Dyeable Hydrophilic Surface Modification for PTFE Substrates by Surface Fluorination
Abstract
1. Introduction
2. Materials and Methods
2.1. Surface Modification of PTFE
2.2. Material Characterization
2.3. Dye Staining of PTFE
3. Results and Discussions
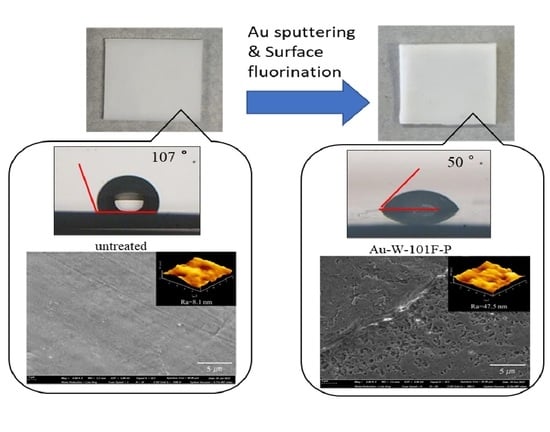
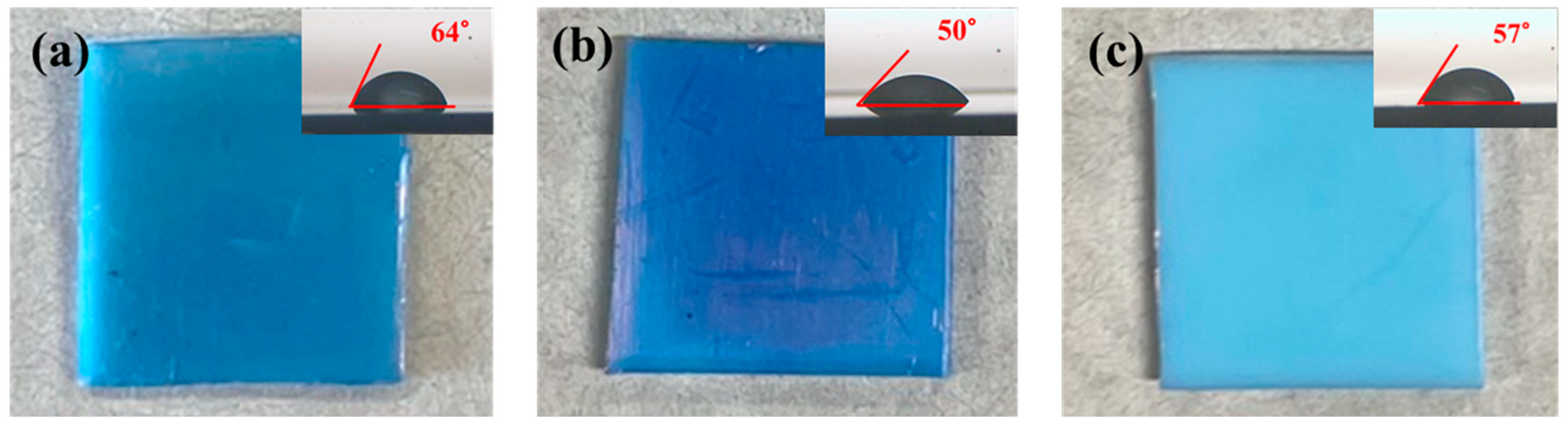
3.1. Surface Modification of PTFE Plates Using Gold Sputtering and Fluorine Gas
3.2. Dyeing of Surface-Modified PTFE Plates
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, J.H.; Kawai, M.; Yonezawa, S.; Takashima, M. Improved thermal stability of crosslinked PTFE using fluorine gas treatment. J. Fluor. Chem. 2008, 129, 654–657. [Google Scholar] [CrossRef]
- Bauer, J.J.; Salky, B.A.; Gelernt, I.M.; Kreel, I. Repair of large abdominal wall defects with expanded polytetrafluoroethylene (PTFE). Ann. Surg. 1987, 206, 765–769. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Lee, J.H.; Kim, C.H.; Kim, Y.J. Fabrication of polytetrafluoroethylene nanofibrous membranes for guided bone regeneration. RSC Adv. 2018, 8, 34359–34369. [Google Scholar] [CrossRef] [PubMed]
- Imbrogno, J.; Rogers, L.; Thomas, D.A.; Jensen, K.F. Continuous purification of active pharmaceutical ingredients utilizing polymer membrane surface wettability. Chem. Commun. 2018, 54, 70–73. [Google Scholar] [CrossRef]
- Budnik, O.A.; Sviderskii, V.A.; Budnik, A.F.; Berladir, K.V.; Rudenko, P.V. Mathematical Modeling of the Mechanical Characteristic of the Activated PTFE-Matrix Using the Method of Planning the Experiment. Chem. Petrol. Eng. 2016, 52, 63–68. [Google Scholar] [CrossRef]
- Extrand, C.W. The use of fluoropolymers to protect semiconductor materials. J. Fluor. Chem. 2003, 122, 121–124. [Google Scholar] [CrossRef]
- Ozeki, K.; Nagashima, I.; Ohgoe, Y.; Hirakuri, K.K.; Mukaibayashi, H.; Masuzawa, T. Gas barrier properties of diamond-like carbon films coated on PTFE. Appl. Surf. Sci. 2009, 255, 7286–7290. [Google Scholar] [CrossRef]
- Khumalo, N.P.; Nthunya, L.N.; Canck, E.D.; Derese, S.; Verliefde, A.R.; Kuvarega, A.T.; Mamba, B.B.; Mhlanga, S.D.; Dlamini, D.S. Congo red dye removal by direct membrane distillation using PVDF/PTFE membrane. Sep. Purif. Technol. 2019, 211, 578–586. [Google Scholar] [CrossRef]
- Ozdemir, S.S.; Buonomenna, M.G.; Drioli, E. Catalytic polymeric membranes: Preparation and application. Appl. Cat. A Gen. 2006, 307, 167–183. [Google Scholar] [CrossRef]
- Oss, C.J.V.; Good, R.J.; Chaudhury, M.K. The role of van der waals forces and hydrogen bonds in “hydrophobic interations” between biopolymers and low energy surfaces. J. Colloid Interface Sci. 1986, 3, 378–390. [Google Scholar]
- Warsinger, D.M.; Chakraborty, S.; Tow, E.W.; Plumlee, M.H.; Bellona, C.; Loutatidou, S.; Karimi, L.; Mikelonis, A.M.; Achilli, A.; Ghassemi, A.; et al. A review of polymeric membranes and processes for potable water reuse. Prog. Polym. Sci. 2018, 81, 209–237. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Yu, H.; Zhu, L.; Xue, L.; Wu, D.; Chen, H. Durable hydrophilic surface modification for PTFE hollow fiber membranes. React. Funct. Polym. 2017, 114, 110–117. [Google Scholar] [CrossRef]
- Shin, J.P.; Chang, B.J.; Kim, J.H.; Lee, S.B.; Dong, H.S. Sulfonated polystyrene/PTFE composite membranes. J. Membr. Sci. 2005, 251, 247–254. [Google Scholar] [CrossRef]
- Liu, F.; Yi, B.; Xing, D.; Yu, J.; Zhang, H. Nafion/PTFE composite membranes for fuel cell applications. J. Membr. Sci. 2004, 212, 213–223. [Google Scholar] [CrossRef]
- Sawyer, W.G.; Freudenberg, K.D.; Bhimaraj, P.; Schadler, L.S. A study on the friction and wear behavior of PTFE filled with alumina nanoparticles. Wear 2004, 254, 573–580. [Google Scholar] [CrossRef]
- Tu, C.Y.; Liu, Y.L.; Lee, K.R.; Lai, J.Y. Hydrophilic surface-grafted poly(tetrafluoroethylene) membranes using in pervaporation dehydration processes. J. Membr. Sci. 2006, 274, 47–55. [Google Scholar] [CrossRef]
- Giorgi, L.; Antolini, E.; Pozio, A.; Passalacqua, E. Influence of the PTFE content in the diffusion layer of low-Pt loading electrodes for polymer electrolyte fuel cells. Electrochim. Acta 1998, 43, 3675–3680. [Google Scholar] [CrossRef]
- Bendavid, R. Composite mesh (polypropylene-e-PTFE) in the intraperitoneal position. Hernia 1997, 1, 5–8. [Google Scholar] [CrossRef]
- Chong, K.C.; Lai, S.O.; Thiam, H.S.; Lee, S.S.; Lau, W.J.; Mokhtar, N.M. Reactive blue dye removal by membrane distillation using PVDF membrane. Indian J. of Sci. Technol. 2016, 9, 1–5. [Google Scholar] [CrossRef]
- Mitsuya, K.; Goto, S.; Otsuka, Y.; Kawano, Y.; Hanawa, T. Saturated adsorption of lidocaine and coal tar dyes onto porous polytetrafluoroethylen. RSC Adv. 2022, 12, 1914–1921. [Google Scholar] [CrossRef]
- Kinoshita, H.; Yonezawa, S.; Kim, J.H.; Kawai, M.; Takashima, M.; Tsukatani, T. Electroless Ni Plating on PTFE fine particles. J. Fluor. Chem. 2008, 129, 416–423. [Google Scholar] [CrossRef]
- Kinoshita, H.; Yonezawa, S.; Kim, J.H.; Kawai, M.; Takashima, M.; Tsukatani, T. Preparation and characterization of Ni plated polytetrafluoroethylene plate as an electrode for alkaline fuel cell. J. Power Sources 2008, 183, 464–470. [Google Scholar] [CrossRef]
- Kim, J.H.; Yonezawa, S.; Takashima, M. Preparation and characterization of Ni-PTFE plate as an electrode for alkaline fuel cell: Effects of conducting materials on the performance of electrode. Int. J. Hydrogen Energy 2010, 35, 8707–8714. [Google Scholar] [CrossRef]
- Kim, J.H.; Yonezawa, S.; Takashima, M. Preparation and characterization of C/Ni-PTFE electrode using Ni-PTFE composite plating for alkaline fuel cells. Int. J. Hydrogen Energy 2011, 36, 1720–1729. [Google Scholar] [CrossRef]
- Kim, J.H.; Yonezawa, S.; Takashima, M. Preparation and characterization of carbon composite plates using Ni-PTFE composite nano-plating. Appl. Surf. Sci. 2013, 279, 329–333. [Google Scholar] [CrossRef]
- Marchesi, J.T.; Keith, H.D.; Garton, A. Adhesion of sodium naphthalenide treated fluoropolymers, Part III. Mechanism of Adhesion. J. Adhes. 1992, 39, 185–205. [Google Scholar] [CrossRef]
- Dwight, D.W.; Riggs, W.M. Fluoropolymer surface studies. J. Colloid Interface Sci. 1974, 47, 650–660. [Google Scholar] [CrossRef]
- Ohkubo, Y.; Okazaki, Y.; Shibahara, M.; Nishino, M.; Seto, Y.; Endo, K. Effects of He and Ar heat-assisted plasma treatments on the adhesion properties of polytetrafluoroethylene (PTFE). Polymers 2021, 13, 4266. [Google Scholar] [CrossRef] [PubMed]
- Omichi, M.; Yamashita, S.; Okura, Y.; Ikutomo, R.; Ueki, Y.; Seko, N.; Kakuchi, R. Surface engineering of fluoropolymer films vis the attachment of crown ether derivatives based on the combination of radiation-induced graft polymerization and the kabachnik-fields reaction. Polymers 2019, 11, 1337. [Google Scholar] [CrossRef]
- Primc, G. Recent advances in surface activation of polytetrafluoroethylene (PTFE) by gaseous plasma treatments. Polymers 2020, 12, 2295. [Google Scholar] [CrossRef]
- Kolska, Z.; Reznickova, A.; Hnatowicz, V.; Svorcik, V. PTFE surface modification by Ar plasma and its characterization. Vaccum 2012, 86, 643–647. [Google Scholar] [CrossRef]
- Rao, X.; Zhou, Q.; Wen, Q.; Ou, Z.; Fu, L.; Gong, Y.; Du, X.; Huo, C. High performance and water resistance PVA based films modified by air plasma treatment. Membranes 2022, 12, 249. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.; Zhao, Y.; Zhang, M.; Li, X.; Li, H. Surface modification of ETTE membrane and PTFE membrane by atmospheric DBD plasma. Membranes 2022, 12, 510. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Namie, M.; Yonezawa, S. Enhanced adhesion between polyethylene terephthalate and metal film by surface fluorination. Compos. Commun. 2018, 10, 205–208. [Google Scholar] [CrossRef]
- Kim, J.H.; Mishina, T.; Namie, M.; Nishimura, F.; Yonezawa, S. Effects of suface fluorination on the dyeing of polycarbonate (PC) resin. J. Coat. Technol. Res. 2021, 19, 617–624. [Google Scholar] [CrossRef]
- Namie, M.; Kim, J.H.; Yonezawa, S. Improving the dyeing of polypropylene by surface fluorination. Colorants 2022, 1, 121–131. [Google Scholar] [CrossRef]
- Tanaike, O.; Hatori, H.; Yamada, Y.; Shiraishi, S.; Oya, A. Preparation and pore control of highly mesoporous carbon from defluorinated PTFE. Carbon 2003, 41, 1759–1764. [Google Scholar] [CrossRef]
- Shiraishi, S.; Kurihara, H.; Tsubota, H.; Oya, A.; Liang, T.T.; Yamada, Y. Electric double layer capacitance of highly porous carbon derived from lithium metal and polytetrafluoroethylene. Electrochem. Solid-State Lett. 2001, 4, A5–A8. [Google Scholar] [CrossRef]
- Shiraishi, S.; Aoyama, Y.; Kurihara, H.; Oya, A.; Yamada, Y. Double layer capacitance of porous carbons derived from defluorination of PTFE. Mol. Cryst. Liq. Cryst. 2002, 388, 129–135. [Google Scholar] [CrossRef]
- Kim, J.H.; Umeda, H.; Ohe, M.; Yonezawa, S.; Takashima, M. Preparation of pure LiPF6 using fluorine gas at room temperature. Chem. Lett. 2011, 40, 360–361. [Google Scholar] [CrossRef]
- Ohkubo, Y.; Ishihara, K.; Shibahara, M.; Nagatani, A.; Honda, K.; Endo, K.; Yamamura, K. Drastic improvement in adhesion property of polytetrafluoroethylene (PTFE) via heat-assisted plasma treatment using a heater. Sci. Rep. 2017, 7, 9476. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.R.; Wakida, T. Studies on the surface free energy and surface structure of PTFE film treated with low temperature plasma. J. Appl. Polym. Sci. 1997, 63, 1733–1739. [Google Scholar]

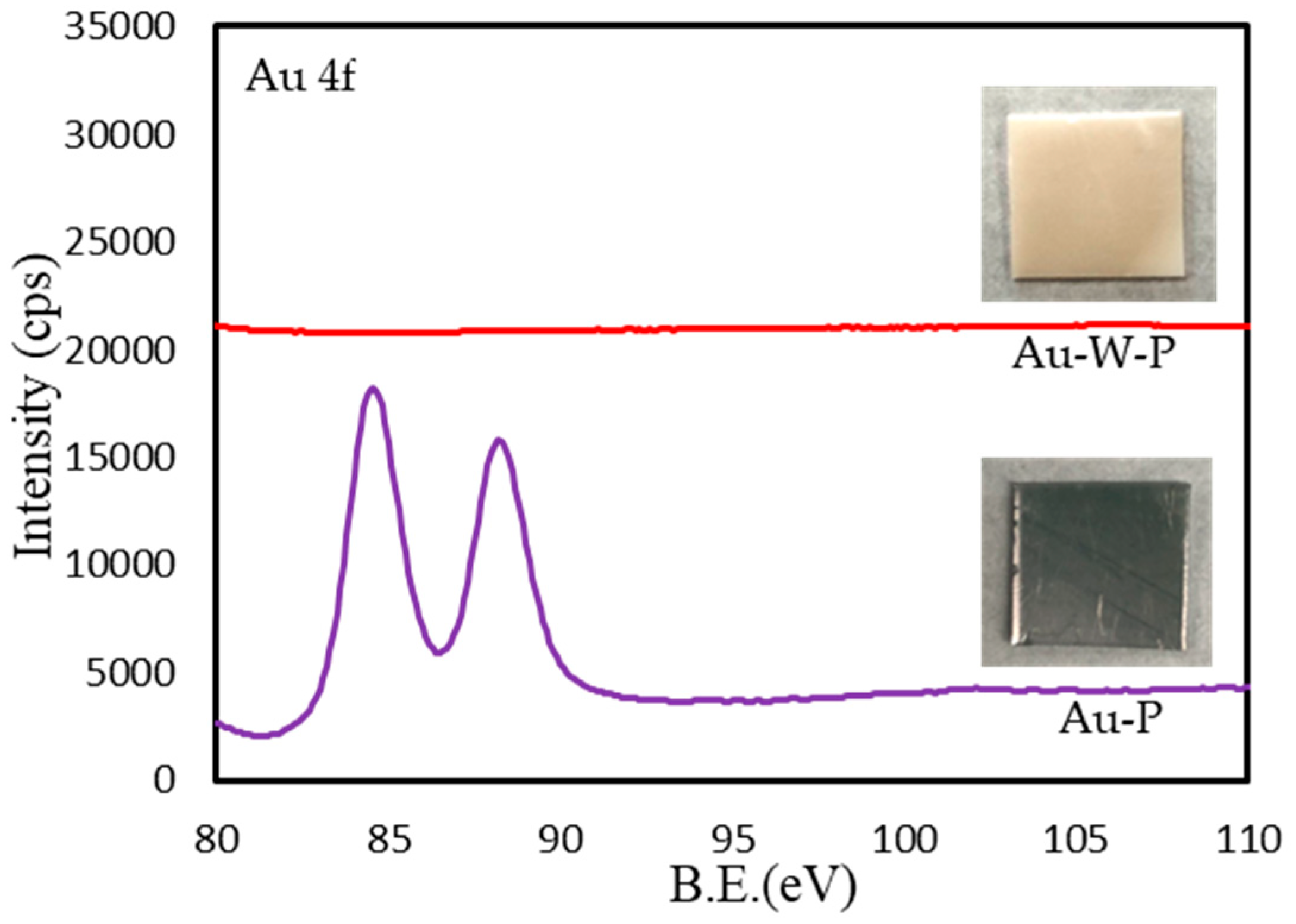
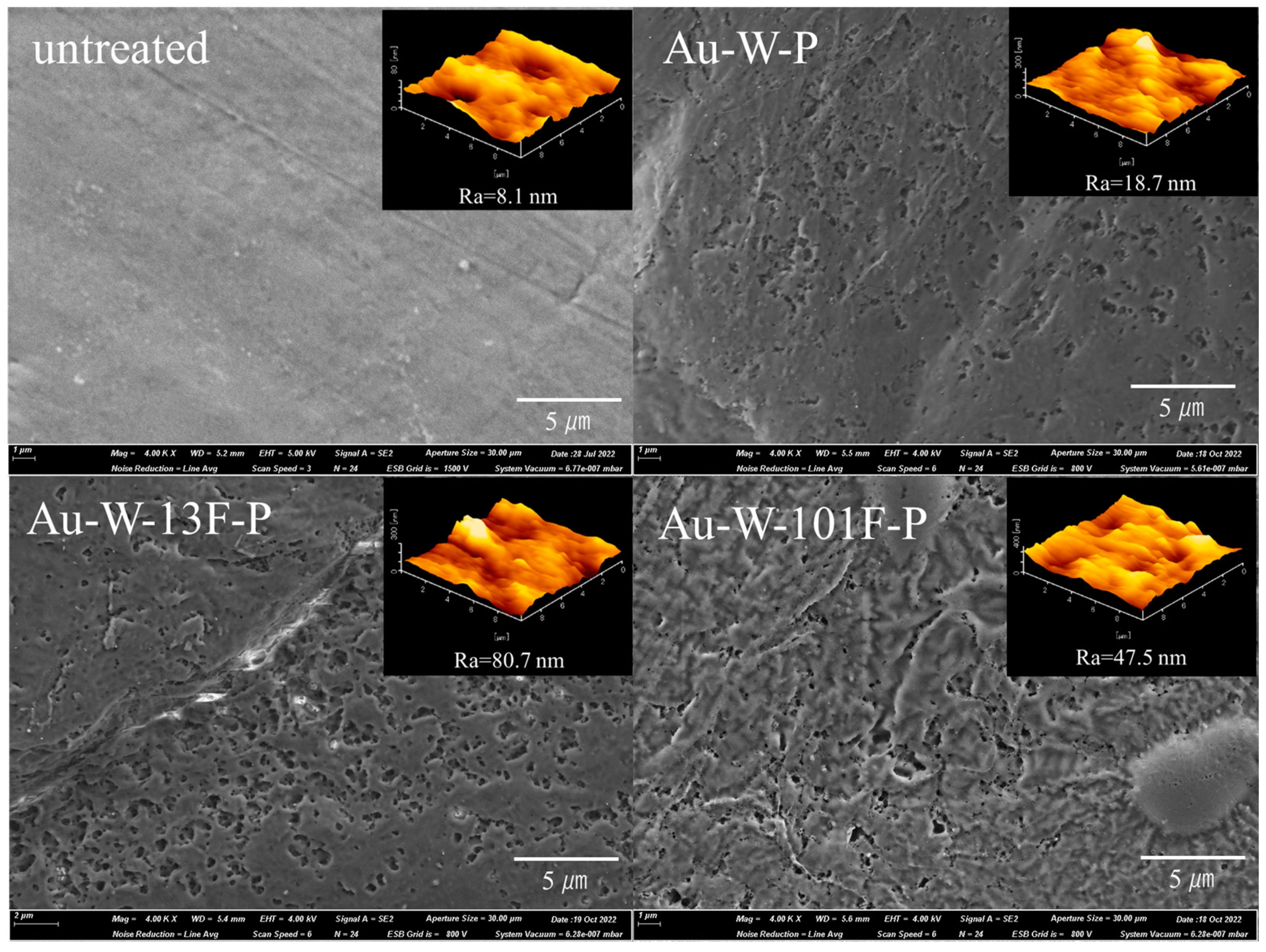
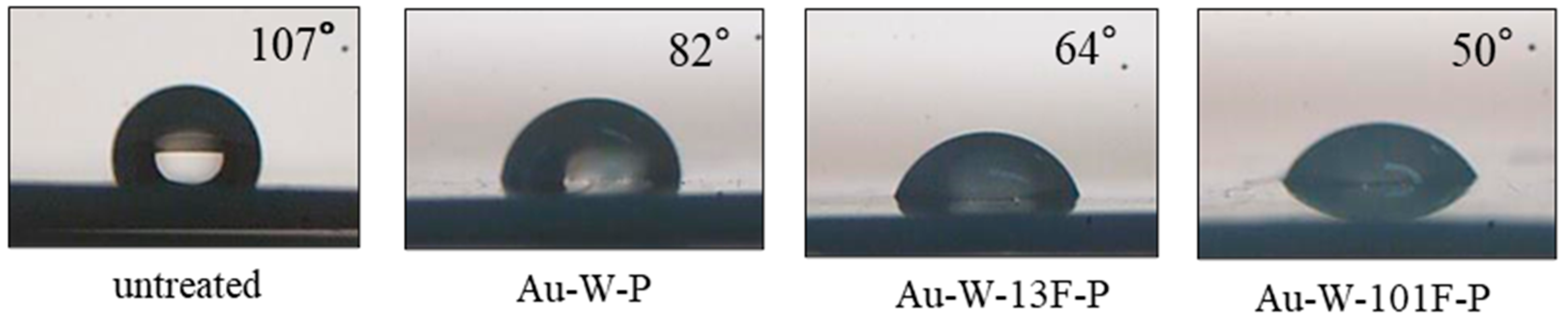
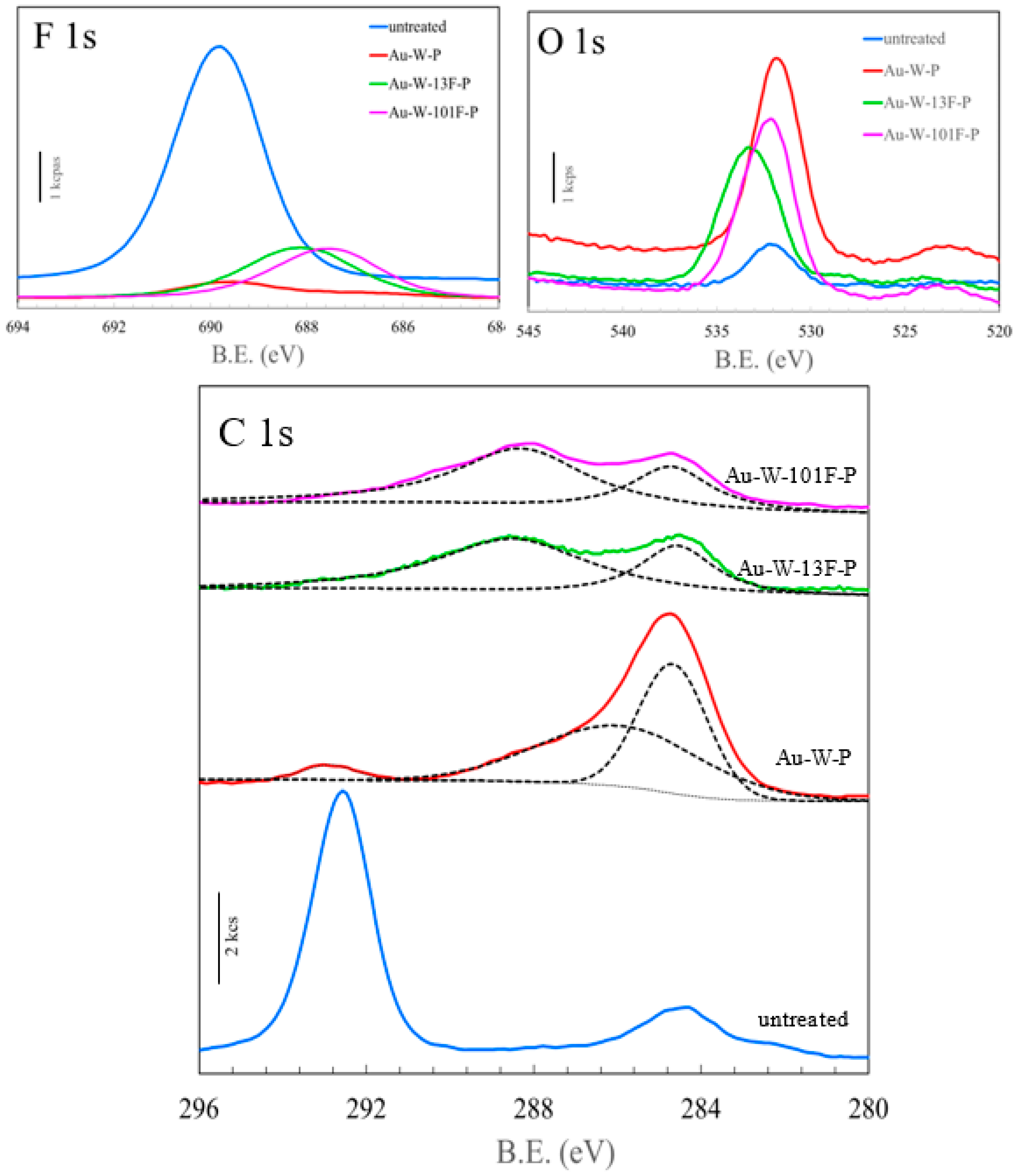


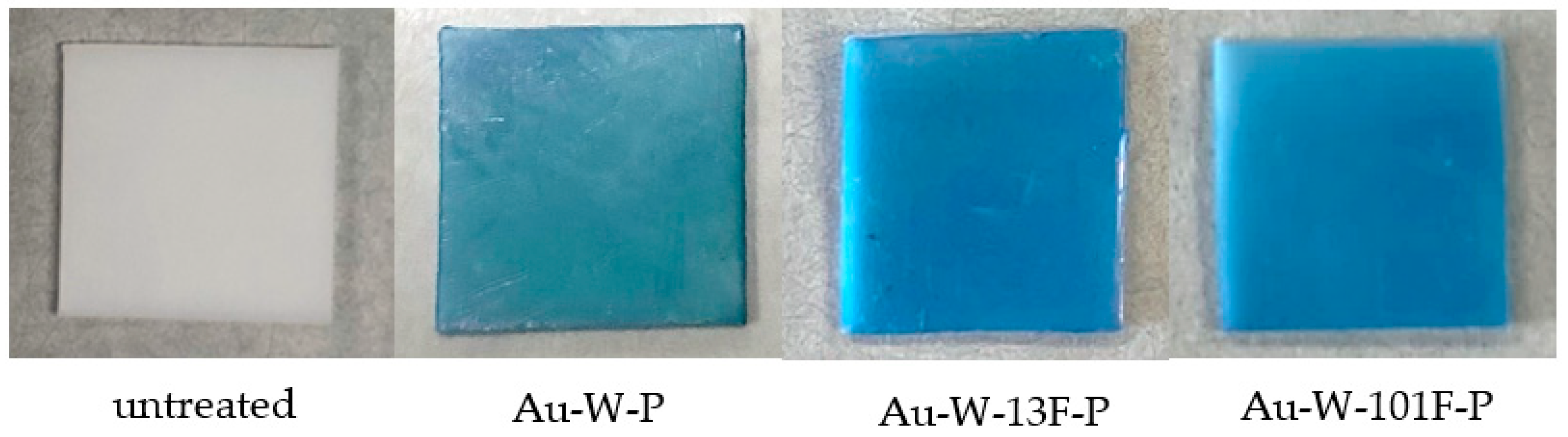
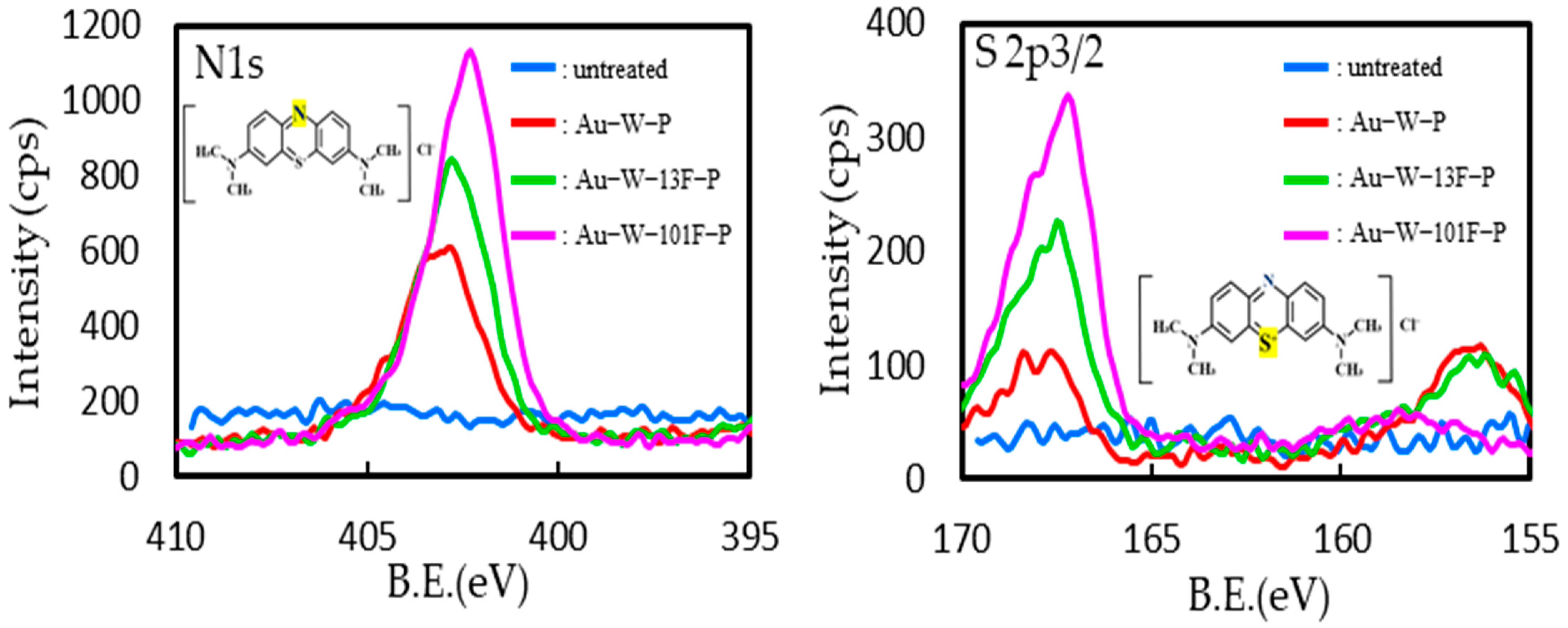
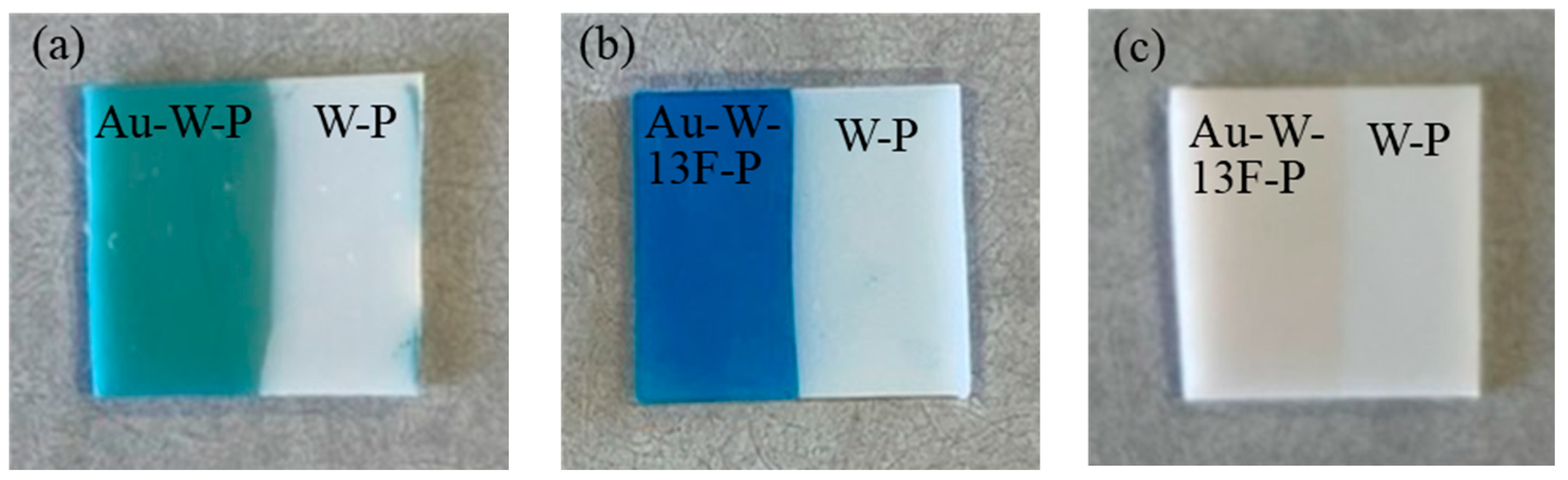
| Sample Name | Au Coating | Washing by Aqua Regia | Surface Fluorination | ||
|---|---|---|---|---|---|
| Temp. (°C) | Time (h) | Pressure (kPa) | |||
| untreated | - | - | - | - | - |
| Au-P | with | without | - | - | - |
| Au-W-P | with | with | - | - | - |
| Au-W-13F-P | with | with | 25 | 1 | 13 |
| Au-W-101F-P | with | with | 101 | ||
| Sample Name | Surface Composition Ratio (%) | F/C | ||
|---|---|---|---|---|
| C | O | F | ||
| untreated | 29.19 | 4.93 | 65.87 | 2.26 |
| Au-W-P | 52.67 | 25.53 | 21.8 | 0.41 |
| Au-W-13F-P | 27.73 | 23.23 | 49.04 | 1.77 |
| Au-W-101F-P | 28.67 | 24.18 | 47.16 | 1.64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobayashi, M.; Nishimura, F.; Kim, J.-H.; Yonezawa, S. Dyeable Hydrophilic Surface Modification for PTFE Substrates by Surface Fluorination. Membranes 2023, 13, 57. https://doi.org/10.3390/membranes13010057
Kobayashi M, Nishimura F, Kim J-H, Yonezawa S. Dyeable Hydrophilic Surface Modification for PTFE Substrates by Surface Fluorination. Membranes. 2023; 13(1):57. https://doi.org/10.3390/membranes13010057
Chicago/Turabian StyleKobayashi, Mizuki, Fumihiro Nishimura, Jae-Ho Kim, and Susumu Yonezawa. 2023. "Dyeable Hydrophilic Surface Modification for PTFE Substrates by Surface Fluorination" Membranes 13, no. 1: 57. https://doi.org/10.3390/membranes13010057
APA StyleKobayashi, M., Nishimura, F., Kim, J.-H., & Yonezawa, S. (2023). Dyeable Hydrophilic Surface Modification for PTFE Substrates by Surface Fluorination. Membranes, 13(1), 57. https://doi.org/10.3390/membranes13010057