Combination of Zinc Oxide Photocatalysis with Membrane Filtration for Surface Water Disinfection
Abstract
1. Introduction
2. Materials and Methods
2.1. Photocatalytic Membrane System
2.2. Nanoparticles and Membrane Modification
2.3. Safety and Recovery of Catalyst in Different Configurations
- (1)
- Recovery of catalyst in 250 mL of suspension (0.5 g/L);
- (2)
- Recovery of catalyst in 250 mL of suspension (0.5 g/L) with the UV-LED light emitting at 365 nm;
- (3)
- Stability of catalyst in the modified membrane after filtration of 250 mL of DI water (DIW);
- (4)
- Stability of catalyst in the modified membrane after filtration of 250 mL of DI water with the UV-LED light emitting at 365 nm.
2.4. Treatment Performance of Real Surface Water Samples
2.5. Quantification of Total Coliforms and Escherichia coli in Water Samples
3. Results
3.1. Recovery of Catalyst
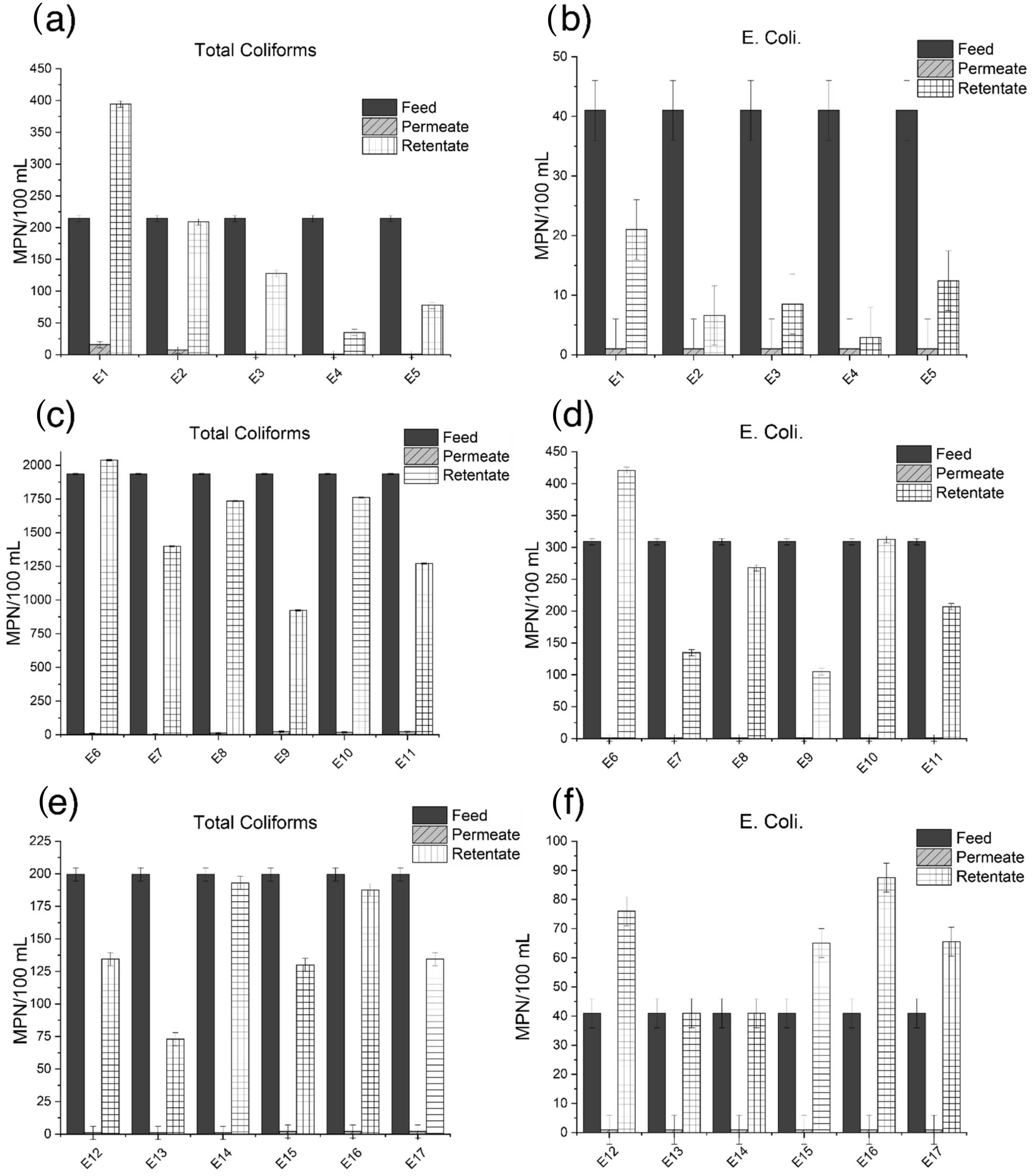
3.2. Treatment Performance
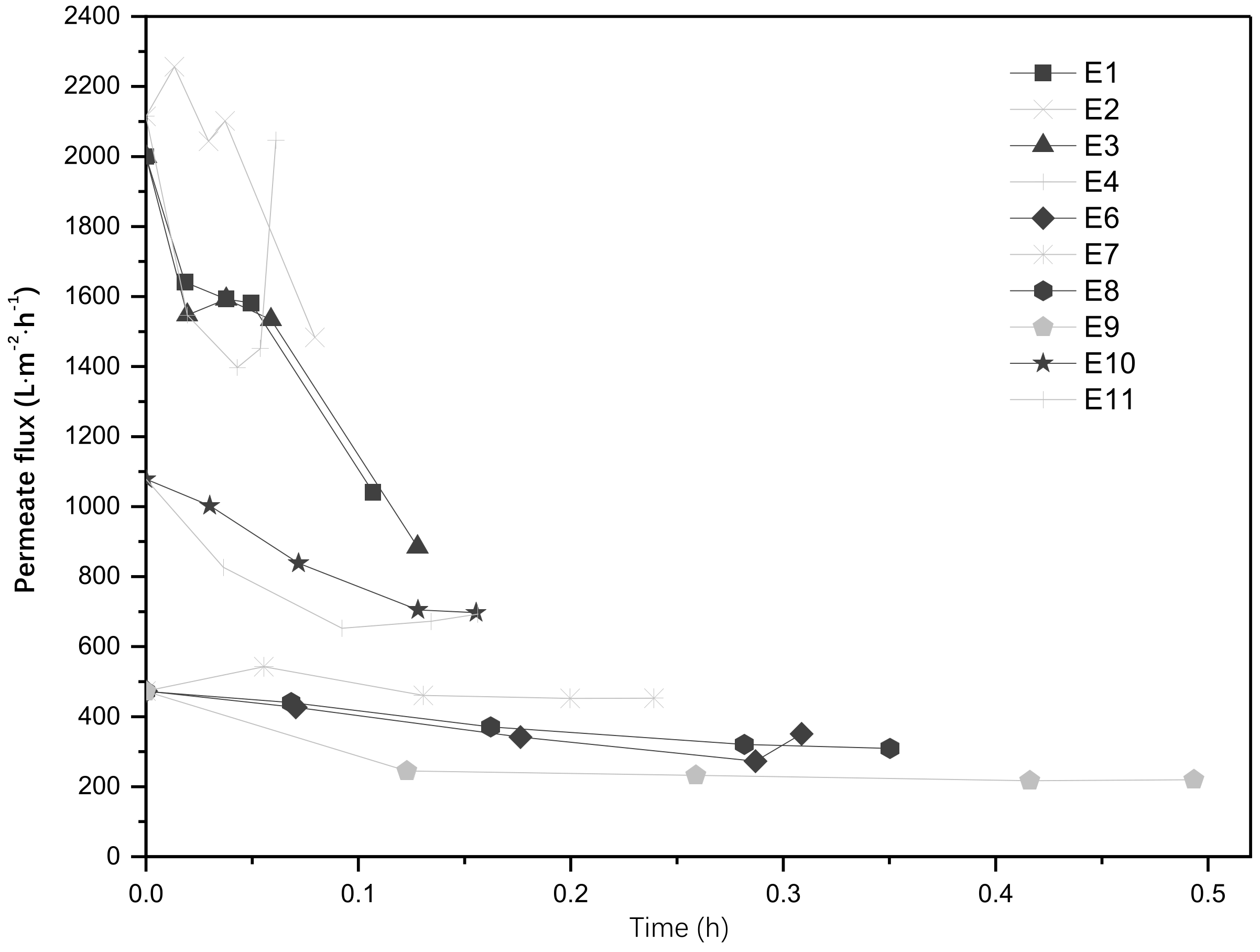
3.3. Impact of Catalyst Load on the Permeate Flux
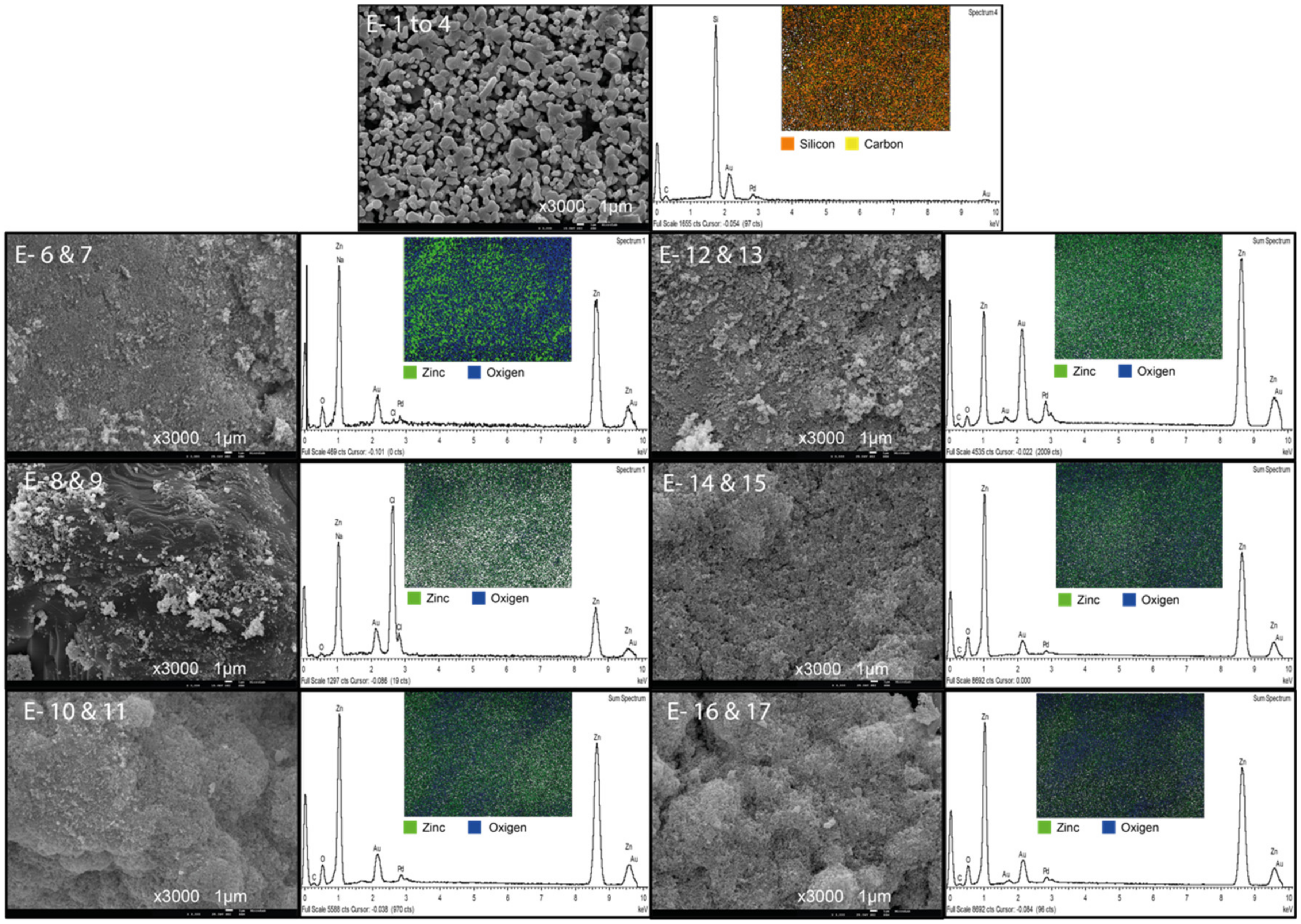
3.4. Characterization of the Membranes
3.5. Membrane Surface Porosity and Particle Size
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guppy, L.; Anderson, K. Water Crisis Report; United Nations University Institute for Water, Environment and Health: Hamilton, ON, Canada, 2017. [Google Scholar]
- IPCC. Climate Change 2014: Synthesis Report; Contribution of Working Groups I, II and III to the Fifth; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- Seyboth, K.; Plattner, G.-K. Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- United Nations World Water Assessment Programme. The United Nations World Water Development Report, Wastewater: The Untapped Resource; United Nations World Water Assessment Programme: Geneva, Switzerland, 2017. [Google Scholar]
- United Nations Environment Programme. The UN-Water Status Report on the Application of Integrated. Approaches to Water Resources Management; United Nations Environment Programme: Geneva, Switzerland, 2012. [Google Scholar]
- Rosegrant, M.W.; Cai, X.; Cline, S.A. World Water and Food to 2025: Dealing with Scarcity; International Food Policy Research Institute: Washington, DC, USA, 2002. [Google Scholar]
- UNEP. A Snapshot of the World’s Water Quality: Towards a Global Assessment; UNEP: Geneva, Switzerland, 2016. [Google Scholar]
- European Commission. Report on the Implementation of the Water Framework Directive River Basin. Management Plans Member State: PORTUGAL; European Commission: Brussels, Belgium, 2015.
- OECD. OECD Economic Outlook, Interim Report March 2021; OECD: Paris, France, 2021. [Google Scholar]
- UNEP. GEO-6: Regional Assessment for Latin America and the Caribbean; UNEP: Nairobi, Kenya, 2016. [Google Scholar]
- Chahal, C.; Van Den Akker, B.; Young, F.; Franco, C.; Blackbeard, J.; Monis, P. Pathogen and Particle Associations in Wastewater: Significance and Implications for Treatment and Disinfection Processes. Adv. Appl. Microbiol. 2016, 97, 63–119. [Google Scholar] [PubMed]
- Li, C.; Sun, W.; Lu, Z.; Ao, X.; Yang, C.; Li, S. Systematic evaluation of TiO2-GO-modified ceramic membranes for water treatment: Retention properties and fouling mechanisms. Chem. Eng. J. 2019, 378, 122138. [Google Scholar] [CrossRef]
- Li, N.N.; Fane, A.G.; Ho, W.S.W.; Matsuura, T. Advanced Membrane Technology and Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008. [Google Scholar] [CrossRef]
- Guo, W.; Ngo, H.H.; Li, J. A mini-review on membrane fouling. Bioresour. Technol. 2012, 122, 27–34. [Google Scholar] [CrossRef]
- Argurio, P.; Fontananova, E.; Molinari, R.; Drioli, E. Photocatalytic Membranes in Photocatalytic Membrane Reactors. Process 2018, 6, 162. [Google Scholar] [CrossRef]
- Mozia, S. Photocatalytic membrane reactors (PMRs) in water and wastewater treatment. A review. Sep. Purif. Technol. 2010, 73, 71–91. [Google Scholar] [CrossRef]
- Kuiper, D.; Bom, C.A.; Hezel, J.L.; Van Verdouw, J. The use of reverse osmosis for the treatment of Rhine river water. Part I. Desalination 1974, 14, 163–172. [Google Scholar] [CrossRef]
- Hofs, B.; Ogier, J.; Vries, D.; Beerendonk, E.F.; Cornelissen, E.R. Comparison of ceramic and polymeric membrane permeability and fouling using surface water. Sep. Purif. Technol. 2011, 79, 365–374. [Google Scholar] [CrossRef]
- Harman, B.I.; Koseoglu, H.; Yigit, N.O.; Beyhan, M.; Kitis, M. The use of iron oxide-coated ceramic membranes in removing natural organic matter and phenol from waters. Desalination 2010, 261, 27–33. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, W.; Lin, Y.; Cai, Y.; Qiu, M.; Fan, Y. Preparation of high-flux γ-alumina nanofiltration membranes by using a modified sol-gel method. Microporous Mesoporous Mater. 2015, 214, 195–203. [Google Scholar] [CrossRef]
- Alem, A.; Sarpoolaky, H.; Keshmiri, M. Titania ultrafiltration membrane: Preparation, characterization and photocatalytic activity. J. Eur. Ceram. Soc. 2009, 29, 629–635. [Google Scholar] [CrossRef]
- Hristov, P.; Yoleva, A.; Djabazov, S.; Chukovska, I.; Dimitrov, D. Preparation and characterization of porous ceramic membranes for micro-filtration from natural zeolite. J. Univ. Chem. Technol. Metall. 2012, 47, 476–480. [Google Scholar]
- Benfer, S.; Árki, P.; Tomandl, G. Ceramic Membranes for Filtration Applications—Preparation and Characterization. Adv. Eng. Mater. 2004, 6, 495–500. [Google Scholar] [CrossRef]
- Mohammadi, T.; Maghsoodloorad, H. Synthesis and Characterization of Ceramic Membranes (W-Type Zeolite Membranes). Int. J. Appl. Ceram. Technol. 2013, 10, 365–375. [Google Scholar] [CrossRef]
- Zhang, H.; Wan, Y.; Luo, J.; Darling, S.B. Drawing on Membrane Photocatalysis for Fouling Mitigation. ACS Appl. Mater. Interfaces 2021, 13, 14844–14865. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental Applications of Semiconductor Photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Palmisano, G.; Garcia-Lopez, E.; Marci, G.; Loddo, V.; Yurdakal, S.; Augugliaro, V.; Palmisano, L. Advances in selective conversions by heterogeneous photocatalysis. Chem. Commun. 2010, 46, 7074–7089. [Google Scholar] [CrossRef]
- Fraga, M.C.; Huertas, R.M.; Crespo, J.G.; Pereira, V.J. Novel Submerged Photocatalytic Membrane Reactor for Treatment of Olive Mill Wastewaters. Catalysts 2019, 9, 769. [Google Scholar] [CrossRef]
- Chong, M.N.; Jin, B.; Chow, C.W.K.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef]
- Mozia, S.; Darowna, D.; Przepiórski, J.; Morawski, A.W. Evaluation of performance of hybrid photolysis-DCMD and photocatalysis-DCMD systems utilizing UV-C radiation for removal of diclofenac sodium salt from water. Polish J. Chem. Technol. 2013, 15, 51–60. [Google Scholar] [CrossRef]
- Horovitz, I.; Avisar, D.; Luster, E.; Lozzi, L.; Luxbacher, T.; Mamane, H. MS2 bacteriophage inactivation using a N-doped TiO2-coated photocatalytic membrane reactor: Influence of water-quality parameters. Chem. Eng. J. 2018, 354, 995–1006. [Google Scholar] [CrossRef]
- Huo, Y.; Xie, Z.; Wang, X.; Li, H.; Hoang, M.; Caruso, R.A. Methyl orange removal by combined visible-light photocatalysis and membrane distillation. Dye. Pigment. 2013, 98, 106–112. [Google Scholar] [CrossRef]
- Cai, L.; Zhang, T. Detecting human bacterial pathogens in wastewater treatment plants by a high-throughput shotgun sequencing technique. Environ. Sci. Technol. 2013, 47, 5433–5441. [Google Scholar] [CrossRef] [PubMed]
- Molinari, R.; Argurio, P.; Bellardita, M.; Palmisano, L. 3.5 Photocatalytic Processes in Membrane Reactors. In Comprehensive Membrane Science and Engineering; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar] [CrossRef]
- Razmjou, A.; Resosudarmo, A.; Holmes, R.L.; Li, H.; Mansouri, J.; Chen, V. The effect of modified TiO 2 nanoparticles on the polyethersulfone ultrafiltration hollow fiber membranes. Desalination 2012, 287, 271–280. [Google Scholar] [CrossRef]
- Tan, B.Y.L.; Juay, J.; Liu, Z.; Sun, D. Flexible Hierarchical TiO2/Fe2O3 Composite Membrane with High Separation Efficiency for Surfactant-Stabilized Oil-Water Emulsions. Chem. Asian J. 2016, 11, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Di Valentin, C.; Pacchioni, G.; Selloni, A. Theory of carbon doping of titanium dioxide. Chem. Mater. 2005, 17, 6656–6665. [Google Scholar] [CrossRef]
- Ani, I.J.; Akpan, U.G.; Olutoye, M.A.; Hameed, B.H. Photocatalytic degradation of pollutants in petroleum refinery wastewater by TiO2-and ZnO-based photocatalysts: Recent development. J. Clean. Prod. 2018, 205, 930–954. [Google Scholar] [CrossRef]
- Qi, K.; Cheng, B.; Yu, J.; Ho, W. Review on the improvement of the photocatalytic and antibacterial activities of ZnO. J. Alloy. Compd. 2017, 727, 792–820. [Google Scholar] [CrossRef]
- Lee, K.M.; Lai, C.W.; Ngai, K.S.; Juan, J.C. Recent developments of zinc oxide based photocatalyst in water treatment technology: A review. Water Res. 2016, 88, 428–448. [Google Scholar] [CrossRef]
- Fatehah, M.O.; Aziz, H.A.; Stoll, S. Stability of ZnO Nanoparticles in Solution. Influence of pH, Dissolution, Aggregation and Disaggregation Effects. J. Colloid Sci. Biotechnol. 2014, 3, 75–84. [Google Scholar] [CrossRef]
- Domingos, R.F.; Rafiei, Z.; Monteiro, C.E.; Khan, M.A.; Wilkinson, K.J. Agglomeration and dissolution of zinc oxide nanoparticles: Role of pH, ionic strength and fulvic acid. Environ. Chem. 2013, 10, 306–312. [Google Scholar] [CrossRef]
- Rajeswari, A.; Vismaiya, S.; Pius, A. Preparation, characterization of nano ZnO-blended cellulose acetate-polyurethane membrane for photocatalytic degradation of dyes from water. Chem. Eng. J. 2017, 313, 928–937. [Google Scholar] [CrossRef]
- Abràmoff, M.D.; Ram, S.J. Image Processing with ImageJ. Biophotonics Int. 2004, 11, 36–42. [Google Scholar]
- Huertas, R.M.; Fraga, M.C.; Crespo, J.G.; Pereira, V.J. Solvent-free process for the development of photocatalytic membranes. Molecules 2019, 24, 4481. [Google Scholar] [CrossRef]
- Huertas, R.M.; Fraga, M.C.; Crespo, J.G.; Pereira, V.J. Sol-gel membrane modification for enhanced photocatalytic activity. Sep. Purif. Technol. 2017, 180, 69–81. [Google Scholar] [CrossRef]
- AlMarzooqi, F.A.; Bilad, M.R.; Mansoor, B.; Arafat, H.A. A comparative study of image analysis and porometry techniques for characterization of porous membranes. J. Mater. Sci. 2016, 514, 2017–2032. [Google Scholar] [CrossRef]
- Grove, C.; Jerram, D.A. jPOR: An ImageJ macro to quantify total optical porosity from blue-stained thin sections. Comput. Geosci. 2011, 37, 1850–1859. [Google Scholar] [CrossRef]
- Singhapong, W.; Srinophakun, P.; Jaroenworaluck, A. Influence of pore characteristics on the properties of porous mullite ceramics. J. Aust. Ceram. Soc. 2017, 53, 811–820. [Google Scholar] [CrossRef]
- Sun, W.; Chen, T.; Chen, C.; Li, J. A study on membrane morphology by digital image processing. J. Membr. Sci. 2007, 305, 93–102. [Google Scholar] [CrossRef]
- Van der Marel, P.; Zwijnenburg, A.; Kemperman, A.; Wessling, M.; Temmink, H.; van der Meer, W. Influence of membrane properties on fouling in submerged membrane bioreactors. J. Membr. Sci. 2010, 348, 66–74. [Google Scholar] [CrossRef]
- Manassero, A.; Satuf, M.L.; Alfano, O.M. Photocatalytic reactors with suspended and immobilized TiO2: Comparative efficiency evaluation. Chem. Eng. J. 2017, 326, 29–36. [Google Scholar] [CrossRef]
- Mendes, C.R.; Dilarri, G.; Forsan, C.F.; Sapata, V.D.M.R.; Lopes, P.R.M.; de Moraes, P.B.; Montagnolli, R.N.; Ferreira, H.; Bidoia, E.D. Antibacterial action and target mechanisms of zinc oxide nanoparticles against bacterial pathogens. Sci. Rep. 2022, 12, 2658. [Google Scholar] [CrossRef] [PubMed]
- Pant, B.; Park, M.; Kim, H.-Y.; Park, S.-J. Ag-ZnO photocatalyst anchored on carbon nanofibers: Synthesis, characterization, and photocatalytic activities. Synth. Met. 2016, 220, 533–537. [Google Scholar] [CrossRef]
- Ilyas, S.U.; Pendyala, R.; Marneni, N. Dispersion behaviour and agglomeration effects of zinc oxide nanoparticles in ethanol–water mixtures. Mater. Res. Innov. 2014, 18, S6-179–S6-183. [Google Scholar] [CrossRef]

| Sample ID | Membrane Type (ZnO2) | Thermal Treatment (°C) | Amount of ZnO (mg) | LED-UV Light |
|---|---|---|---|---|
| E-1 | unmodified | No | 0 | No |
| E-2 | unmodified | No | 0 | Yes |
| E-3 | unmodified/ZnO2 in suspension (S) | No | 125 | No |
| E-4 | unmodified/ZnO2 in suspension (S) | No | 125 | Yes |
| E-5 | modified/ZnO2 in layer (L) | No | 125 | Yes |
| E-6 | modified/ZnO2 in layer (L) | 100 | 62.5 | No |
| E-7 | modified/ZnO2 in layer (L) | 100 | 62.5 | Yes |
| E-8 | modified/ZnO2 in layer (L) | 100 | 125 | No |
| E-9 | modified/ZnO2 in layer (L) | 100 | 125 | Yes |
| E-10 | modified/ZnO2 in layer (L) | 100 | 250 | No |
| E-11 | modified/ZnO2 in layer (L) | 100 | 250 | Yes |
| E-12 | modified/ZnO2 in layer (L) | 300 | 125 | No |
| E-13 | modified/ZnO2 in layer (L) | 300 | 125 | Yes |
| E-14 | modified/ZnO2 in layer (L) | 500 | 125 | No |
| E-15 | modified/ZnO2 in layer (L) | 500 | 125 | Yes |
| E-16 | modified/ZnO2 in layer (L) | 700 | 125 | No |
| E-17 | modified/ZnO2 in layer (L) | 700 | 125 | Yes |
| Experiment | ZnO Recovered (%) |
|---|---|
| SiC-ZnO (S) | 73.5 ± 3.3 |
| SiC-ZnO (S)-UV | 78.4 ± 2.5 |
| SiC-ZnO (L) | 99.7 ± 0.2 |
| SiC-ZnO (L)-UV | 91.3 ± 0.2 |
| Characteristics | Concentration (mg/L) |
|---|---|
| Catalyst in suspension (S) | 0.55 |
| Catalyst in suspension (S) + UV | 0.55 |
| Catalyst in layer (L) | 0.05 |
| Catalyst in layer (L) + UV | 0.06 |
| Total Coliforms | E. coli | |||
|---|---|---|---|---|
| Experiment | % Rejection | % Treatment | % Rejection | % Treatment |
| E1 | 92.6 | 70.9 | 97.6 | 89.3 |
| E2 | 96.5 | 86.3 | 97.6 | 96.9 |
| E3 | 99.5 | 91.9 | >97.6 | 95.9 |
| E4 | >99.6 | 99.0 | >97.6 | 99 |
| E5 | >99.6 | 97.0 | >97.6 | 95.9 |
| E6 | 99.5 | 69.3 | >99.6 | 57.0 |
| E7 | >99.9 | 81.3 | >99.6 | 87.8 |
| E8 | 99.4 | 76.7 | 99.6 | 75.6 |
| E9 | 98.8 | 87.6 | >99.6 | 90.5 |
| E10 | 99.1 | 76.3 | 99.7 | 71.6 |
| E11 | 98.9 | 82.9 | >99.6 | 81.2 |
| E12 | >99.5 | 87.5 | >97.6 | 65.2 |
| E13 | >99.5 | 92.5 | >97.6 | 79.2 |
| E14 | 99.5 | 81.7 | >97.6 | 80.8 |
| E15 | 98.9 | 87.1 | >97.6 | 68.3 |
| E16 | 98.9 | 80.3 | >97.6 | 54.7 |
| E17 | >99.5 | 87.0 | >97.6 | 68.7 |
| Experiment | ZnO Layer Thickness (µm) |
|---|---|
| E 1–4 | N/A |
| E 6–7 | 0.8 ± 0.1 |
| E 8–9 | 26.4 ± 0.6 |
| E 10–11 | 58.7 ± 1.3 |
| E 12–13 | 31.3 ± 1.1 |
| E 14–15 | 42.3 ± 0.2 |
| E 16–17 | 31.6 ± 0.8 |
| Name | Membrane Surface | |||||
|---|---|---|---|---|---|---|
| Mean Particle Size (nm) | Mean Pore Size (nm) | Pore Density (1/nm2) | Porosity (%) | Pore Circularity | Feret’s Diameter of Pores (nm) | |
| E 1–4 | 484.4 | 371.8 | 1.1 × 10−6 | 17.5 | 0.6 | 546.6 |
| E 6–7 | 42.8 | 38.7 | 1.3 × 10−4 | 24.7 | 0.7 | 54.9 |
| E 8–9 | 34.9 | 56.7 | 3.4 × 10−5 | 16.7 | 0.7 | 81.9 |
| E 10–11 | 61.8 | 49.7 | 4.8 × 10−5 | 10.2 | 0.5 | 62.3 |
| E 12–13 | 59.1 | 41.9 | 6.2 × 10−5 | 14.7 | 0.7 | 61.7 |
| E 14–15 | 53.0 | 41.3 | 9.0 × 10−5 | 20.1 | 0.7 | 60.2 |
| E 16–17 | 60.7 | 48.6 | 4.8 × 10−5 | 14.4 | 0.6 | 70.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sosa, S.M.; Huertas, R.; Pereira, V.J. Combination of Zinc Oxide Photocatalysis with Membrane Filtration for Surface Water Disinfection. Membranes 2023, 13, 56. https://doi.org/10.3390/membranes13010056
Sosa SM, Huertas R, Pereira VJ. Combination of Zinc Oxide Photocatalysis with Membrane Filtration for Surface Water Disinfection. Membranes. 2023; 13(1):56. https://doi.org/10.3390/membranes13010056
Chicago/Turabian StyleSosa, Santiago Martínez, Rosa Huertas, and Vanessa Jorge Pereira. 2023. "Combination of Zinc Oxide Photocatalysis with Membrane Filtration for Surface Water Disinfection" Membranes 13, no. 1: 56. https://doi.org/10.3390/membranes13010056
APA StyleSosa, S. M., Huertas, R., & Pereira, V. J. (2023). Combination of Zinc Oxide Photocatalysis with Membrane Filtration for Surface Water Disinfection. Membranes, 13(1), 56. https://doi.org/10.3390/membranes13010056








