Recovery of Isoamyl Alcohol by Graphene Oxide Immobilized Membrane and Air-Sparged Membrane Distillation
Abstract
1. Introduction
2. Experimental
2.1. Chemicals and Materials
2.2. Membrane Fabrication
2.3. Experimental Set-Up
2.4. Flux and Separation Factor in Membrane Distillation
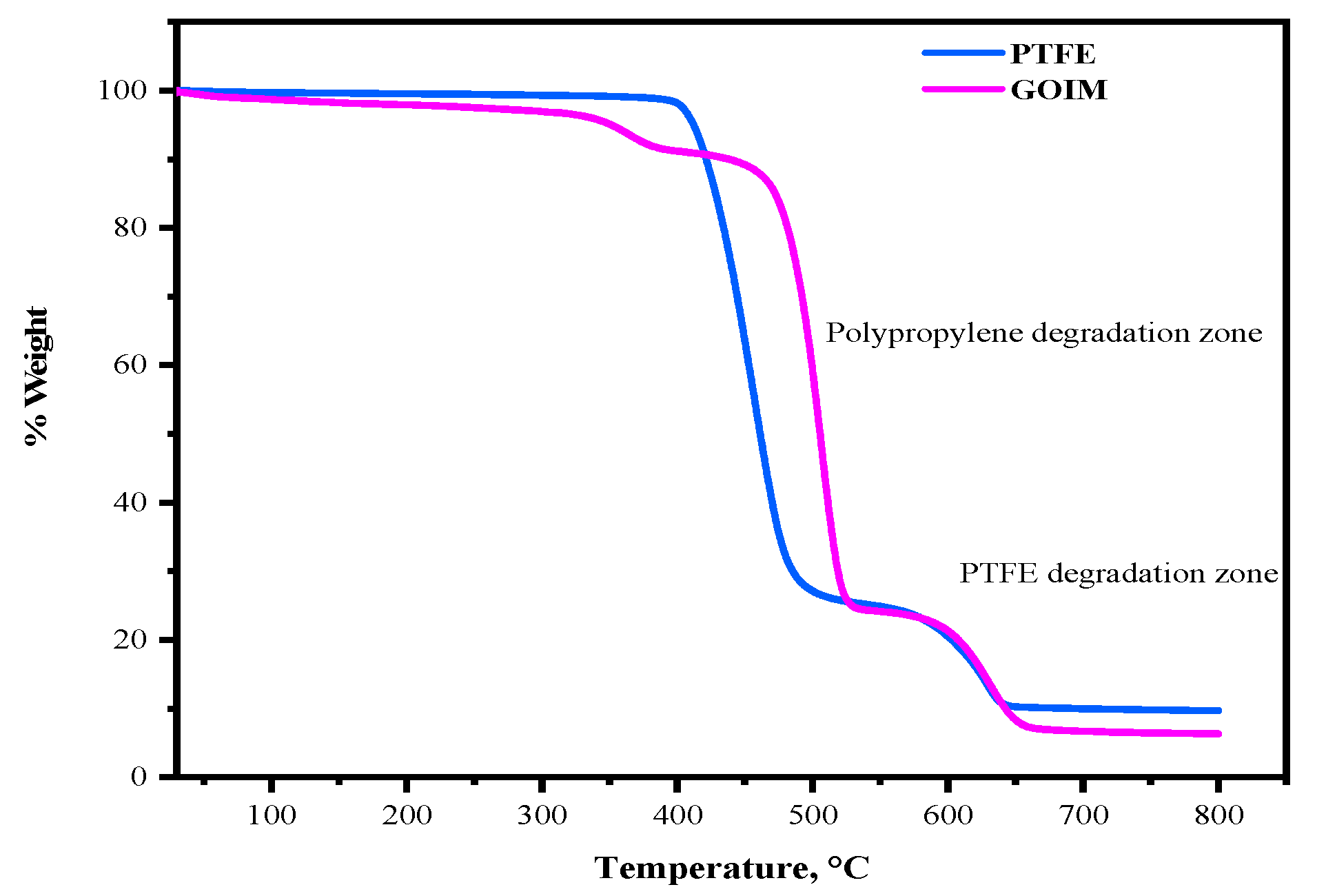
3. Membrane Characterization
4. Results and Discussion
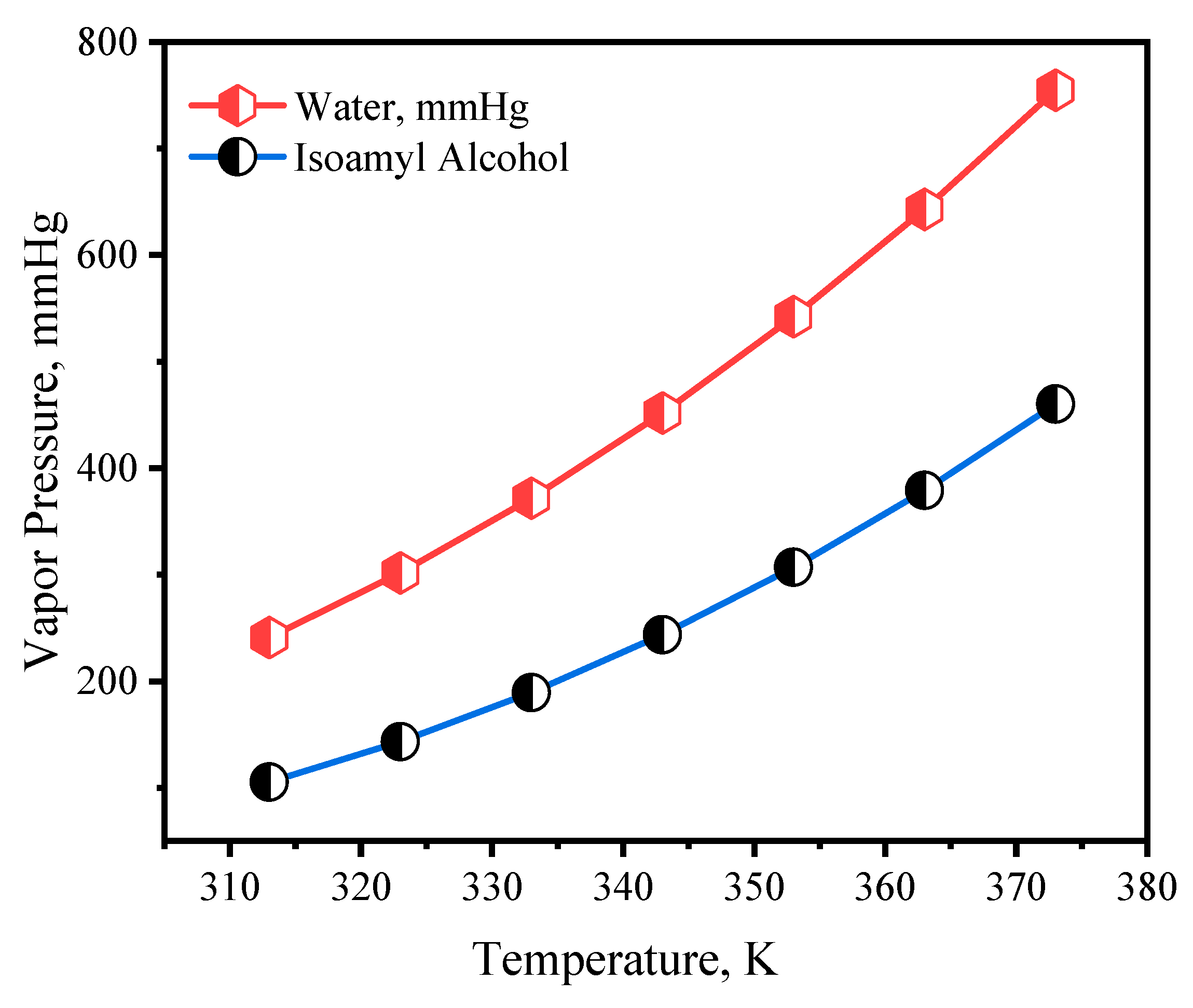
4.1. Antoine Equation for the Isoamyl Alcohol–Water System:
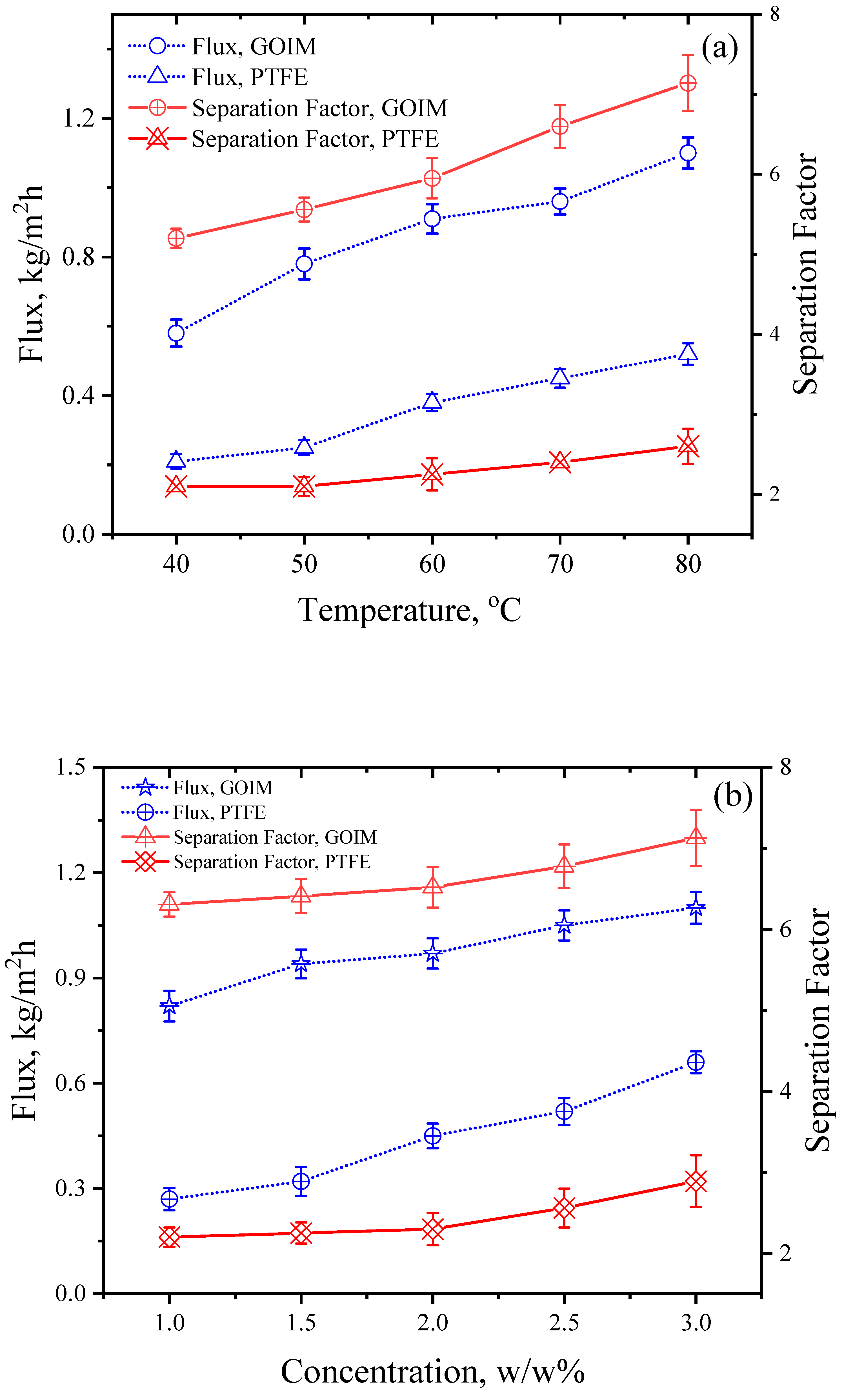
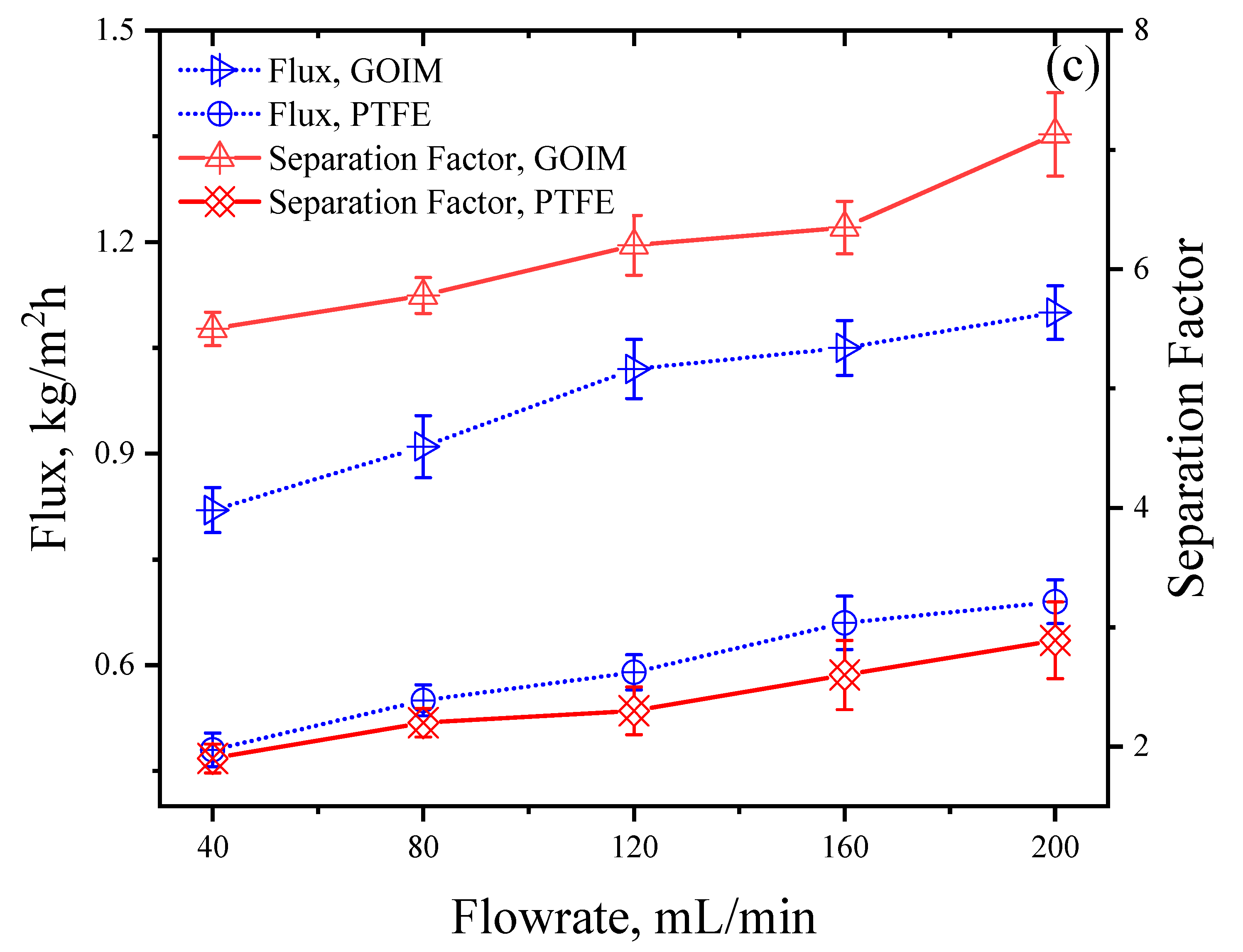
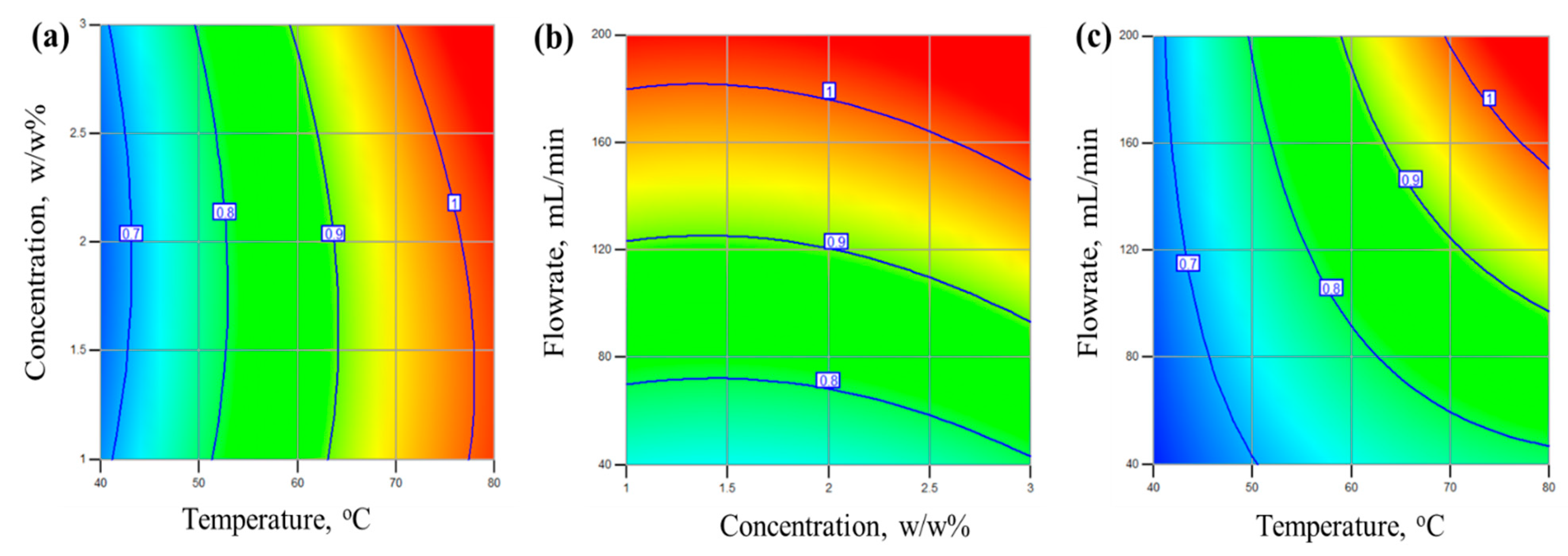
4.2. Effect of Operating Variables on Flux and Separation Factor
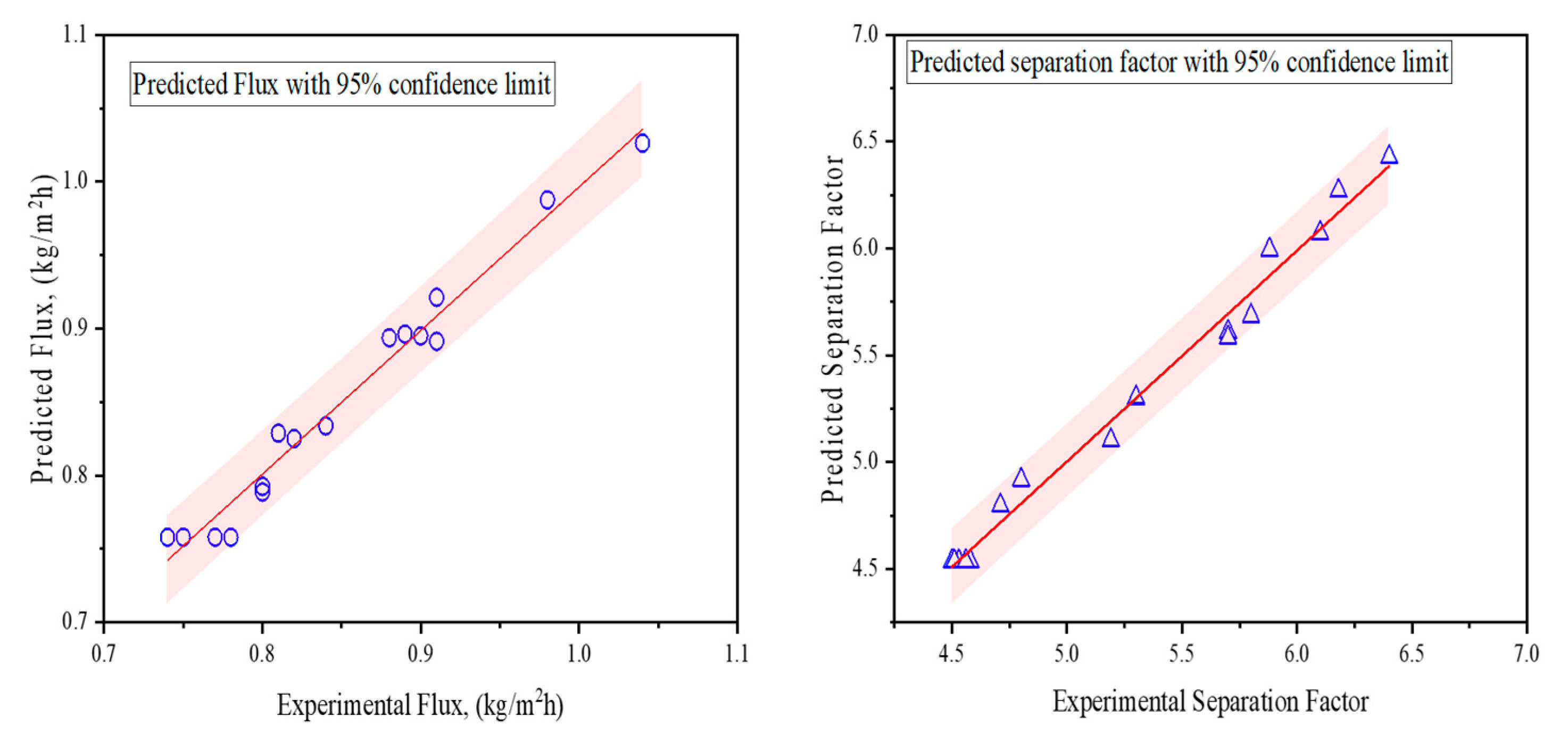
4.3. Prediction of Flux and Separation Factor
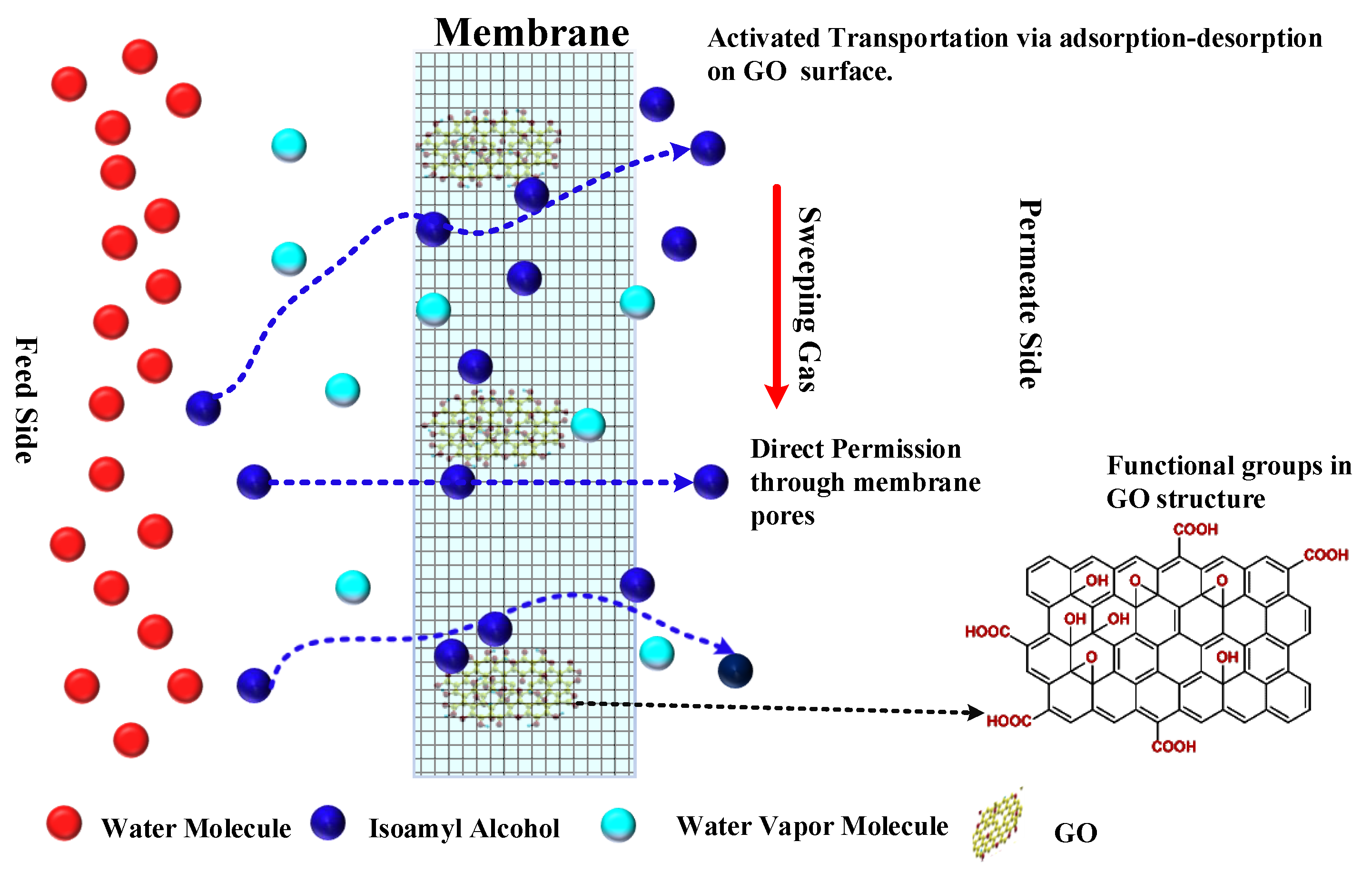
4.4. Mechanism of Isoamyl Alcohol Enrichment
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liew, W.H.; Hassim, M.H.; Ng, D.K.S. Review of evolution, technology and sustainability assessments of biofuel production. J. Clean. Prod. 2014, 71, 11–29. [Google Scholar] [CrossRef]
- Priya; Deora, P.S.; Verma, Y.; Muhal, R.A.; Goswami, C.; Singh, T. Biofuels: An alternative to conventional fuel and energy source. Mater. Today Proc. 2021, 48, 1178–1184. [Google Scholar] [CrossRef]
- Ambaye, T.G.; Vaccari, M.; Bonilla-Petriciolet, A.; Prasad, S.; van Hullebusch, E.D.; Rtimi, S. Emerging technologies for biofuel production: A critical review on recent progress, challenges and perspectives. J. Environ. Manag. 2021, 290, 112627. [Google Scholar] [CrossRef]
- Joshi, G.; Pandey, J.K.; Rana, S.; Rawat, D.S. Challenges and opportunities for the application of biofuel. Renew. Sustain. Energy Rev. 2017, 79, 850–866. [Google Scholar] [CrossRef]
- Muthuraman, V.S.; Kasianantham, N. Valorization opportunities and adaptability assessment of algae based biofuels for futuristic sustainability-A review. Process Saf. Environ. Prot. 2023, 174, 694–721. [Google Scholar] [CrossRef]
- Razzak, S.A.; Lucky, R.A.; Hossain, M.M.; Delasa, H. Valorization of Microalgae Biomass to Biofuel Production: A review. Energy Nexus 2022, 7, 100139. [Google Scholar] [CrossRef]
- Pan, S.; Zabed, H.M.; Wei, Y.; Qi, X. Technoeconomic and environmental perspectives of biofuel production from sugarcane bagasse: Current status, challenges and future outlook. Ind. Crops Prod. 2022, 188, 115684. [Google Scholar] [CrossRef]
- Sandaka, B.P.; Kumar, J. Alternative vehicular fuels for environmental decarbonization: A critical review of challenges in using electricity, hydrogen, and biofuels as a sustainable vehicular fuel. Chem. Eng. J. Adv. 2023, 14, 100442. [Google Scholar] [CrossRef]
- Shokravi, H.; Shokravi, Z.; Heidarrezaei, M.; Ong, H.C.; Koloor, S.S.R.; Petru, M.; Lau, W.J.; Ismail, A.F. Fourth generation biofuel from genetically modified algal biomass: Challenges and future directions. Chemosphere 2021, 285, 131535. [Google Scholar] [CrossRef]
- Singh, R.; Langyan, S.; Rohtagi, B.; Darjee, S.; Khandelwal, A.; Shrivastava, M.; Kothari, R.; Mohan, H.; Raina, S.; Kaur, J.; et al. Production of biofuels options by contribution of effective and suitable enzymes: Technological developments and challenges. Mater. Sci. Energy Technol. 2022, 5, 294–310. [Google Scholar] [CrossRef]
- Ji, X.; Long, X. A review of the ecological and socioeconomic effects of biofuel and energy policy recommendations. Renew. Sustain. Energy Rev. 2016, 61, 41–52. [Google Scholar] [CrossRef]
- Kumar, R.; Ghosh, A.K.; Pal, P. Synergy of biofuel production with waste remediation along with value-added co-products recovery through microalgae cultivation: A review of membrane-integrated green approach. Sci. Total Environ. 2020, 698, 134169. [Google Scholar] [CrossRef]
- Maurya, R.; Gohil, N.; Nixon, S.; Kumar, N.; Noronha, S.B.; Dhali, D.; Trabelsi, H.; Alzahrani, K.J.; Reshamwala, S.M.; Awasthi, M.K.; et al. Rewiring of metabolic pathways in yeasts for sustainable production of biofuels. Bioresour. Technol. 2023, 372, 128668. [Google Scholar] [CrossRef]
- Sadykov, V.A.; Eremeev, N.F.; Sadovskaya, E.M.; Shlyakhtina, A.V.; Pikalova, E.Y.; Osinkin, D.A.; Yaremchenko, A.A. Design of materials for solid oxide fuel cells, permselective membranes, and catalysts for biofuel transformation into syngas and hydrogen based on fundamental studies of their real structure, transport properties, and surface reactivity. Curr. Opin. Green Sustain. Chem. 2022, 33, 100558. [Google Scholar] [CrossRef]
- Sundarsingh, T.J.A.; Ameen, F.; Ranjitha, J.; Raghavan, S.; Shankar, V. Engineering microbes for sustainable biofuel production and extraction of lipids—Current research and future perspectives. Fuel 2024, 355, 129532. [Google Scholar] [CrossRef]
- Khan, S.; Naushad, M.; Iqbal, J.; Bathula, C.; Al-Muhtaseb, A.H. Challenges and perspectives on innovative technologies for biofuel production and sustainable environmental management. Fuel 2022, 325, 124845. [Google Scholar] [CrossRef]
- Wancura, J.H.C.; Brondani, M.; Vezaro, F.D.; Martins-Vieira, J.C.; Moreira, B.P.; dos Santos, M.S.N.; Abaide, E.R.; de Castilhos, F.; Mayer, F.D. Motivations to produce biofuels from rice bran: An overview involving a recent panorama. Ind. Crops Prod. 2023, 203, 117170. [Google Scholar] [CrossRef]
- Wu, S.; Wang, Q.; Fang, M.; Wu, D.; Cui, D.; Pan, S.; Bai, J.; Xu, F.; Wang, Z. Hydrothermal carbonization of food waste for sustainable biofuel production: Advancements, challenges, and future prospects. Sci. Total Environ. 2023, 897, 165327. [Google Scholar] [CrossRef]
- Gómez-Castro, F.I.; Gutiérrez-Antonio, C.; Romero-Izquierdo, A.G.; May-Vázquez, M.M.; Hernández, S. Intensified technologies for the production of triglyceride-based biofuels: Current status and future trends. Renew. Sustain. Energy Rev. 2023, 184, 113580. [Google Scholar] [CrossRef]
- Jiang, L.Y.; Wang, Y.; Chung, T.-S.; Qiao, X.Y.; Lai, J.-Y. Polyimides membranes for pervaporation and biofuels separation. Prog. Progress. Polym. Sci. 2009, 34, 1135–1160. [Google Scholar] [CrossRef]
- Kim, S.; Im, H.; Yu, J.; Kim, K.; Kim, M.; Lee, T. Biofuel production from Euglena: Current status and techno-economic perspectives. Bioresour. Technol. 2023, 371, 128582. [Google Scholar] [CrossRef]
- Fu, C.; Li, Z.; Song, W.; Yi, C.; Xie, S. A new process for separating biofuel based on the salt + 1-butanol + water system. Fuel 2020, 278, 118402. [Google Scholar] [CrossRef]
- Kang, A.; Mendez-Perez, D.; Goh, E.B.; Baidoo, E.E.K.; Benites, V.T.; Beller, H.R.; Keasling, J.D.; Adams, P.D.; Mukhopadhyay, A.; Lee, T.S. Optimization of the IPP-bypass mevalonate pathway and fed-batch fermentation for the production of isoprenol in Escherichia coli. Metab. Eng. 2019, 56, 85–96. [Google Scholar] [CrossRef]
- Monroe, E.; Gladden, J.; Albrecht, K.O.; Bays, J.T.; McCormick, R.; Davis, R.W.; George, A. Discovery of novel octane hyperboosting phenomenon in prenol biofuel/gasoline blends. Fuel 2019, 239, 1143–1148. [Google Scholar] [CrossRef]
- Woodroffe, J.-D.; Harvey, B.G. Thermal cyclodimerization of isoprene for the production of high-performance sustainable aviation fuel. Energy Adv. 2022, 1, 338–343. [Google Scholar] [CrossRef]
- Gil, A. Challenges on waste-to-energy for the valorization of industrial wastes: Electricity, heat and cold, bioliquids and biofuels. Environ. Nanotechnol. Monit. Manag. 2022, 17, 100615. [Google Scholar] [CrossRef]
- Alam, S.N.; Singh, B.; Guldhe, A. Aquatic weed as a biorefinery resource for biofuels and value-added products: Challenges and recent advancements. Clean. Eng. Technol. 2021, 4, 100235. [Google Scholar] [CrossRef]
- Patra, S.; Verma, J.; Mishra, Y.K.; Kurinec, S.; Wang, Q.; Syväjärvi, M.; Tiwari, A. The positioning of biofuel cells-based biobatteries for net-zero energy future. J. Energy Storage 2023, 72, 107919. [Google Scholar] [CrossRef]
- Velvizhi, G.; Jacqueline, P.J.; Shetti, N.P.; Latha, K.; Mohanakrishna, G.; Aminabhavi, T.M. Emerging trends and advances in valorization of lignocellulosic biomass to biofuels. J. Environ. Manag. 2023, 345, 118527. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Sun, Y.; Rathour, R.; Pandey, A.; Thakur, I.S.; Tsang, D.C.W. Algae as potential feedstock for the production of biofuels and value-added products: Opportunities and challenges. Sci. Total Environ. 2020, 716, 137116. [Google Scholar] [CrossRef] [PubMed]
- Baral, N.R.; Yang, M.; Harvey, B.G.; Simmons, B.A.; Mukhopadhyay, A.; Lee, T.S.; Scown, C.D. Production Cost and Carbon Footprint of Biomass-Derived Dimethylcyclooctane as a High-Performance Jet Fuel Blendstock. ACS Sustain. Chem. Eng. 2021, 9, 11872–11882. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Tamura, M.; Tomishige, K. Recent development of production technology of diesel- and jet-fuel-range hydrocarbons from inedible biomass. Fuel Process. Technol. 2019, 193, 404–422. [Google Scholar] [CrossRef]
- Ağbulut, Ü.; Sarıdemir, S.; Karagöz, M. Experimental investigation of fusel oil (isoamyl alcohol) and diesel blends in a CI engine. Fuel 2020, 267, 117042. [Google Scholar] [CrossRef]
- Rosenkoetter, K.E.; Kennedy, C.R.; Chirik, P.J.; Harvey, B.G. [4+4]-Cycloaddition of Isoprene for the Production of High-Performance Bio-Based Jet Fuel. Green Chem. 2019, 21, 5616–5623. [Google Scholar] [CrossRef]
- Davis, M.C.; Prokai, L.; Woodroffe, J.D. Oligo (amylene) from the reaction of fusel oil with zinc dihalide. RSC Adv. 2021, 11, 1960–1968. [Google Scholar] [CrossRef]
- Dahman, Y.; Syed, K.; Begum, S.; Roy, P.; Mohtasebi, B. Biofuels: Their characteristics and analysis. In Biomass, Biopolymer-Based Materials, and Bioenergy; Elsevier: Amsterdam, The Netherlands, 2019; pp. 277–325. [Google Scholar]
- BBC Research. Biorefinary Products: Global Market; BCC Report EGY117D; BCC Publishing: Boston, MA, USA, 2021. [Google Scholar]
- Subhash, G.V.; Rajvanshi, M.; Kumar, G.R.K.; Sagaram, U.S.; Prasad, V.; Govindachary, S.; Dasgupta, S. Challenges in microalgal biofuel production: A perspective on techno economic feasibility under biorefinery stratagem. Bioresour. Technol. 2022, 343, 126155. [Google Scholar]
- Aliyu, A.; Lee, J.; Harvey, A. Microalgae for biofuels: A review of thermochemical conversion processes and associated opportunities and challenges. Bioresour. Technol. Rep. 2021, 15, 100694. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, Q.; Li, L.; Qin, W.; Yang, J.; Zhang, H.; Jiang, X.; Cheng, T.; Liu, W.; Xu, X.; et al. Metabolic engineering of Escherichia coli for high-specificity production of isoprenol and prenol as next generation of biofuels. Biotechnol. Biofuels 2013, 6, 57. [Google Scholar] [CrossRef]
- Zhang, X.; Lei, H.; Zhu, L.; Wei, Y.; Liu, Y.; Yadavalli, G.; Yan, D.; Wu, J.; Chen, S. Production of renewable jet fuel range alkanes and aromatics via integrated catalytic processes of intact biomass. Fuel 2015, 160, 375–385. [Google Scholar] [CrossRef]
- Demirbas, A. Biofuels sources, biofuel policy, biofuel economy and global biofuel projections. Energy Convers. Manag. 2008, 49, 2106–2116. [Google Scholar] [CrossRef]
- Wang, Y.; Qiu, B.; Fan, S.; Liu, J.; Qin, Y.; Jian, S.; Wang, Y.; Xiao, Z. Membrane Distillation of Butanol from Aqueous Solution with Polytetrafluoroethylene Membrane. Chem. Eng. Technol. 2020, 43, 1160–1166. [Google Scholar] [CrossRef]
- Bamasag, A.; Almatrafi, E.; Alqahtani, T.; Phelan, P.; Ullah, M.; Mustakeem, M.; Ghaffour, N. Recent advances and future prospects in direct solar desalination systems using membrane distillation technology. J. Clean. Prod. 2023, 385, 135737. [Google Scholar] [CrossRef]
- Paul, S.; Bhoumick, M.C.; Roy, S.; Mitra, S. Carbon nanotube enhanced membrane filtration for trace level dewatering of hydrocarbons. Sep. Purif. Technol. 2022, 292, 121047. [Google Scholar] [CrossRef]
- Paul, S.; Roy, S.; Mitra, S. Carbon nanotube enhanced selective micro filtration of butanol. Sep. Purif. Technol. 2024, 330, 125462. [Google Scholar] [CrossRef]
- Abid, M.B.; Wahab, R.A.; Salam, M.A.; Moujdin, I.A.; Gzara, L. Desalination technologies, membrane distillation, and electrospinning, an overview. Heliyon 2023, 9, e12810. [Google Scholar] [CrossRef]
- Bhoumick, M.C.; Roy, S.; Mitra, S. Synergistic effect of air Sparging in Direct Contact Membrane Distillation to Control Membrane Fouling and Enhancing Flux. Sep. Purif. Technol. 2021, 272, 118681. [Google Scholar] [CrossRef]
- Presson, L.K.; Felix, V.; Hardikar, M.; Achilli, A.; Hickenbottom, K.L. Fouling characterization and treatment of water reuse concentrate with membrane distillation: Do organics really matter. Desalination 2023, 553, 116443. [Google Scholar] [CrossRef]
- Babiker, D.M.D.; Zhu, L.; Yagoub, H.; Lin, F.; Altam, A.A.; Liang, S.; Jin, Y.; Yang, S. The change from hydrophilicity to hydrophobicity of HEC/PAA complex membrane for water-in-oil emulsion separation: Thermal versus chemical treatment. Carbohydr. Polym. 2020, 241, 116343. [Google Scholar]
- Peng, Y.; Fan, H.; Dong, Y.; Song, Y.; Han, H. Effects of exposure time on variations in the structure and hydrophobicity of polyvinylidene fluoride membranes prepared via vapor-induced phase separation. Appl. Surf. Sci. 2012, 258, 7872–7881. [Google Scholar] [CrossRef]
- Sun, X.; Xue, B.; Yang, S.; Guo, Y.; Qin, S. Controllable surficial and internal hierarchical structures of porous poly (L-lactic acid) membranes for hydrophobicity and potential application in oil-water separation. Surf. Interfaces 2021, 24, 101147. [Google Scholar] [CrossRef]
- Ghaffour, N.; Bundschuh, J.; Mahmoudi, H.; Goosen, M.F.A. Renewable energy-driven desalination technologies: A comprehensive review on challenges and potential applications of integrated systems. Desalination 2015, 356, 94–114. [Google Scholar] [CrossRef]
- Laqbaqbi, M.; García-Payo, M.; Khayet, M.; El Kharraz, J.; Chaouch, M. Application of direct contact membrane distillation for textile wastewater treatment and fouling study. Sep. Purif. Technol. 2019, 209, 815–825. [Google Scholar] [CrossRef]
- Li, F.; Huang, J.; Xia, Q.; Lou, M.; Yang, B.; Tian, Q.; Liu, Y. Direct contact membrane distillation for the treatment of industrial dyeing wastewater and characteristic pollutants. Sep. Purif. Technol. 2018, 195, 83–91. [Google Scholar] [CrossRef]
- Alawad, S.M.; Khalifa, A.E. Development of an efficient compact multistage membrane distillation module for water desalination. Case Stud. Therm. Eng. 2021, 25, 100979. [Google Scholar] [CrossRef]
- Alawad, S.M.; Khalifa, A.E. Performance and energy evaluation of compact multistage air gap membrane distillation system: An experimental investigation. Sep. Purif. Technol. 2021, 268, 118594. [Google Scholar] [CrossRef]
- Alawad, S.M.; Khalifa, A.E.; Antar, M.A. Performance analysis of multistage water gap membrane distillation system with economic evaluation. Appl. Therm. Eng. 2021, 184, 116297. [Google Scholar] [CrossRef]
- Bauer, A.; Wagner, M.; Horn, H.; Saravia, F. Operation conditions affecting scale formation in membrane distillation—An in situ scale study based on optical coherence tomography. J. Membr. Sci. 2021, 623, 118989. [Google Scholar] [CrossRef]
- Bin Bandar, K.; Alsubei, M.D.; Aljlil, S.A.; Bin Darwish, N.; Hilal, N. Membrane distillation process application using a novel ceramic membrane for Brackish water desalination. Desalination 2021, 500, 114906. [Google Scholar] [CrossRef]
- Grasso, G.; Galiano, F.; Yoo, M.J.; Mancuso, R.; Park, H.B.; Gabriele, B.; Figoli, A.; Drioli, E. Development of graphene-PVDF composite membranes for membrane distillation. J. Membr. Sci. 2020, 604, 118017. [Google Scholar] [CrossRef]
- Charfi, K.; Khayet, M.; Safi, M.J. Numerical simulation and experimental studies on heat and mass transfer using sweeping gas membrane distillation. Desalination 2010, 259, 84–96. [Google Scholar] [CrossRef]
- Shukla, S.; Benes, N.E.; Vankelecom, I.; Méricq, J.P.; Belleville, M.P.; Hengl, N.; Marcano, J.S. Sweep gas membrane distillation in a membrane contactor with metallic hollow-fibers. J. Membr. Sci. 2015, 493, 167–178. [Google Scholar] [CrossRef]
- Shukla, S.; Méricq, J.P.; Belleville, M.P.; Hengl, N.; Benes, N.E.; Vankelecom, I.; Marcano, J.S. Process intensification by coupling the Joule effect with pervaporation and sweeping gas membrane distillation. J. Membr. Sci. 2018, 545, 150–157. [Google Scholar] [CrossRef]
- Xie, Z.; Duong, T.; Hoang, M.; Nguyen, C.; Bolto, B. Ammonia removal by sweep gas membrane distillation. Water Res. 2009, 43, 1693–1699. [Google Scholar] [CrossRef]
- Bhoumick, M.C.; Li, C.; Roy, S.; Sundstrom, E.; Harvey, B.G.; Mitra, S. Enhanced Recovery of Aviation Biofuel Precursor Isoprenol Using Nanocarbon-Immobilized Membrane-Based Membrane Distillation. Energy Fuels 2023, 37, 2875–2885. [Google Scholar] [CrossRef]
- Roy, S.; Petrova, R.S.; Mitra, S. Effect of carbon nanotube (CNT) functionalization in Epoxy-CNT composites. Nanotechnol. Rev. 2018, 7, 475–485. [Google Scholar] [CrossRef]
- Ihsanullah, I. Carbon nanotube membranes for water purification: Developments, challenges, and prospects for the future. Sep. Purif. Technol. 2019, 209, 307–337. [Google Scholar] [CrossRef]
- Li, J.; Ren, L.F.; Huang, M.; Yang, J.; Shao, J.; He, Y. Facile preparation of omniphobic PDTS-ZnO-PVDF membrane with excellent anti-wetting property in direct contact membrane distillation (DCMD). J. Membr. Sci. 2022, 650, 120404. [Google Scholar] [CrossRef]
- Liu, J.; Xie, B.; Mushtaq, N.; Xu, G.; Bar-Zeev, E.; Hu, Y. New insights into the role of carbon nanotubes spray-coated on both sides of the PTFE membrane in suppressing temperature polarization and enhancing water flux in direct contact membrane distillation. J. Membr. Sci. 2024, 689, 122184. [Google Scholar] [CrossRef]




| Coefficient | b0 | k1 | k2 | k3 | k4 | k5 | k6 | k7 | k8 | k9 |
|---|---|---|---|---|---|---|---|---|---|---|
| Flux | 0.76 | 0.066 | 0.031 | 0.001 | 0.015 | 0.052 | 0.00025 | 0.081 | 0.36 | 0.069 |
| Selectivity | 4.54 | 0.088 | 0.24 | 0.090 | 0.050 | 0.48 | 0.15 | 1.01 | 0.34 | 0.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhoumick, M.C.; Paul, S.; Roy, S.; Harvey, B.G.; Mitra, S. Recovery of Isoamyl Alcohol by Graphene Oxide Immobilized Membrane and Air-Sparged Membrane Distillation. Membranes 2024, 14, 49. https://doi.org/10.3390/membranes14020049
Bhoumick MC, Paul S, Roy S, Harvey BG, Mitra S. Recovery of Isoamyl Alcohol by Graphene Oxide Immobilized Membrane and Air-Sparged Membrane Distillation. Membranes. 2024; 14(2):49. https://doi.org/10.3390/membranes14020049
Chicago/Turabian StyleBhoumick, Mitun Chandra, Sumona Paul, Sagar Roy, Benjamin G. Harvey, and Somenath Mitra. 2024. "Recovery of Isoamyl Alcohol by Graphene Oxide Immobilized Membrane and Air-Sparged Membrane Distillation" Membranes 14, no. 2: 49. https://doi.org/10.3390/membranes14020049
APA StyleBhoumick, M. C., Paul, S., Roy, S., Harvey, B. G., & Mitra, S. (2024). Recovery of Isoamyl Alcohol by Graphene Oxide Immobilized Membrane and Air-Sparged Membrane Distillation. Membranes, 14(2), 49. https://doi.org/10.3390/membranes14020049










