Membrane Cascade Fractionation of Tomato Leaf Extracts—Towards Bio-Based Crop Protection
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Aqueous Extraction from the Tomato Leaves
2.2.2. Membrane Process
2.2.3. Quantification of Biomolecules and Solids
2.2.4. Biopesticide Activity of the Collected Fractions
3. Results
3.1. Tomato Leaf Extract Analysis
3.2. Fouling Index and Cleaning Efficiency in Cascade Membrane Processes
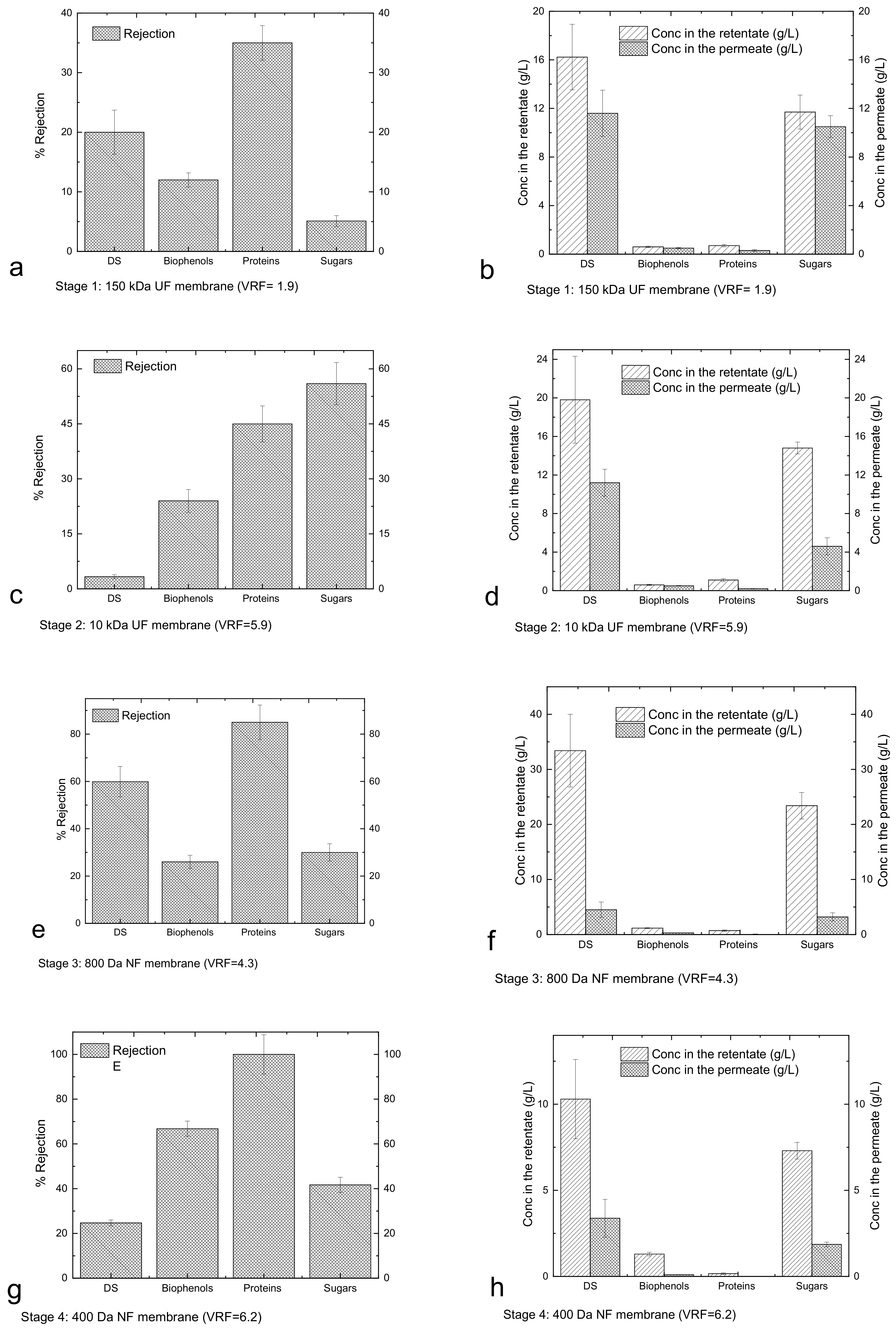
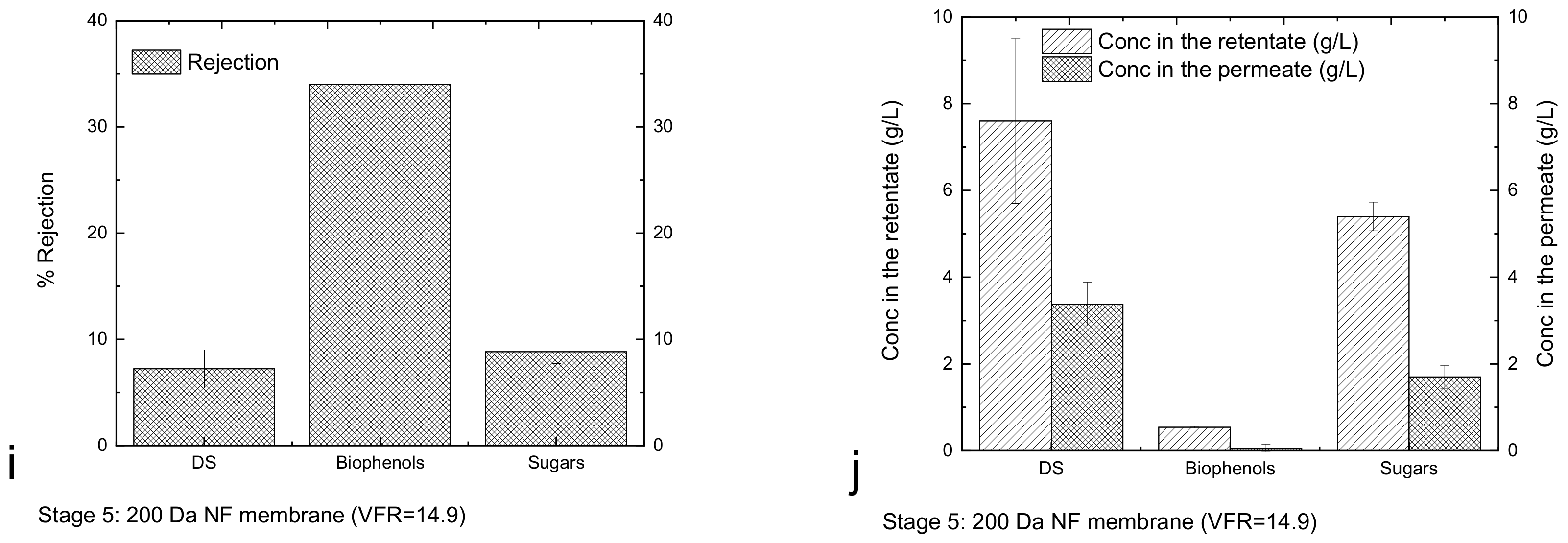
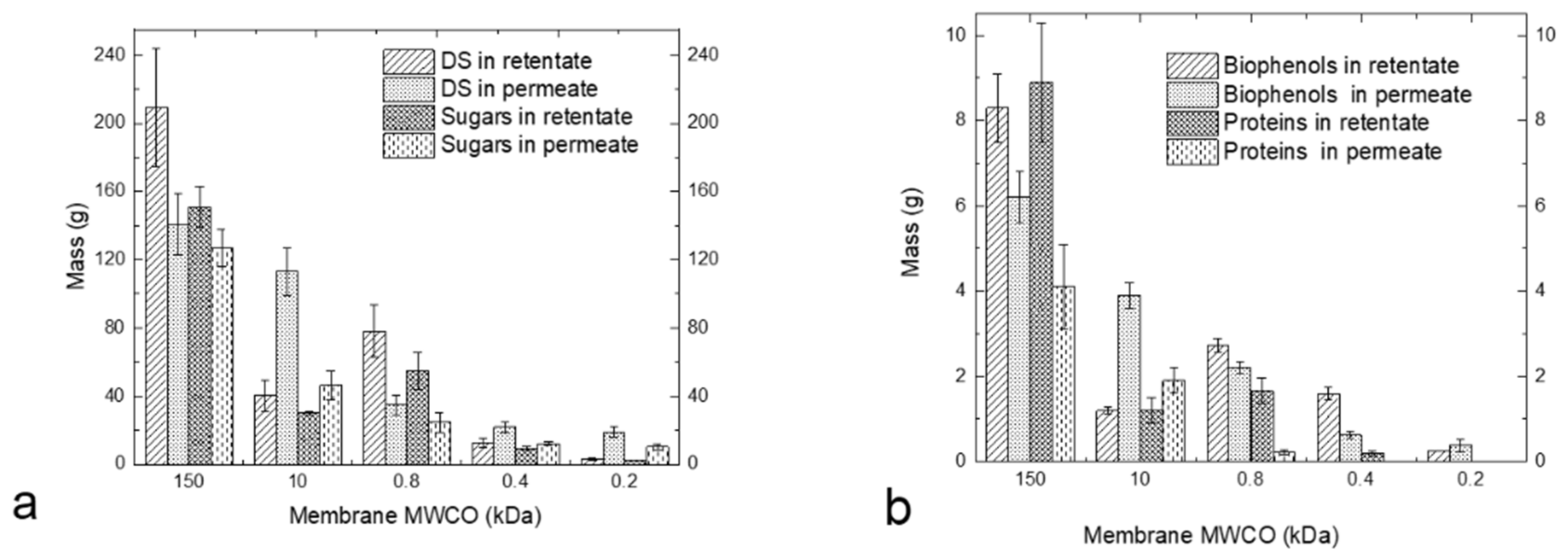
3.3. Performance of Membrane Cascade and Characterisation of Tomato Leaf Fractions
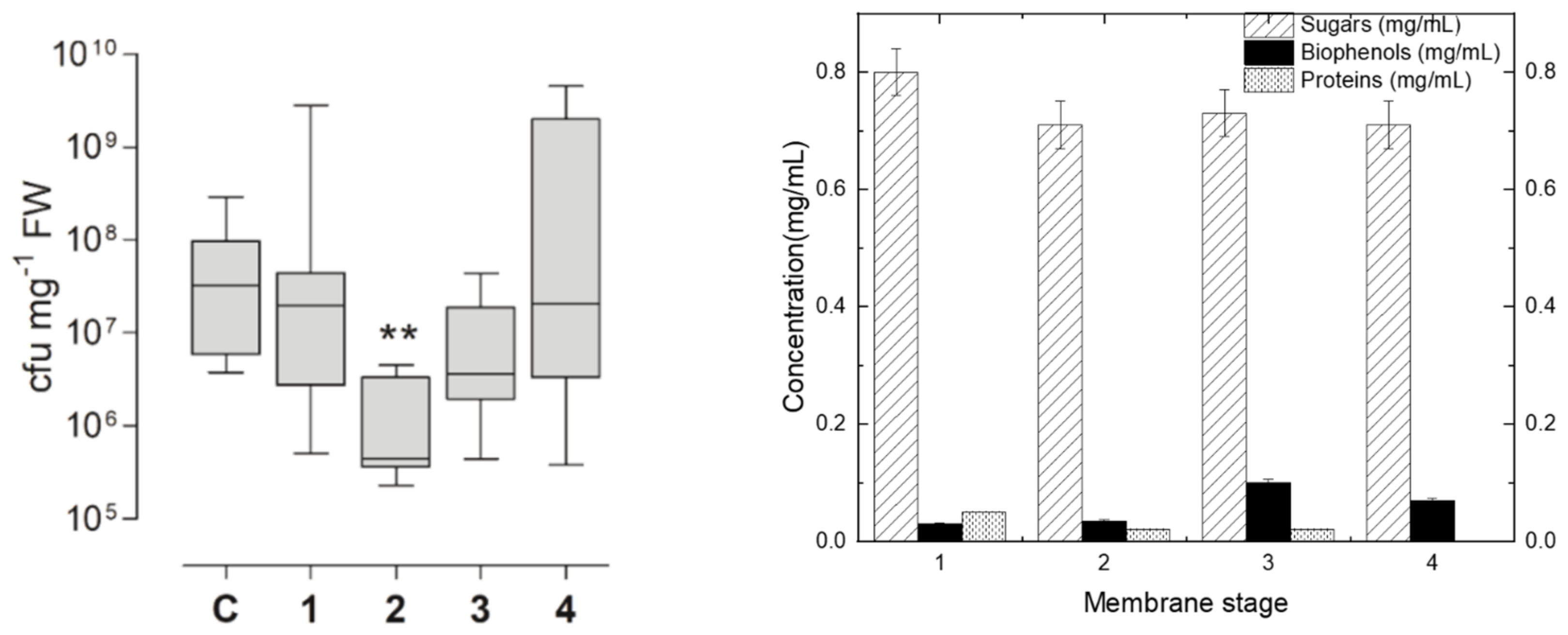
3.4. Elicitor Activity of Tomato Leaf Fractions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bazzarelli, F.; Mazzei, R.; Papaioannou, E.; Giannakopoulos, V.; Roberts, R.R.; Giorno, L. Biorefinery of tomato leaves by integrated extraction and membrane processes to obtain fractions that enhance induced resistance against Pseudomonas syringae infection. Membranes 2022, 12, 585. [Google Scholar] [CrossRef] [PubMed]
- ‘Tomato: Crop Description and Climate’ in Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/land-water/databases-and-software/crop-information/tomato/en/ (accessed on 27 September 2023).
- Poussio, G.B.; Abro, M.A.; Syed, R.N.; Ibrahim, M.K.; Hajano, J.U. Eco-friendly management of tomato wilt disease caused by Fusarium sp. in Sindh Province, Pakistan. Int. J. Recycl. Org. Waste Agric. 2022, 11, 117–129. [Google Scholar]
- Lima, T.S.P.; Borges, M.M.; Buarque, F.S.; de Souza, R.L.; Soares, C.M.F.; Lima, A.S. Purification of vitamins from tomatoes (Solanum lycopersicum) using ethanolic two-phases systems based on ionic liquids and polypropylene glycol. Fluid Phase Equilibria 2022, 557, 113434. [Google Scholar] [CrossRef]
- Arab, M.; Bahramian, B.; Schindeler, A.; Fathi, A.; Valtchev, P.; McConchie, R.; Dehghani, F. A benign process for the recovery of solanesol from tomato leaf waste. Heliyon 2019, 5, e01523. [Google Scholar] [CrossRef] [PubMed]
- Manríquez-Altamirano, A.; Sierra-Pérez, J.; Muñoz, P.; Gabarrell, X. Analysis of urban agriculture solid waste in the frame of circular economy: Case study of tomato crop in integrated rooftop greenhouse. Sci. Total Environ. 2020, 734, 139375. [Google Scholar] [CrossRef]
- Maboko, M.M.; Plooy, C.P.D. Effect of pruning on yield and quality of hydroponically grown cherry tomato (lycopersicon esculentum). S. Afr. J. Plant Soil 2008, 25, 178–181. [Google Scholar] [CrossRef]
- Yu, Y.; Kleuter, M.; Taghian Dinani, S.; Trindade, L.M.; van der Goot, A.J. The role of plant age and leaf position on protein extraction and phenolic compounds removal from tomato (Solanum lycopersicum) leaves using food-grade solvents. Food Chem. 2023, 406, 135072. [Google Scholar] [CrossRef]
- Silva-Beltrán, N.P.; Ruiz-Cruz, S.; Chaidez, C.; Ornelas-Paz Jde, J.; López-Mata, M.A.; Márquez-Ríos, E.; Estrada, M.I. Chemical constitution and effect of extracts of tomato plants byproducts on the enteric viral surrogates. Int. J. Environ. Health Res. 2015, 25, 299–311. [Google Scholar] [CrossRef]
- Papaioannou, E.H.; Mazzei, R.; Bazzarelli, F.; Piacentini, E.; Giannakopoulos, V.; Roberts, M.R.; Giorno, L. Agri-food industry waste as resource of chemicals: The role of membrane technology in their sustainable recycling. Sustainability 2022, 14, 1483. [Google Scholar] [CrossRef]
- Figueiredo-González, M.; Valentão, P.; Andrade, P.B. Tomato plant leaves: From by-products to the management of enzymes in chronic diseases. Ind. Crops Prod. 2016, 94, 621–629. [Google Scholar] [CrossRef]
- Taveira, M.; Ferreres, F.; Gil-Izquierdo, A.; Oliveira, L.; Valentão, P.; Andrade, P.B. Fast determination of bioactive compounds from Lycopersicon esculentum Mill. leaves. Food Chem. 2012, 135, 748–755. [Google Scholar] [CrossRef] [PubMed]
- Papaioannou, E.H.; Mitrouli, S.T.; Patsios, S.I.; Kazakli, M.; Karabelas, A.J. Valorization of pomegranate husk—Integration of extraction with nanofiltration for concentrated polyphenols recovery. J. Environ. Chem. Eng. 2020, 8, 103951. [Google Scholar] [CrossRef]
- Pimentel, D. Pest management and control. In Integrated Pest Management; Peshin, R., Dhawan, A.K., Eds.; Springer: Dordrecht, The Netherlands, 2009; Volume 1, pp. 83–87. [Google Scholar]
- Oerke, E.C. Crop losses to pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Quintana-Rodriguez, E.; Duran-Flores, D.; Heil, M.; Camacho-Coronel, X. Damage-associated molecular patterns (DAMPs) as future plant vaccines that protect crops from pests. Sci. Hortic. 2018, 237, 207–220. [Google Scholar] [CrossRef]
- Moo-Koh, F.A.; Cristóbal-Alejo, J.; Tun-Suárez, J.M.; Medina-Baizabal, I.L.; Arjona-Cruz, A.A.; Gamboa-Angulo, M. Activity of aqueous extracts from native plants of the Yucatan Peninsula against fungal pathogens of tomato in vitro and from Croton chichenensis against Corynespora cassiicola on tomato. Plants 2022, 11, 2821. [Google Scholar] [CrossRef]
- Hermann, S.; Orlik, M.; Boevink, P.; Stein, E.; Scherf, A.; Kleeberg, I.; Schmitt, A.; Schikora, A. Biocontrol of plant diseases using Glycyrrhiza glabra leaf extract. Plant Dis. 2022, 106, 3133–3144. [Google Scholar] [CrossRef]
- O’Malley, M.R.; Kpenu, E.; Peck, S.C.; Anderson, J.C. Plant-exuded chemical signals induce surface attachment of the bacterial pathogen Pseudomonas syringae. PeerJ 2023, 11, e14862. [Google Scholar] [CrossRef]
- Ishiga, Y. Studies on mode of action of phytotoxin coronatine produced by Pseudomonas syringae pv. tomato. J. Gen. Plant Pathol. 2017, 83, 424–426. [Google Scholar] [CrossRef]
- Bacete, L.; Mélida, H.; Miedes, E.; Molina, A. Plant cell wall-mediated immunity: Cell wall changes trigger disease resistance responses. Plant J. 2018, 93, 614–636. [Google Scholar] [CrossRef]
- Boller, T.; Felix, G. A renaissance of elicitors: Perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu. Rev. Plant Biol. 2009, 60, 379–406. [Google Scholar] [CrossRef]
- Aitouguinane, M.; El Alaoui-Talibi, Z.; Rchid, H.; Fendri, I.; Abdelkafi, S.; Ould El-Hadj, M.D.; Boual, Z.; Dubessay, P.; Michaud, P.; Le Cerf, D. A novel sulfated glycoprotein elicitor extracted from the Moroccan green seaweed Codium decorticatum induces natural defenses in tomato. Appl. Sci. 2022, 12, 3643. [Google Scholar] [CrossRef]
- Hernández-Herrera, R.M.; Virgen-Calleros, G.; Ruiz-López, M.; Zañudo-Hernández, J.; Délano-Frier, J.P.; Sánchez-Hernández, C. Extracts from green and brown seaweeds protect tomato (Solanum lycopersicum) against the necrotrophic fungus Alternaria solani. J. Appl. Phycol. 2014, 26, 1607–1614. [Google Scholar] [CrossRef]
- Faccin, D.; Di Piero, R.M. Extracts and fractions of humic substances reduce bacterial spot severity in tomato plants, improve primary metabolism and activate the plant defense system. Physiol. Mol. Plant Pathol. 2022, 121, 101877. [Google Scholar] [CrossRef]
- Simpson, S.D.; Ashford, D.A.; Harvey, D.J.; Bowles, D.J. Short chain oligogalacturonides induce ethylene production and expression of the gene encoding aminocyclopropane 1-carboxylic acid oxidase in tomato plants. Glycobiology 1998, 8, 579–583. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Wang, X.; Wei, T.; Wang, M.; Liu, X.; Hua, L.; Ren, X.; Guo, J.; Li, J. Exogenous salicylic acid regulates cell wall polysaccharides synthesis and pectin methylation to reduce Cd accumulation of tomato. Ecotoxicol. Environ. Saf. 2021, 207, 111550. [Google Scholar] [CrossRef]
- Abels, C.; Carstensen, F.; Wessling, M. Membrane processes in biorefinery applications. J. Membr. Sci. 2013, 444, 285–317. [Google Scholar] [CrossRef]
- Tonova, K.; Lazarova, M.; Dencheva-Zarkova, M.; Paniovska, S.; Tsibranska, I.; Stanoev, V.; Dzhonova, D.; Genova, J. Separation of glucose, other reducing sugars and phenolics from natural extract by nanofiltration: Effect of pressure and cross-flow velocity. Chem. Eng. Res. Des. 2020, 162, 107–116. [Google Scholar] [CrossRef]
- Nazir, A.; Khan, K.; Maan, A.; Zia, R.; Giorno, L.; Schroën, K. Membrane separation technology for the recovery of nutraceuticals from food industrial streams. Trends Food Sci. Technol. 2019, 86, 426–438. [Google Scholar] [CrossRef]
- Cai, M.; Chen, S.; Ma, Q.; Yang, K.; Sun, P. Isolation of crude oligosaccharides from Hericium erinaceus by integrated membrane technology and its proliferative activity. Food Hydrocoll. 2019, 95, 426–431. [Google Scholar] [CrossRef]
- Hajihama, M.; Youravong, W. Concentration and desalination of protein derived from tuna cooking juice by nanofiltration. J. Teknol. 2013, 65, 1–6. [Google Scholar] [CrossRef][Green Version]
- Cassano, A.; Conidi, C.; Figueroa, R.R.; Muñoz, R.C. A two-step nanofiltration process for the production of phenolic-rich fractions from artichoke aqueous extracts. Int. J. Mol. Sci. 2015, 16, 8968–8987. [Google Scholar] [CrossRef] [PubMed]
- Cassano, A.; Conidi, C.; Ruby-Figueroa, R.; Castro-Muñoz, R. Nanofiltration and tight ultrafiltration membranes for the recovery of polyphenols from agro-food by-products. Int. J. Mol. Sci. 2018, 19, 351. [Google Scholar] [CrossRef]
- Gómez, B.; Gullón, B.; Yáñez, R.; Parajó, J.C.; Alonso, J.L. Pectic oligosacharides from lemon peel wastes: Production, purification, and chemical characterization. J. Agric. Food Chem. 2013, 61, 10043–10053. [Google Scholar] [CrossRef] [PubMed]
- Box, J.D. Investigation of the Folin-Ciocalteau phenol reagent for the determination of polyphenolic substances in natural waters. Water Res. 1983, 17, 511–525. [Google Scholar] [CrossRef]
- Magiera, S.; Zaręba, M. Chromatographic determination of phenolic acids and flavonoids in Lycium barbarum L. and evaluation of antioxidant activity. Food Anal. Methods 2015, 8, 2665–2674. [Google Scholar] [CrossRef]
- Mazzei, R.; Gebreyohannes, A.Y.; Papaioannou, E.; Nunes, S.P.; Vankelecom, I.F.J.; Giorno, L. Enzyme catalysis coupled with artificial membranes towards process intensification in biorefinery- a review. Bioresour. Technol. 2021, 335, 125248. [Google Scholar] [CrossRef]
- Butylina, S.; Luque, S.; Nyström, M. Fractionation of whey-derived peptides using a combination of ultrafiltration and nanofiltration. J. Membr. Sci. 2006, 280, 418–426. [Google Scholar] [CrossRef]


| Process | Material | Manufacturer | Configuration (Internal Diameter, mm) | Area (cm2) | MWCO (kDa) |
|---|---|---|---|---|---|
| UF | PES/PVP PVDF/PVP PVDF/PVP | Pentair | Hollow Fiber * (3), Tubular (5) Tubular (8) | 430 180 170 | 150 150 150 |
| PES/SPES | Pentair | Hollow fibre (~0.8) | 690 | 10 | |
| NF | Modified PES | NXFiltration | Hollow fibre (~0.7) | 650 | 0.8 |
| NXFiltration | Hollow fibre (~0.7) | 650 | 0.4 | ||
| NXFiltration | Hollow fibre (~0.7) | 650 | 0.2 |
| Time (min) | Mobile-Phase Composition | Flow Rate (mL/min) | |
|---|---|---|---|
| A (%) | B (%) | ||
| 0 | 98 | 2 | 0.4 |
| 3 | 87 | 13 | 0.8 |
| 6 | 87 | 13 | 0.8 |
| 9 | 85 | 15 | 0.8 |
| 11 | 80 | 20 | 0.8 |
| 13 | 55 | 45 | 0.8 |
| 16 | 25 | 75 | 0.5 |
| 19 | 80 | 20 | 0.5 |
| 21 | 98 | 2 | 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papaioannou, E.H.; Bazzarelli, F.; Mazzei, R.; Giannakopoulos, V.; Roberts, M.R.; Giorno, L. Membrane Cascade Fractionation of Tomato Leaf Extracts—Towards Bio-Based Crop Protection. Membranes 2023, 13, 855. https://doi.org/10.3390/membranes13110855
Papaioannou EH, Bazzarelli F, Mazzei R, Giannakopoulos V, Roberts MR, Giorno L. Membrane Cascade Fractionation of Tomato Leaf Extracts—Towards Bio-Based Crop Protection. Membranes. 2023; 13(11):855. https://doi.org/10.3390/membranes13110855
Chicago/Turabian StylePapaioannou, Emmanouil H., Fabio Bazzarelli, Rosalinda Mazzei, Vasileios Giannakopoulos, Michael R. Roberts, and Lidietta Giorno. 2023. "Membrane Cascade Fractionation of Tomato Leaf Extracts—Towards Bio-Based Crop Protection" Membranes 13, no. 11: 855. https://doi.org/10.3390/membranes13110855
APA StylePapaioannou, E. H., Bazzarelli, F., Mazzei, R., Giannakopoulos, V., Roberts, M. R., & Giorno, L. (2023). Membrane Cascade Fractionation of Tomato Leaf Extracts—Towards Bio-Based Crop Protection. Membranes, 13(11), 855. https://doi.org/10.3390/membranes13110855










