Chromium-Modified Heterogeneous Bipolar Membrane: Structure, Characteristics, and Practical Application in Electrodialysis
Abstract
1. Introduction
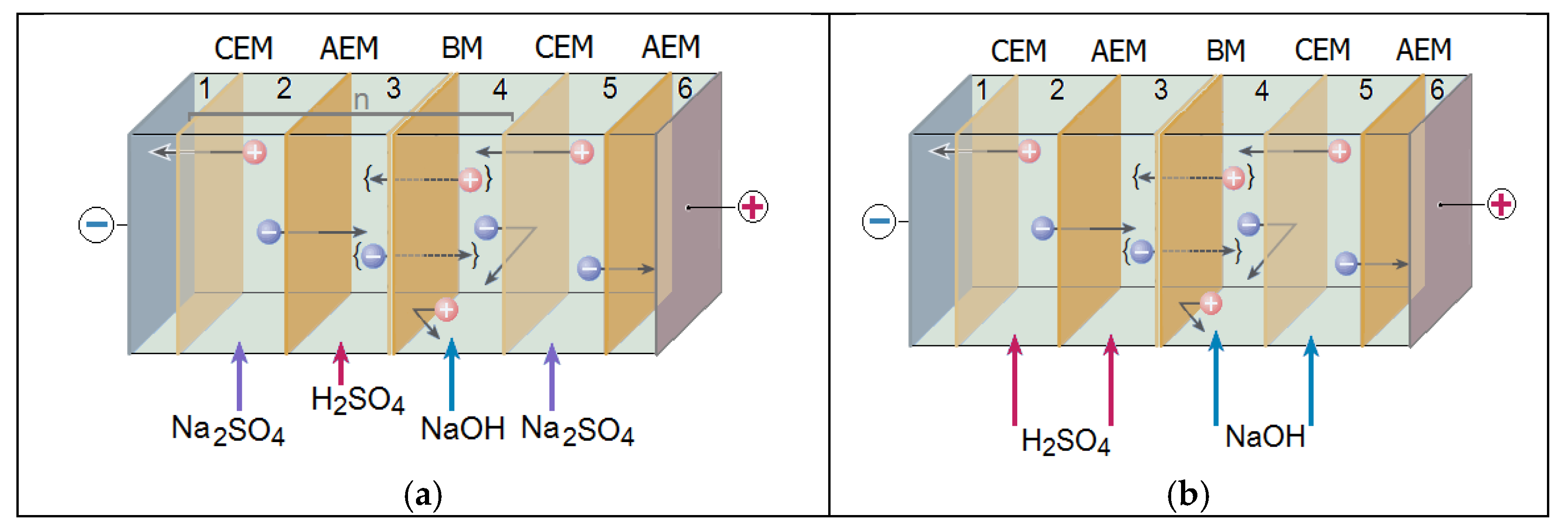
2. Materials and Methods
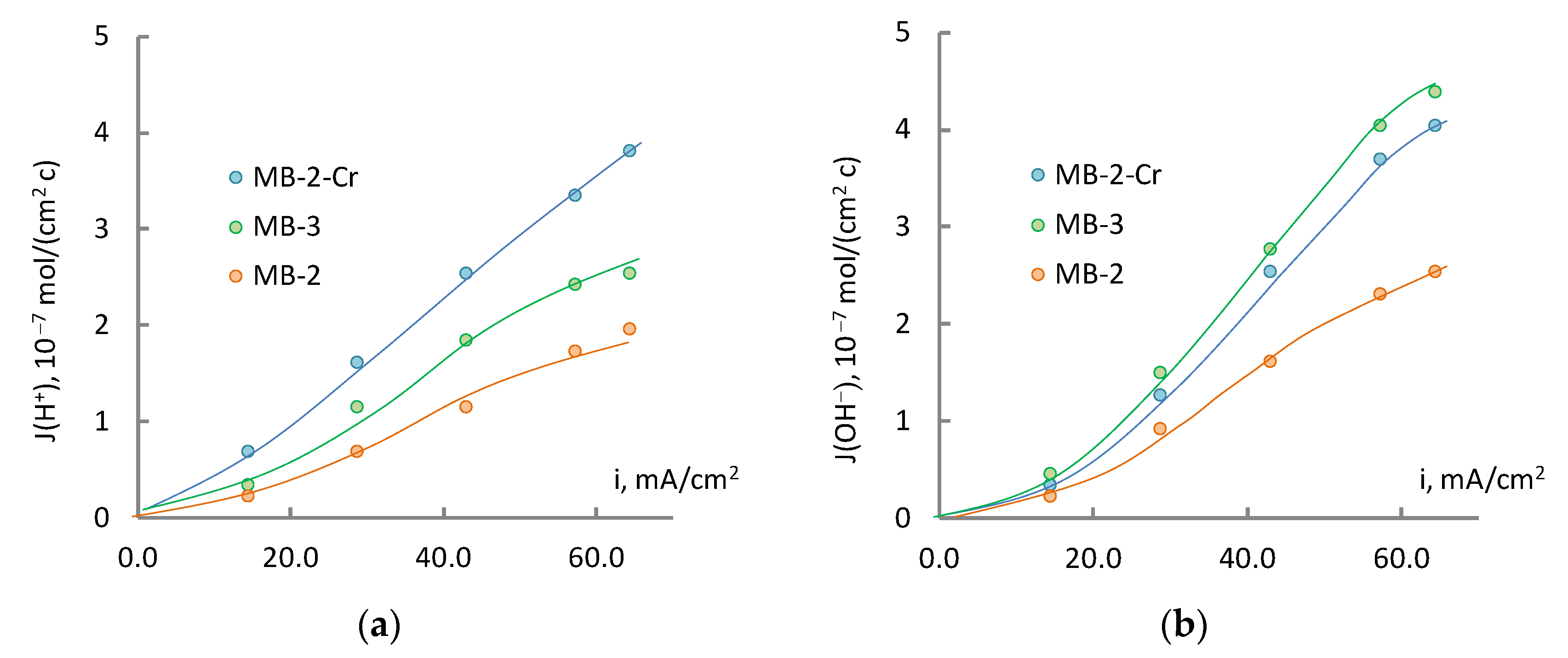
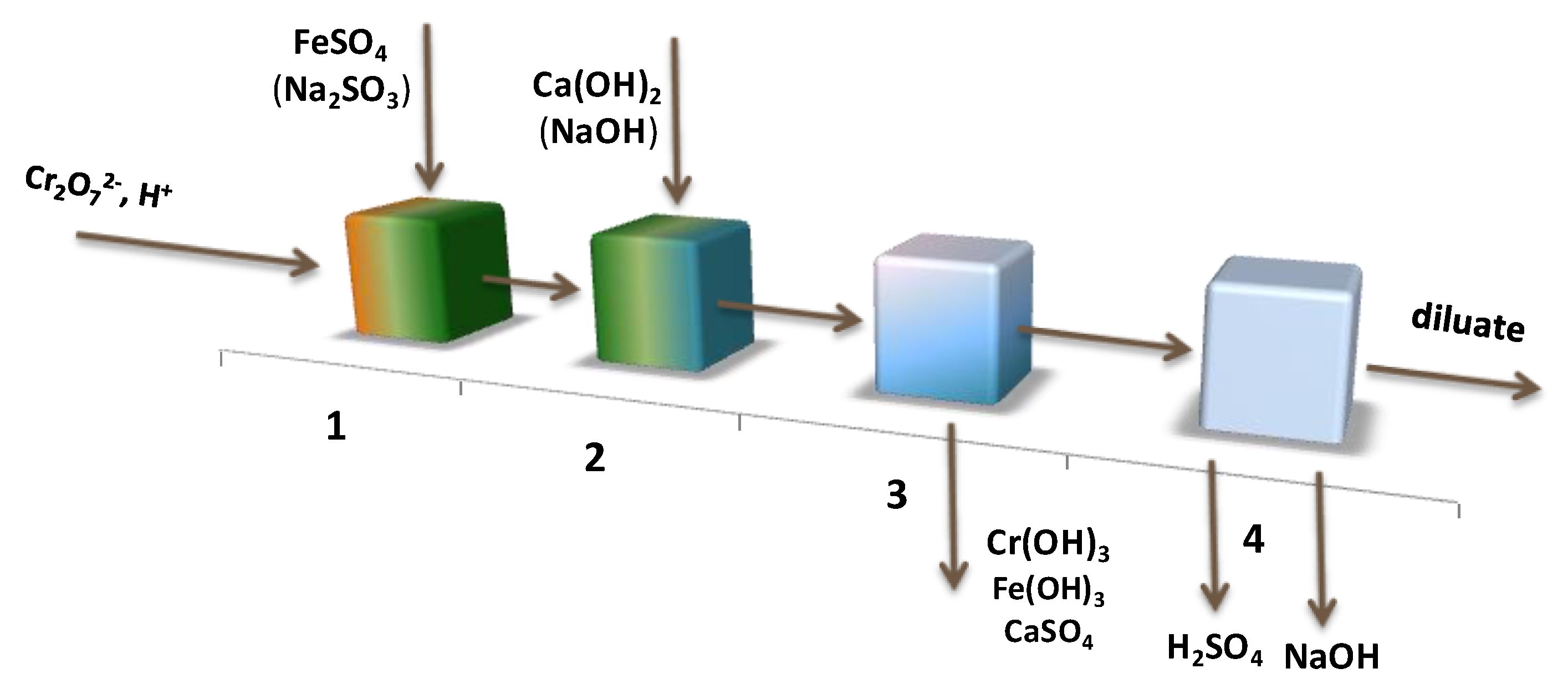
3. Results and Discussion
4. Conclusions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Costa, M.; KleinCrit, C.B. Toxicity and carcinogenicity of chromium compounds in humans. Cit. Rev. Toxicol. 2006, 36, 155–163. [Google Scholar] [CrossRef]
- Golbaz, S.; Jafari, A.J.; Rafiee, M.; Kalantary, R.R. Separate and simultaneous removal of phenol, chromium, and cyanide from aqueous solution by coagulation/precipitation: Mechanisms and theory. Chem. Eng. J. 2014, 253, 251–257. [Google Scholar] [CrossRef]
- Apandi, N.M.; Gani, P.; Sunar, N.M.; Mohamed, R.M.S.R.; AlGheethi, A.; Apandi, A.M.; Nagarajah, R.; Shaari, N.A.R.; Cheong, K.; Rahman, R.A. Scenedesmus sp. Harvesting by Using Natural Coagulant after Phycoremediation of Heavy Metals in Different Concentrations of Wet Market Wastewater for Potential Fish Feeds. Sustainability 2022, 14, 5090. [Google Scholar] [CrossRef]
- Martín-Domínguez, A.; Rivera-Huerta, M.D.L.; Pérez-Castrejón, S.; Garrido-Hoyos, S.E.; Villegas-Mendoza, I.E.; Gelover-Santiago, S.L.; Drogui, P.; Buelna, G. Chromium removal from drinking water by redox-assisted coagulation: Chemical versus electrocoagulation. Sep. Purif. Technol. 2018, 200, 266–272. [Google Scholar] [CrossRef]
- Li, G.; Yang, C.; Yao, Y.; Zeng, M. Electrocoagulation of chromium in tannery wastewater by a composite anode modified with titanium: Parametric and kinetic study. Desalination Water Treat. 2019, 171, 294–301. [Google Scholar] [CrossRef]
- Duan, W.; Chen, G.; Chen, C.; Sanghvi, R.; Iddya, A.; Walker, S.; Liu, H.; Ronen, A.; Jassby, D. Electrochemical removal of hexavalent chromium using electrically conducting carbon nanotube/polymer composite ultrafiltration membranes. J. Membr. Sci. 2017, 531, 160–171. [Google Scholar] [CrossRef]
- Rengaraj, S.; Yeon, K.H.; Moon, S.H. Removal of chromium from water and wastewater by ion exchange resins. J. Hazard. Mater. 2001, 87, 273–287. [Google Scholar] [CrossRef]
- Dehghani, M.H.; Sanaei, D.; Ali, I.; Bhatnagar, A. Removal of chromium (VI) from aqueous solution using treated waste newspaper as a low-cost adsorbent: Kinetic modeling and isotherm studies. J. Mol. Liq. 2016, 215, 671–679. [Google Scholar] [CrossRef]
- Habiba, U.; Siddique, T.A.; Joo, T.C.; Salleh, A.; Ang, B.C.; Afifi, A.M. Synthesis of chitosan/polyvinyl alcohol/zeolite composite for removal of methyl orange, Congo red and chromium (VI) by flocculation/adsorption. Carbohydr. Polym. 2017, 157, 1568–1576. [Google Scholar] [CrossRef]
- Yang, W.; Lei, G.; Quan, S.; Zhang, L.; Wang, B.; Hu, H.; Li, L.; Ma, H.; Yin, C.; Feng, F.; et al. The Removal of Cr(VI) from Aqueous Solutions with Corn Stalk Biochar. Int. J. Environ. Res. Public Health 2022, 19, 14188. [Google Scholar] [CrossRef]
- Sočo, E.; Domoń, A.; Papciak, D.; Michel, M.M.; Cieniek, B.; Pająk, D. Characteristics of the Properties of Absodan Plus Sorbent and Its Ability to Remove Phosphates and Chromates from Aqueous Solutions. Materials 2022, 15, 3540. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Duan, X. Mitigating the Health Effects of Aqueous Cr(VI) with Iron-Modified Biochar. Int. J. Environ. Res. Public Health 2022, 19, 1481. [Google Scholar] [CrossRef] [PubMed]
- Tsybulskaya, O.N.; Ksenik, T.V.; Yudakov, A.A.; Slesarenko, V.V.; Perfiliev, A.V.; Kisel, A.A. Features of reactant treatment of chromium-containing waste water of electroplating industry. Mod. Sci. Res. Ideas Results Technol. 2015, 16, 74–78. [Google Scholar] [CrossRef]
- Ozeryanskaya, V.V.; Rybalkina, I.S.; Filipenko, N.L.; Medvedeva, V.A. Investigation of chromiferous galvanic wastes treatment by reactant and flotation method combination. Adv. Eng. Res. 2011, 11, 1385–1390. [Google Scholar]
- Simons, R. Strong electric field effects on proton transfer between membrane-bound amines and water. Nature 1979, 280, 824–826. [Google Scholar] [CrossRef]
- Simons, R. Electric field effects on proton thansfer between ionizable groups and water in ion exchange membranes. Electrochim. Acta 1984, 29, 131. [Google Scholar] [CrossRef]
- Greben, V.P.; Pivovarov, N.Y.; Kovarskii, N.Y.; Nefedova, G.Z. Influence of ion-exchange resin nature on physic-chemical properties of bipolar membranes. J. Phys. Chem. 1978, 52, 2641. [Google Scholar]
- Zabolotskii, V.I.; Shel′deshov, N.V.; Gnusin, N.P. Dissociation of Water Molecules in Systems with Ion-exchange Membranes. Russ. Chem. Rev. 1988, 57, 801–808. [Google Scholar] [CrossRef]
- Simons, R. High Performance Bipolar Membrane. U.S. Patent US5227040, 13 July 1993. [Google Scholar]
- Melnikov, S.S.; Shapovalova, O.V.; Sheldeshov, N.V.; Zabolotskii, V.I. Effect of d-metal hydroxides on water dissociation in bipolar membranes. Petrol. Chem. 2011, 51, 577–584. [Google Scholar] [CrossRef]
- Shel’deshov, N.V.; Zabolotskii, V.I.; Ganych, V.V. The effect of insoluble metal hydroxides on the reaction rate of water dissociation on a cation-exchange membrane. Russ. J. Electrochem. 1994, 30, 1458–1461. [Google Scholar]
- Huang, M.; Shen, Y.; Cheng, W.; Shao, Y.; Sun, X.; Liu, B.; Dong, S. Nanocomposite films containing Au nanoparticles formed by electrochemical reduction of metal ions in the multilayer films as electrocatalyst for dioxygen reduction. Anal. Chim. Acta. 2005, 535, 15–22. [Google Scholar] [CrossRef]
- Camargo, P.H.C.; Satyanarayana, K.G.; Wypych, F. Nanocomposites: Synthesis, structure, properties and new application opportunities. Mater. Res. 2009, 12, 1–39. [Google Scholar] [CrossRef]
- Domenech, B.; Bastos-Arrieta, J.; Alonso, A.; Macanas, J.; Munoz, M.; Muraviev, D.N. Bifunctional Polymer-Metal Nanocomposite Ion Exchange Materials. In Ion Exchange Technologies; IntechOpen: Rijeka, Croatia, 2012; pp. 35–72. [Google Scholar] [CrossRef]
- Krysanov, V.A.; Plotnikova, N.V.; Kravchenko, T.A. Sorption of Molecular Oxygen by Metal–Ion Exchanger Nanocomposites. Russ. J. Phys. Chem. 2018, 92, 527–531. [Google Scholar] [CrossRef]
- Kang, M.S. Electrochemical characteristics of ion-exchange membranes coated with iron hydroxide/oxide and silica sol. J. Colloid Interface Sci. 2003, 273, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Parnam, R.; Mareev, S.; Nikonenko, V.; Melnikov, S.; Sheldeshov, N.; Zabolotskii, V.; Hamelers, H.V.M.; Tedesco, M. Bipolar membranes: A review on principles, latest developments, and applications. J. Membr. Sci. 2021, 617, 118538. [Google Scholar] [CrossRef]
- Zabolotskii, V.I.; Shel′deshov, N.V. Bipolar ion exchange membranes. Receipt. Properties. Application. In Membranes and Membrane Technologies; Nauchnyi Mir: Moscow, Russia, 2013; pp. 70–125. [Google Scholar]
- Kravchenko, T.A.; Polyanskii, L.N.; Kalinichev, A.I.; Konev, D.V. Nanokompozity Metall-Ionoobmennik (Metal-Ion Exchanger NanoComposites); Nauka: Moscow, Russia, 2009; 391p. [Google Scholar]
- Demina, O.A.; Kononenko, N.A.; Falina, I.V. New approach to the characterization of ion-exchange membranes using a set of model parameters. Pet. Chem. 2014, 54, 515–525. [Google Scholar] [CrossRef]
- Shaposhnik, V.A.; Vasil’eva, V.I.; Grigorchuk, O.V. Yavleniya Perenosa v Ionoobmennykh Membranakh; MFTI: Moscow, Russia, 2001; p. 200. [Google Scholar]
- Bipolyarnye Membrany. Available online: http://www.azotom.ru/ionoobmennye_membrany (accessed on 30 November 2022).
- Graillon, S.; Persin, F.; Pourcelly, G.; Gavach, C. Development of electrodialysis with bipolar membrane for the treatment of concentrated nitrate effluents. Desalination 1996, 107, 159–169. [Google Scholar] [CrossRef]
- Vasil′ev, V.P. Analiticheskaya Khimiya. V 2 ch. Chast′ I. Gravimetricheskii i Titrimetricheskii Metody Analiza; Vyssh. Shkola: Moscow, Russia, 1989; 320p. [Google Scholar]
- NH4+—Selective Electrode of the Volta Series: Instruction Manual; Volta: St. Petersburg, Russia, 2011; p. 6.
- SO42-—Selective Electrode of the Volta Series: Instruction Manual; Volta: St. Petersburg, Russia, 2011; p. 6.
- Kozaderova, O.A. Electrochemical characterization of an MB-2 bipolar membrane modified by nanosized chromium(III) hydroxide. Nanotechnol. Russ. 2018, 13, 508–515. [Google Scholar] [CrossRef]
- Kozaderova, O.A. Synthesis of a chromium (III) hydroxide nanocomposite-KU-2-8 cation exchanger. Sorbtsionnye Khromatograficheskie Protsessy 2020, 5, 608–614. [Google Scholar] [CrossRef]
- Vasil′eva, V.I.; Akberova, E.M.; Malykhin, M.D.; Demina, O.A.; Kononenko, N.A. Effect of thermochemical treatment on conductivity and mechanism of current flow in MK-40 sulfocationite membrane. Russ. J. Electrochem. 2015, 51, 627–637. [Google Scholar] [CrossRef]
- Salmeron-Sanchez, I.; Asenjo-Pascual, J.; Avil’es-Moreno, J.R.; Ocon, P. Microstructural description of ion exchange membranes: The effect of PPy-based modification. J. Membr. Sci. 2022, 659, 120771. [Google Scholar] [CrossRef]
- Zabolotsky, V.I.; Nikonenko, V.V. Effect of structural membrane inhomogeneity on transport properties. J. Membr. Sci. 1993, 79, 181–198. [Google Scholar] [CrossRef]
- León, T.; Shah, S.A.; López, J.; Culcasi, A.; Jofre, L.; Cipollina, A.; Cortina, J.L.; Tamburini, A.; Micale, G. Electrodialysis with Bipolar Membranes for the Generation of NaOH and HCl Solutions from Brines: An Inter-Laboratory Evaluation of Thin and Ultrathin Non-Woven Cloth-Based Ion-Exchange Membranes. Membranes 2022, 12, 1204. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.X.; Yuan, D.X.; Yan, J.M.; Li, Q.L.; Ouyang, T. Electrochemical removal of chromium from aqueous solutions using electrodes of stainless steel nets coated with single wall carbon nanotubes. J. Hazard. Mater. 2011, 186, 473–480. [Google Scholar] [CrossRef] [PubMed]
- De Silva, R.A.; Secco, M.P.; Menezes, J.C.S.S.; Schneider, I.A.H.; Thomas, R. Reduction of High-Chromium-Containing Wastewater in the Leaching of Pyritic Waste Rocks from Coal Mines. Sustainability 2022, 14, 11814. [Google Scholar] [CrossRef]
- Wu, X.; Zhu, H.; Liu, Y.; Chen, R.; Qian, Q.; Van der Bruggen, B. Cr(III) recovery in form of Na2CrO4 from aqueous solution using improved bipolar membrane electrodialysis. J. Membr. Sci. 2020, 604, 118097. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, H.; Zhang, M.; Chen, R.; Chen, X.; Zheng, X.; Jin, Y. Cr(VI) recovery from chromite ore processing residual using an enhanced electrokinetic process by bipolar membranes. J. Membr. Sci. 2018, 566, 190–196. [Google Scholar] [CrossRef]






| Heterogeneous Membranes | Functional Groups | Thickness, mm | |
|---|---|---|---|
| monopolar | MK-40 | -SO3H | <0.45 |
| MA-41 | -N+(CH3)3 | <0.45 | |
| bipolar | MB-2 | -SO3H -N+(CH3)3 | 0.5–0.8 |
| MB-3 | -PO3H2 -N+(CH3)3 | 0.5–0.8 | |
| Phase | Atomic Fraction of an Element in Each Phase, % | ||||
|---|---|---|---|---|---|
| C | O | S | Cr | Na | |
| Phase I | 68.3 | 9.4 | 11.5 | 1.4 | 9.4 |
| Phase II | 70.9 | 12.8 | 5.7 | 6.0 | 4.6 |
| Phase III | 67.6 | 15.1 | 5.9 | 0.8 | 10.6 |
| Process | Without Recycling | With Recycling | ||||||
|---|---|---|---|---|---|---|---|---|
| Membranes | MB-2 | MB-3 | MB-2-Cr | MB-2-Cr | ||||
| Products | NaOH | H2SO4 | NaOH | H2SO4 | NaOH | H2SO4 | NaOH | H2SO4 |
| Δc, mol-equiv /dm3 | 0.22 | 0.17 | 0.38 | 0.22 | 0.35 | 0.33 | 1.50 | 0.95 |
| η, % | 38 | 30 | 66 | 38 | 61 | 57 | 51 | 31 |
| W, kW⋅h/kg | 34.3 | 38.6 | 15.9 | 23.8 | 19.3 | 17.9 | 18.1 | 23.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozaderova, O. Chromium-Modified Heterogeneous Bipolar Membrane: Structure, Characteristics, and Practical Application in Electrodialysis. Membranes 2023, 13, 172. https://doi.org/10.3390/membranes13020172
Kozaderova O. Chromium-Modified Heterogeneous Bipolar Membrane: Structure, Characteristics, and Practical Application in Electrodialysis. Membranes. 2023; 13(2):172. https://doi.org/10.3390/membranes13020172
Chicago/Turabian StyleKozaderova, Olga. 2023. "Chromium-Modified Heterogeneous Bipolar Membrane: Structure, Characteristics, and Practical Application in Electrodialysis" Membranes 13, no. 2: 172. https://doi.org/10.3390/membranes13020172
APA StyleKozaderova, O. (2023). Chromium-Modified Heterogeneous Bipolar Membrane: Structure, Characteristics, and Practical Application in Electrodialysis. Membranes, 13(2), 172. https://doi.org/10.3390/membranes13020172





