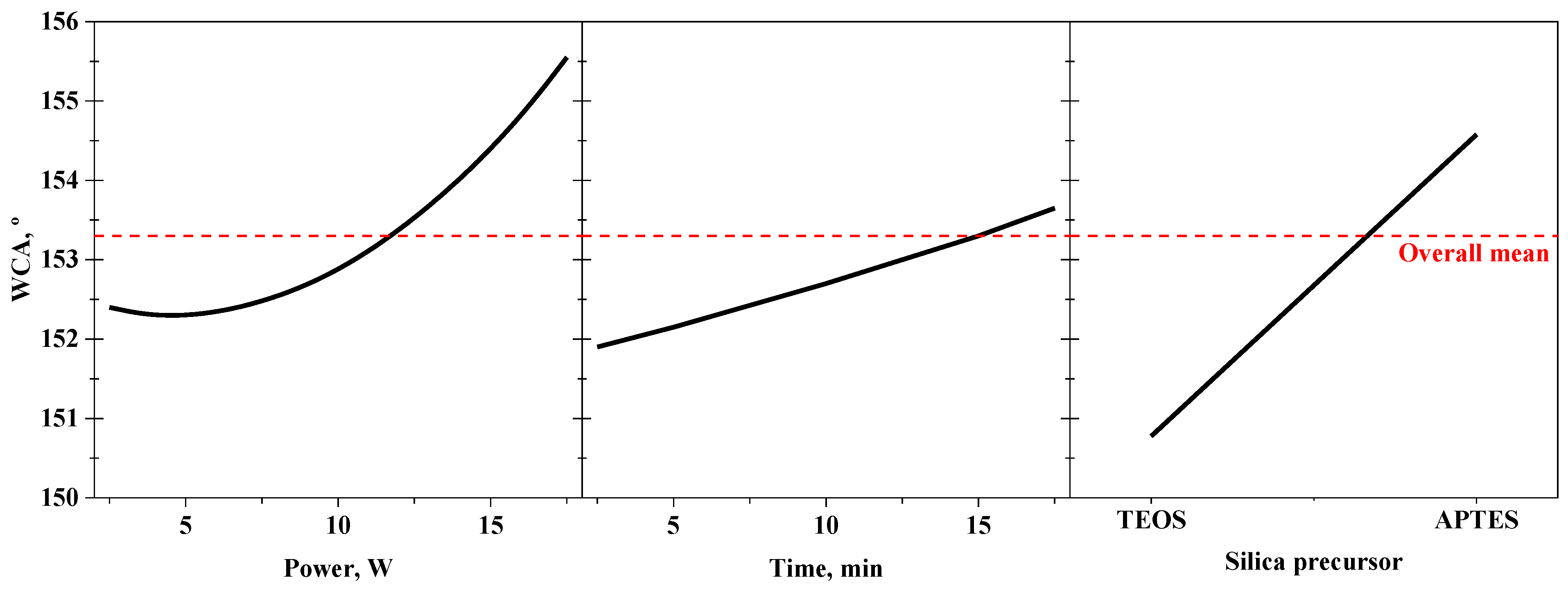
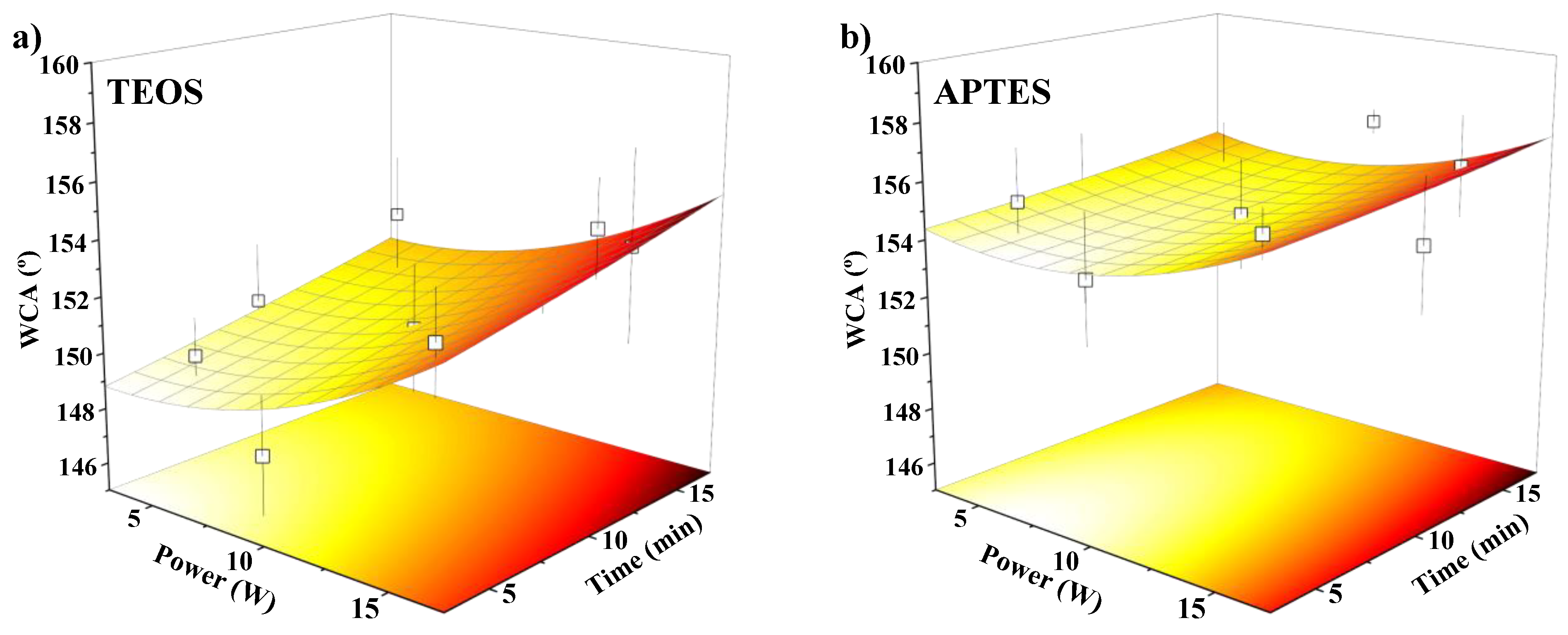
3.1. Effect of the Oxygen Plasma Activation and Organofluorosilanisation on Membrane Hydrophobicity
3.2. Membrane Stability Tests in Long-Term Operation
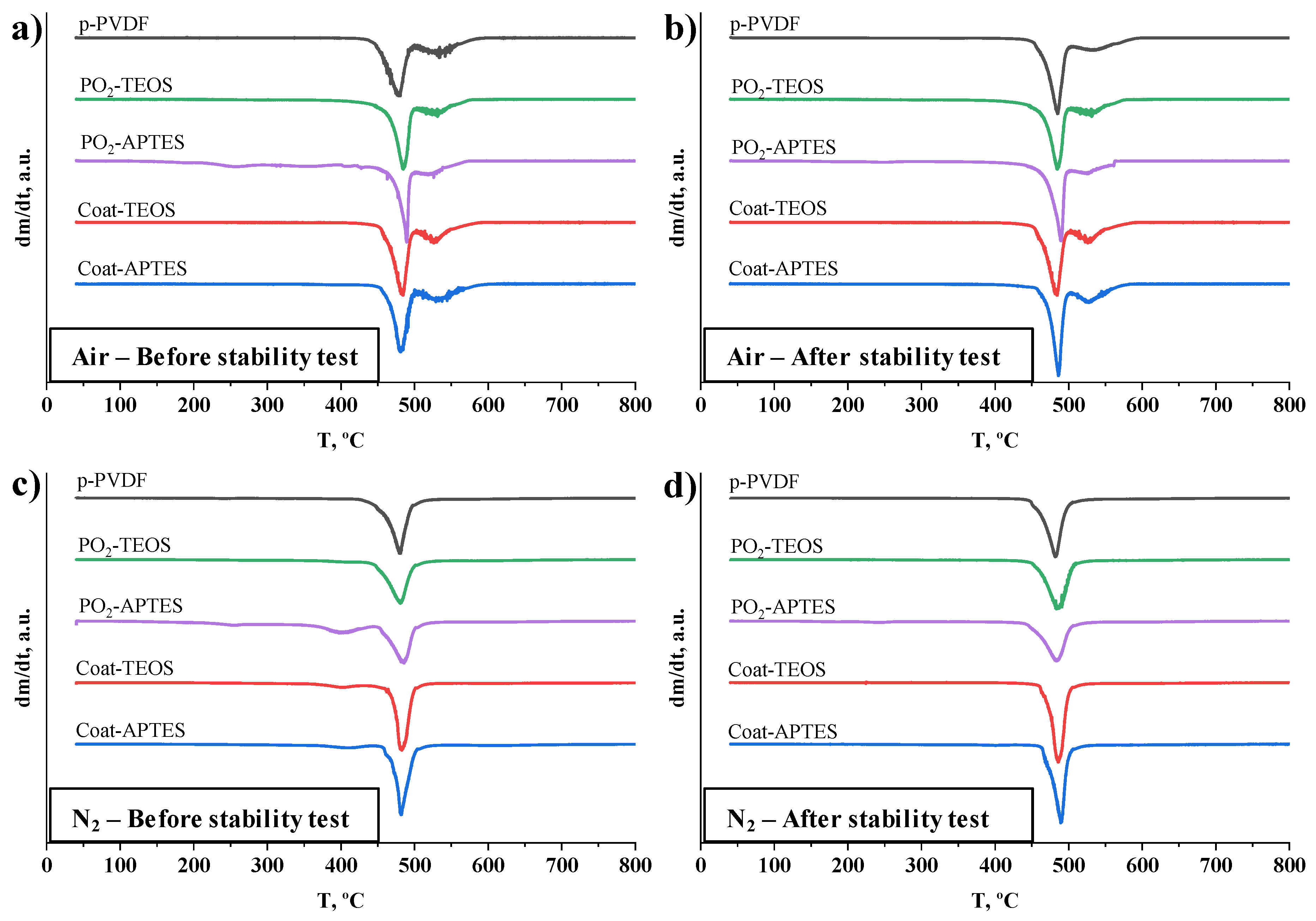
3.2.1. Stability of the Bulk Properties after Operation
3.2.2. Stability of the Surface Hydrophobicity in Operation
3.3. Structure and Chemical Composition of the Modified Membranes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stazi, V.; Tomei, M.C. Dissolved methane in anaerobic effluents: A review on sustainable strategies for optimization of energy recovery or internal process reuse. J. Clean. Prod. 2021, 317, 128359. [Google Scholar] [CrossRef]
- Lee, Y.; Yun, K.H.; Sethunga, D.; Bae, T.H. Membrane contactors for maximizing biomethane recovery in anaerobic wastewater treatments: Recent efforts and future prospect. Appl. Sci. 2021, 11, 1372. [Google Scholar] [CrossRef]
- Yang, X.; Wang, R.; Shi, L.; Fane, A.G.; Debowski, M. Performance improvement of PVDF hollow fiber-based membrane distillation process. J. Memb. Sci. 2011, 369, 437–447. [Google Scholar] [CrossRef]
- Jeong, S.; Shin, B.; Jo, W.; Kim, H.Y.; Moon, M.W.; Lee, S. Nanostructured PVDF membrane for MD application by an O2 and CF4 plasma treatment. Desalination 2016, 399, 178–184. [Google Scholar] [CrossRef]
- Woo, Y.C.; Chen, Y.; Tijing, L.D.; Phuntsho, S.; He, T.; Choi, J.S.; Kim, S.H.; Shon, H.K. CF4 plasma-modified omniphobic electrospun nanofiber membrane for produced water brine treatment by membrane distillation. J. Memb. Sci. 2017, 529, 234–242. [Google Scholar] [CrossRef]
- Klaassen, R.; Feron, P.H.M.; Jansen, A.E. Membrane contactors in industrial applications. Chem. Eng. Res. Des. 2005, 83, 234–246. [Google Scholar] [CrossRef]
- Zhang, N.; Pan, Z.; Zhang, Z.; Zhang, W.; Zhang, L.; Baena-Moreno, F.M.; Lichtfouse, E. CO2 capture from coalbed methane using membranes: A review. Environ. Chem. Lett. 2020, 18, 79–96. [Google Scholar] [CrossRef]
- Zhu, H.; Li, X.; Pan, Y.; Liu, G.; Wu, H.; Jiang, M.; Jin, W. Fluorinated PDMS membrane with anti-biofouling property for in-situ biobutanol recovery from fermentation-pervaporation coupled process. J. Memb. Sci. 2020, 609, 118225. [Google Scholar] [CrossRef]
- Henares, M.; Izquierdo, M.; Penya-Roja, J.M.; Martínez-Soria, V. Comparative study of degassing membrane modules for the removal of methane from Expanded Granular Sludge Bed anaerobic reactor effluent. Sep. Purif. Technol. 2016, 170, 22–29. [Google Scholar] [CrossRef]
- Jiménez-Robles, R.; Martínez-Soria, V.; Izquierdo, M. Fouling characterization in PVDF membrane contactors for dissolved methane recovery from anaerobic effluents: Effect of surface organofluorosilanisation. Environ. Sci. Pollut. Res. 2022. [Google Scholar] [CrossRef]
- Pan, J.; Zhang, F.; Wang, Z.; Sun, S.P.; Cui, Z.; Jin, W.; Bamaga, O.; Abulkhair, H.; Albeirutty, M.; Drioli, E. Enhanced anti-wetting and anti-fouling properties of composite PFPE/PVDF membrane in vacuum membrane distillation. Sep. Purif. Technol. 2022, 282, 1–11. [Google Scholar] [CrossRef]
- Liu, L.; Xiao, Z.; Liu, Y.; Li, X.; Yin, H.; Volkov, A.; He, T. Understanding the fouling/scaling resistance of superhydrophobic/omniphobic membranes in membrane distillation. Desalination 2021, 499, 114864. [Google Scholar] [CrossRef]
- Sethunga, G.S.M.D.P.; Karahan, H.E.; Wang, R.; Bae, T.H. Wetting- and fouling-resistant hollow fiber membranes for dissolved methane recovery from anaerobic wastewater treatment effluents. J. Memb. Sci. 2021, 617, 118621. [Google Scholar] [CrossRef]
- Henares, M.; Ferrero, P.; San-Valero, P.; Martínez-Soria, V.; Izquierdo, M. Performance of a polypropylene membrane contactor for the recovery of dissolved methane from anaerobic effluents: Mass transfer evaluation, long-term operation and cleaning strategies. J. Memb. Sci. 2018, 563, 926–937. [Google Scholar] [CrossRef]
- Al-Juboori, R.A.; Yusaf, T. Biofouling in RO system: Mechanisms, monitoring and controlling. Desalination 2012, 302, 1–23. [Google Scholar] [CrossRef]
- Wang, J.; Chen, X.; Reis, R.; Chen, Z.; Milne, N.; Winther-Jensen, B.; Kong, L.; Dumée, L.F. Plasma modification and synthesis of membrane materials—A mechanistic review. Membranes 2018, 8, 56. [Google Scholar] [CrossRef]
- Zhao, X.; Xuan, H.; Qin, A.; Liu, D.; He, C. Improved antifouling property of PVDF ultrafiltration membrane with plasma treated PVDF powder. RSC Adv. 2015, 5, 64526–64533. [Google Scholar] [CrossRef]
- Sethunga, G.S.M.D.P.; Lee, J.; Wang, R.; Bae, T.H. Influence of membrane characteristics and operating parameters on transport properties of dissolved methane in a hollow fiber membrane contactor for biogas recovery from anaerobic effluents. J. Memb. Sci. 2019, 589, 117263. [Google Scholar] [CrossRef]
- Liu, L.; Charlton, L.; Song, Y.; Li, T.; Li, X.; Yin, H.; He, T. Scaling resistance by fluoro-treatments: The importance of wetting states. J. Mater. Chem. A 2022, 10, 3058–3068. [Google Scholar] [CrossRef]
- Jothi Prakash, C.G.; Prasanth, R. Approaches to design a surface with tunable wettability: A review on surface properties. J. Mater. Sci. 2021, 56, 108–135. [Google Scholar] [CrossRef]
- Rana, D.; Matsuura, T. Surface modifications for antifouling membranes. Chem. Rev. 2010, 110, 2448–2471. [Google Scholar] [CrossRef] [PubMed]
- Ebert, D.; Bhushan, B. Transparent, superhydrophobic, and wear-resistant surfaces using deep reactive ion etching on PDMS substrates. J. Colloid Interface Sci. 2016, 481, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wu, Z.; Cen, X.; Liu, Y.; Zhang, Y.; Liu, D.; Chen, Z. Efficient Production of 1,3-Propanediol from Diverse Carbohydrates via a Non-natural Pathway Using 3-Hydroxypropionic Acid as an Intermediate. ACS Synth. Biol. 2021, 10, 478–486. [Google Scholar] [CrossRef]
- Yang, C.; Tian, M.; Xie, Y.; Li, X.M.; Zhao, B.; He, T.; Liu, J. Effective evaporation of CF4 plasma modified PVDF membranes in direct contact membrane distillation. J. Memb. Sci. 2015, 482, 25–32. [Google Scholar] [CrossRef]
- Kaur, S.; Ma, Z.; Gopal, R.; Singh, G.; Ramakrishna, S.; Matsuura, T. Plasma-induced graft copolymerization of poly(methacrylic acid) on electrospun poly(vinylidene fluoride) nanofiber membrane. Langmuir 2007, 23, 13085–13092. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Bui, H.M.; Wang, Y.F.; You, S.J. Non-fluoroalkyl functionalized hydrophobic surface modifications used in membrane distillation for cheaper and more environmentally friendly applications: A mini-review. Sustain. Chem. Pharm. 2022, 28, 100714. [Google Scholar] [CrossRef]
- Kang, G.d.; Cao, Y.M. Application and modification of poly(vinylidene fluoride) (PVDF) membranes—A review. J. Memb. Sci. 2014, 463, 145–165. [Google Scholar] [CrossRef]
- Oldani, V.; Sergi, G.; Pirola, C.; Sacchi, B.; Bianchi, C.L. Sol-gel hybrid coatings containing silica and a perfluoropolyether derivative with high resistance and anti-fouling properties in liquid media. J. Fluor. Chem. 2016, 188, 43–49. [Google Scholar] [CrossRef]
- Taurino, R.; Fabbri, E.; Messori, M.; Pilati, F.; Pospiech, D.; Synytska, A. Facile preparation of superhydrophobic coatings by sol-gel processes. J. Colloid Interface Sci. 2008, 325, 149–156. [Google Scholar] [CrossRef]
- Xie, M.; Luo, W.; Gray, S.R. Surface pattern by nanoimprint for membrane fouling mitigation: Design, performance and mechanisms. Water Res. 2017, 124, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Won, Y.-J.; Lee, J.; Choi, D.-C.; Chae, H.R.; Kim, I.; Lee, C.-H.; Kim, I.-C. Preparation and application of patterned membranes for wastewater treatment. Environ. Sci. Pollut. Res. 2012, 46, 11021–11027. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.; Choi, D.C.; Lee, J.; Chae, H.R.; Hee Jang, J.; Lee, C.H.; Park, P.K.; Won, Y.J. Preparation and application of patterned hollow-fiber membranes to membrane bioreactor for wastewater treatment. J. Memb. Sci. 2015, 490, 190–196. [Google Scholar] [CrossRef]
- Palumbo, F.; Di Mundo, R.; Cappelluti, D.; Dagostino, R. SuperHydrophobic and SuperHydrophilic polycarbonate by tailoring chemistry and nano-texture with plasma processing. Plasma Process. Polym. 2011, 8, 118–126. [Google Scholar] [CrossRef]
- Zhao, S.; Liao, Z.; Fane, A.; Li, J.; Tang, C.; Zheng, C.; Lin, J.; Kong, L. Engineering antifouling reverse osmosis membranes: A review. Desalination 2021, 499, 114857. [Google Scholar] [CrossRef]
- Liu, L.; Shen, F.; Chen, X.; Luo, J.; Su, Y.; Wu, H.; Wan, Y. A novel plasma-induced surface hydrophobization strategy for membrane distillation: Etching, dipping and grafting. J. Memb. Sci. 2016, 499, 544–554. [Google Scholar] [CrossRef]
- Gryta, M. Surface modification of polypropylene membrane by helium plasma treatment for membrane distillation. J. Memb. Sci. 2021, 628, 119265. [Google Scholar] [CrossRef]
- Miller, D.J.; Dreyer, D.R.; Bielawski, C.W.; Paul, D.R.; Freeman, B.D. Surface Modification of Water Purification Membranes. Angew. Chemie—Int. Ed. 2017, 56, 4662–4711. [Google Scholar] [CrossRef]
- Correia, D.M.; Nunes-Pereira, J.; Alikin, D.; Kholkin, A.L.; Carabineiro, S.A.C.; Rebouta, L.; Rodrigues, M.S.; Vaz, F.; Costa, C.M.; Lanceros-Méndez, S. Surface wettability modification of poly(vinylidene fluoride) and copolymer films and membranes by plasma treatment. Polymer 2019, 169, 138–147. [Google Scholar] [CrossRef]
- Sairiam, S.; Loh, C.H.; Wang, R.; Jiraratananon, R. Surface modification of PVDF hollow fiber membrane to enhance hydrophobicity using organosilanes. J. Appl. Polym. Sci. 2013, 130, 610–621. [Google Scholar] [CrossRef]
- Cortese, G.; Martina, F.; Vasapollo, G.; Cingolani, R.; Gigli, G.; Ciccarella, G. Modification of micro-channel filling flow by poly(dimethylsiloxane) surface functionalization with fluorine-Substituted aminonaphthols. J. Fluor. Chem. 2010, 131, 357–363. [Google Scholar] [CrossRef]
- Arima, V.; Bianco, M.; Zacheo, A.; Zizzari, A.; Perrone, E.; Marra, L.; Rinaldi, R. Fluoropolymers coatings on polydimethylsiloxane for retarding swelling in toluene. Thin Solid Films 2012, 520, 2293–2300. [Google Scholar] [CrossRef]
- Han, Y.; Song, S.; Lu, Y.; Zhu, D. A method to modify PVDF microfiltration membrane via ATRP with low-temperature plasma pretreatment. Appl. Surf. Sci. 2016, 379, 474–479. [Google Scholar] [CrossRef]
- Lin, S.H.; Tung, K.L.; Chang, H.W.; Lee, K.R. Influence of fluorocarbon flat-membrane hydrophobicity on carbon dioxide recovery. Chemosphere 2009, 75, 1410–1416. [Google Scholar] [CrossRef]
- Juang, R.S.; Huang, C.; Hsieh, C.L. Surface modification of PVDF ultrafiltration membranes by remote argon/methane gas mixture plasma for fouling reduction. J. Taiwan Inst. Chem. Eng. 2014, 45, 2176–2186. [Google Scholar] [CrossRef]
- Meyyappan, M. Plasma nanotechnology: Past, present and future. J. Phys. D. Appl. Phys. 2011, 44, 174002. [Google Scholar] [CrossRef]
- Plasma Technology, 4th ed.; Diener Electronic GmbH + Co. KG: Ebhausen, Germany, 2011; Available online: http://lampx.tugraz.at/~hadley/semi/ch9/instruments/Diener/Plasmatechnik_eng.pdf (accessed on 5 March 2023).
- Duca, M.D.; Plosceanu, C.L.; Pop, T. Surface modifications of polyvinylidene fluoride (PVDF) under rf Ar plasma. Polym. Degrad. Stab. 1998, 61, 65–72. [Google Scholar] [CrossRef]
- Vesel, A.; Zaplotnik, R.; Primc, G.; Mozetič, M.; Katan, T.; Kargl, R.; Mohan, T.; Kleinschek, K.S. Non-equilibrium plasma methods for tailoring surface properties of polyvinylidene fluoride: Review and challenges. Polymers 2021, 13, 4243. [Google Scholar] [CrossRef]
- Dumée, L.F.; Alglave, H.; Chaffraix, T.; Lin, B.; Magniez, K.; Schütz, J. Morphology-properties relationship of gas plasma treated hydrophobic meso-porous membranes and their improved performance for desalination by membrane distillation. Appl. Surf. Sci. 2016, 363, 273–285. [Google Scholar] [CrossRef]
- Jafari, R.; Asadollahi, S.; Farzaneh, M. Applications of plasma technology in development of superhydrophobic surfaces. Plasma Chem. Plasma Process. 2013, 33, 177–200. [Google Scholar] [CrossRef]
- Huang, C.; Lin, P.J.; Tsai, C.Y.; Juang, R.S. Electrospun microfibrous membranes with atmospheric-pressure plasma surface modification for the application in dye-sensitized solar cells. Plasma Process. Polym. 2013, 10, 938–947. [Google Scholar] [CrossRef]
- Cardinaud, C.; Peignon, M.C.; Tessier, P.Y. Plasma etching: Principles, mechanisms, application to micro- and nano-technologies. Appl. Surf. Sci. 2000, 164, 72–83. [Google Scholar] [CrossRef]
- Park, Y.W.; Inagaki, N. Surface modification of poly (vinylidene fluoride) film by remote Ar, H2, and O2 plasmas. Polymer 2003, 44, 1569–1575. [Google Scholar] [CrossRef]
- Vlachopoulou, M.E.; Tsougeni, K.; Petrou, P.; Kakabakos, S.; Tserepi, A.; Gogolides, E. High-Aspect-Ratio Plasma Induced Nanotexturing of Polymers (PDMS, PMMA, PEEK,...) for protein adsorption applications. In Proceedings of the International Conference on Electron, Ion and Photon Beam Technology and Nanofabrication, Denver, CO, USA, 29 May–1 June 2007. [Google Scholar]
- Bhushan, B.; Hansford, D.; Lee, K.K. Surface modification of silicon and polydimethylsiloxane surfaces with vapor-phase-deposited ultrathin fluorosilane films for biomedical nanodevices. J. Vac. Sci. Technol. A Vacuum Surfaces Film. 2006, 24, 1197–1202. [Google Scholar] [CrossRef]
- Lin, S.-H.; Tung, K.-L.; Chen, W.-J.; Chang, H.-W. Absorption of carbon dioxide by mixed piperazine–alkanolamine absorbent in a plasma-modified polypropylene hollow fiber contactor. J. Memb. Sci. 2009, 333, 30–37. [Google Scholar] [CrossRef]
- Akamatsu, K.; Furue, T.; Han, F.; Nakao, S.I. Plasma graft polymerization to develop low-fouling membranes grafted with poly(2-methoxyethylacrylate). Sep. Purif. Technol. 2013, 102, 157–162. [Google Scholar] [CrossRef]
- Di Mundo, R.; Troia, M.; Palumbo, F.; Trotta, M.; D’Agostino, R. Nano-texturing of transparent polymers with plasma etching: Tailoring topography for a low reflectivity. Plasma Process. Polym. 2012, 9, 947–954. [Google Scholar] [CrossRef]
- Yalcinkaya, F.; Yalcinkaya, B.; Pazourek, A.; Mullerova, J.; Stuchlik, M.; Maryska, J. Surface Modification of Electrospun PVDF/PAN Nanofibrous Layers by Low Vacuum Plasma Treatment. Int. J. Polym. Sci. 2016, 2016, 4671658. [Google Scholar] [CrossRef]
- Xu, W.T.; Zhao, Z.P.; Liu, M.; Chen, K.C. Morphological and hydrophobic modifications of PVDF flat membrane with silane coupling agent grafting via plasma flow for VMD of ethanol-water mixture. J. Memb. Sci. 2015, 491, 110–120. [Google Scholar] [CrossRef]
- Liang, S.; Kang, Y.; Tiraferri, A.; Giannelis, E.P.; Huang, X.; Elimelech, M. Highly hydrophilic polyvinylidene fluoride (PVDF) ultrafiltration membranes via postfabrication grafting of surface-tailored silica nanoparticles. ACS Appl. Mater. Interfaces 2013, 5, 6694–6703. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Li, X.M.; Gilron, J.; Kong, D.F.; Yin, Y.; Oren, Y.; Linder, C.; He, T. CF4 plasma-modified superhydrophobic PVDF membranes for direct contact membrane distillation. J. Memb. Sci. 2014, 456, 155–161. [Google Scholar] [CrossRef]
- Liu, F.; Hashim, N.A.; Liu, Y.; Abed, M.R.M.; Li, K. Progress in the production and modification of PVDF membranes. J. Memb. Sci. 2011, 375, 1–27. [Google Scholar] [CrossRef]
- Jiménez-Robles, R.; Moreno-Torralbo, B.M.; Badia, J.D.; Martínez-Soria, V.; Izquierdo, M. Flat PVDF membrane with enhanced hydrophobicity through alkali activation and organofluorosilanisation for dissolved methane recovery. Membranes 2022, 12, 426. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Robles, R.; Gabaldón, C.; Badia, J.D.; Izquierdo, M.; Martínez-Soria, V. Recovery of dissolved methane through a flat sheet module with PDMS, PP, and PVDF membranes. Sep. Purif. Technol. 2022, 282, 120057. [Google Scholar] [CrossRef]
- Fabbri, P.; Messori, M.; Montecchi, M.; Pilati, F.; Taurino, R.; Tonelli, C.; Toselli, M. Surface properties of fluorinated hybrid coatings. J. Appl. Polym. Sci. 2006, 102, 1483–1488. [Google Scholar] [CrossRef]
- Correia, D.M.; Ribeiro, C.; Sencadas, V.; Botelho, G.; Carabineiro, S.A.C.; Ribelles, J.L.G.; Lanceros-Méndez, S. Influence of oxygen plasma treatment parameters on poly(vinylidene fluoride) electrospun fiber mats wettability. Prog. Org. Coatings 2015, 85, 151–158. [Google Scholar] [CrossRef]
- Gregorio, R.; Cestari, M. Effect of crystallization temperature on the crystalline phase content and morphology of poly(vinylidene fluoride). J. Polym. Sci. Part B Polym. Phys. 1994, 32, 859–870. [Google Scholar] [CrossRef]
- Martins, P.; Lopes, A.C.; Lanceros-Mendez, S. Electroactive phases of poly(vinylidene fluoride): Determination, processing and applications. Prog. Polym. Sci. 2014, 39, 683–706. [Google Scholar] [CrossRef]
- Hebbar, R.S.; Isloor, A.M.; Ismail, A.F. Contact Angle Measurements. In Membrane Characterization; Elsevier: Amsterdam, The Netherlands, 2017; pp. 219–255. ISBN 9780444637918. [Google Scholar]
- Abbasgholipourghadim, M.; Mailah, M.B.; Zaurah, I.; Ismail, A.F.; Rezaei Dashtarzhandi, M.; Abbasgholipourghadim, M. Porosity and pore area determination of hollow fiber membrane incorporating digital image processing. In Proceedings of the Recent Advances in Mechanics and Mechanical Engineering, Kuala Lumpur, Malaysia, 23–25 April 2015; pp. 118–123. [Google Scholar]
- Tserepi, A.D.; Vlachopoulou, M.E.; Gogolides, E. Nanotexturing of poly (dimethylsiloxane) in plasmas for creating robust super-hydrophobic surfaces. Nanotechnology 2006, 17, 3977. [Google Scholar] [CrossRef]
- Kim, E.S.; Kim, Y.J.; Yu, Q.; Deng, B. Preparation and characterization of polyamide thin-film composite (TFC) membranes on plasma-modified polyvinylidene fluoride (PVDF). J. Memb. Sci. 2009, 344, 71–81. [Google Scholar] [CrossRef]
- Hanh Le, T.M.; Singto, S.; Sajomsang, W.; Mongkolnavin, R.; Nuisin, R.; Painmanakul, P.; Sairiam, S. Hydrophobic PVDF hollow fiber membrane modified with pulse inductively coupling plasma activation and chloroalkylsilanes for efficient dye wastewater treatment by ozonation membrane contactor. J. Memb. Sci. 2021, 635, 119443. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, R. Novel method for incorporating hydrophobic silica nanoparticles on polyetherimide hollow fiber membranes for CO2 absorption in a gas-liquid membrane contactor. J. Memb. Sci. 2014, 452, 379–389. [Google Scholar] [CrossRef]
- Badia, J.D.; Gil-Castell, O.; Ribes-Greus, A. Long-term properties and end-of-life of polymers from renewable resources. Polym. Degrad. Stab. 2017, 137, 35–57. [Google Scholar] [CrossRef]
- Badía, J.D.; Vilaplana, F.; Karlsson, S.; Ribes-Greus, A. Thermal analysis as a quality tool for assessing the influence of thermo-mechanical degradation on recycled poly(ethylene terephthalate). Polym. Test. 2009, 28, 169–175. [Google Scholar] [CrossRef]




| Property | Value |
|---|---|
| Structure | Microporous |
| Thickness, µm | 125 a |
| Pore diameter, µm | 0.22 a |
| Bubble point, bar | ≥1.24 a |
| Porosity, % | 75 a |
| Static water contact angle, ° | 119.4 ± 1.7 b |
| Independent Variables (Factors) | Levels | |||||
|---|---|---|---|---|---|---|
| −α | −1 | 0 | +1 | +α | ||
| A | Power (W) | 3 | 5 | 10 | 15 | 17 |
| B | Time (min) | 3 | 5 | 10 | 15 | 17 |
| C | Silica precursor (SiP) * | TEOS—APTES | ||||
| Before Stability Test | After Stability Test | |||||
|---|---|---|---|---|---|---|
| Tm, °C | Δhm, J g−1 | χ, % | Tm, °C | Δhm, J g−1 | χ, % | |
| p-PVDF | 162.3 | 59.3 | 60.6 | 162.0 | 62.2 | 63.5 |
| Coat-TEOS | 164.0 | 53.0 | 54.1 | 164.0 | 54.9 | 56.1 |
| Coat-APTES | 164.3 | 55.0 | 56.2 | 162.6 | 54.7 | 55.9 |
| PO2-TEOS | 162.3 | 54.2 | 55.4 | 161.8 | 58.1 | 59.3 |
| PO2-APTES | 162.9 | 57.0 | 58.2 | 163.4 | 58.9 | 60.2 |
| Membrane | Before Stability Test | After Stability Test |
|---|---|---|
| p-PVDF | 11 ± 3% | 5 ± 1% |
| Coat-TEOS | 8 ± 1% | 4 ± 2% |
| Coat-APTES | 10 ± 2% | 3 ± 3% |
| PO2-TEOS | 15 ± 4% | 6 ± 3% |
| PO2-APTES | 15 ± 3% | 7 ± 1% |
| Before Stability Test | After Stability Test | |||||||
|---|---|---|---|---|---|---|---|---|
| Membrane | F/C | O/C | Si/C | N/C | F/C | O/C | Si/C | N/C |
| p-PVDF | 0.92 | - | - | - | 0.90 | - | - | - |
| Coat-TEOS | 0.95 | 0.02 | <0.01 | - | 0.94 | 0.02 | <0.01 | - |
| Coat-APTES | 0.95 | 0.03 | <0.01 | 0.03 | 0.91 | 0.04 | 0.01 | <0.01 |
| PO2-TEOS | 0.95 | 0.04 | 0.01 | - | 0.80 | 0.03 | <0.01 | - |
| PO2-APTES | 0.99 | 0.05 | 0.01 | 0.04 | 0.85 | 0.09 | 0.02 | 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez-Robles, R.; Izquierdo, M.; Martínez-Soria, V.; Martí, L.; Monleón, A.; Badia, J.D. Stability of Superhydrophobicity and Structure of PVDF Membranes Treated by Vacuum Oxygen Plasma and Organofluorosilanisation. Membranes 2023, 13, 314. https://doi.org/10.3390/membranes13030314
Jiménez-Robles R, Izquierdo M, Martínez-Soria V, Martí L, Monleón A, Badia JD. Stability of Superhydrophobicity and Structure of PVDF Membranes Treated by Vacuum Oxygen Plasma and Organofluorosilanisation. Membranes. 2023; 13(3):314. https://doi.org/10.3390/membranes13030314
Chicago/Turabian StyleJiménez-Robles, Ramón, Marta Izquierdo, Vicente Martínez-Soria, Laura Martí, Alicia Monleón, and José David Badia. 2023. "Stability of Superhydrophobicity and Structure of PVDF Membranes Treated by Vacuum Oxygen Plasma and Organofluorosilanisation" Membranes 13, no. 3: 314. https://doi.org/10.3390/membranes13030314
APA StyleJiménez-Robles, R., Izquierdo, M., Martínez-Soria, V., Martí, L., Monleón, A., & Badia, J. D. (2023). Stability of Superhydrophobicity and Structure of PVDF Membranes Treated by Vacuum Oxygen Plasma and Organofluorosilanisation. Membranes, 13(3), 314. https://doi.org/10.3390/membranes13030314