Non-Supported and PET-Supported Chitosan Membranes for Pervaporation: Production, Characterization, and Performance
Abstract
1. Introduction
2. Materials and Methods
2.1. Membrane Preparation
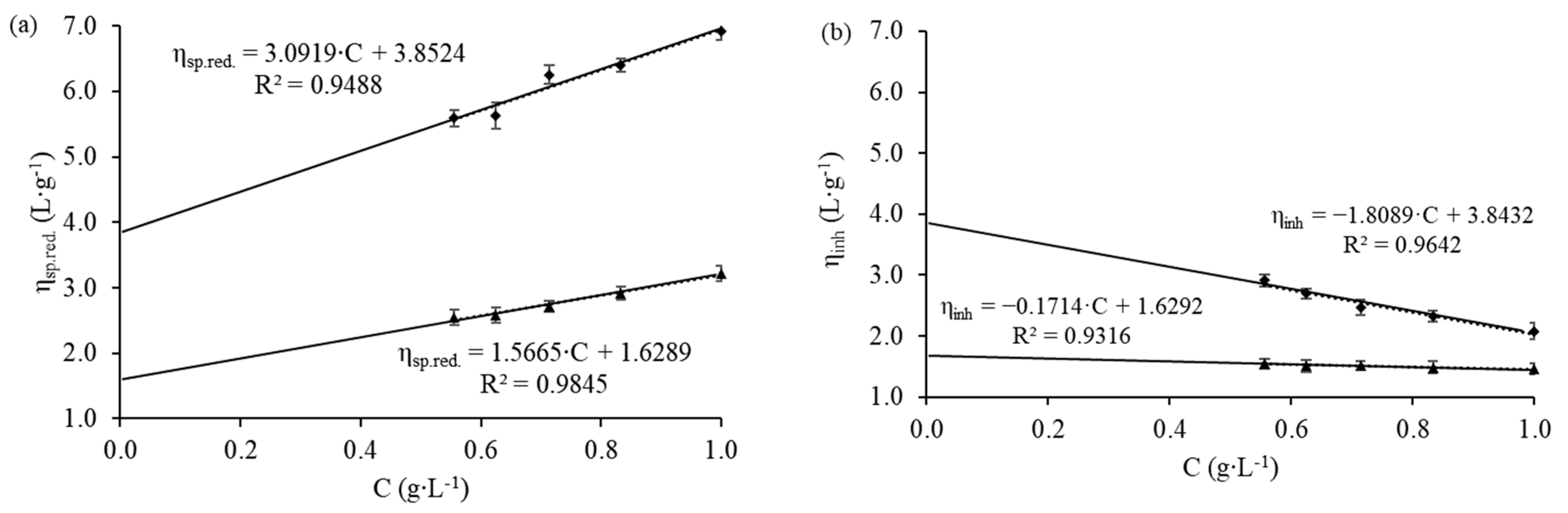
2.2. Characterization of the Polymeric Solution
2.3. Membrane Characterization
2.4. Pervaporation Tests
2.5. Chromatographic Analyses
2.6. Experimental Design and Statistical Analysis
3. Results and Discussion
3.1. Characterization of the Polymeric Solution
3.2. Characterization of the Membranes
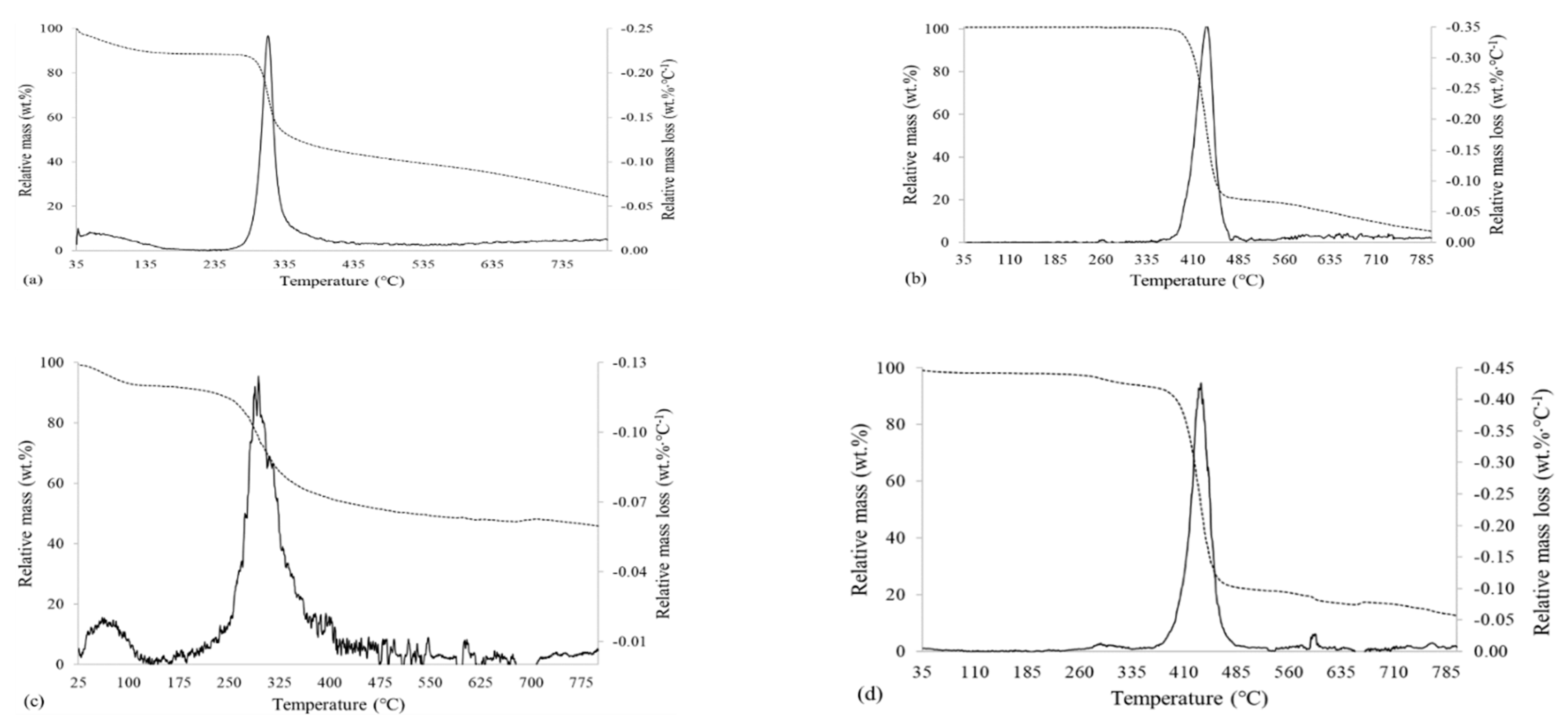
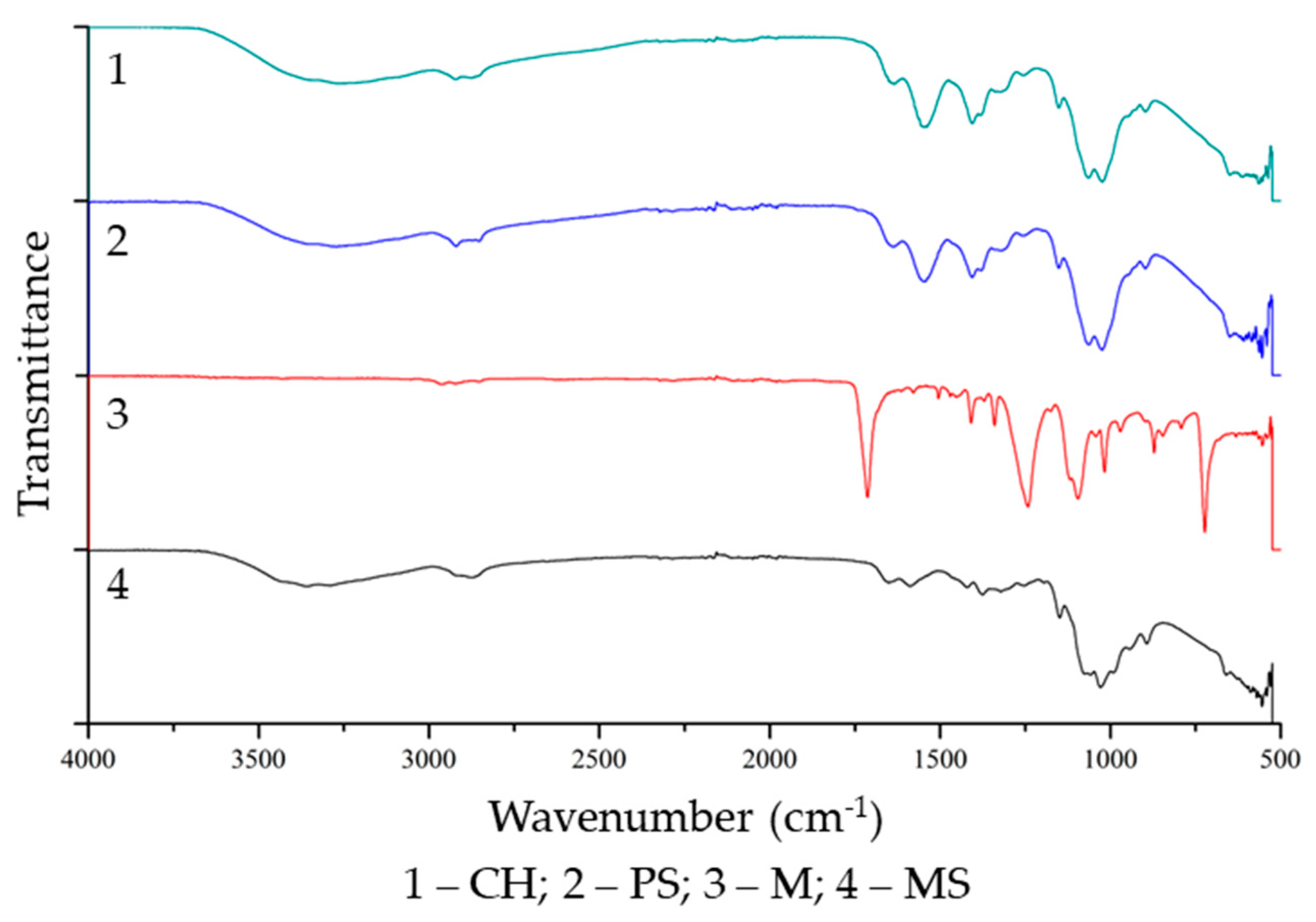
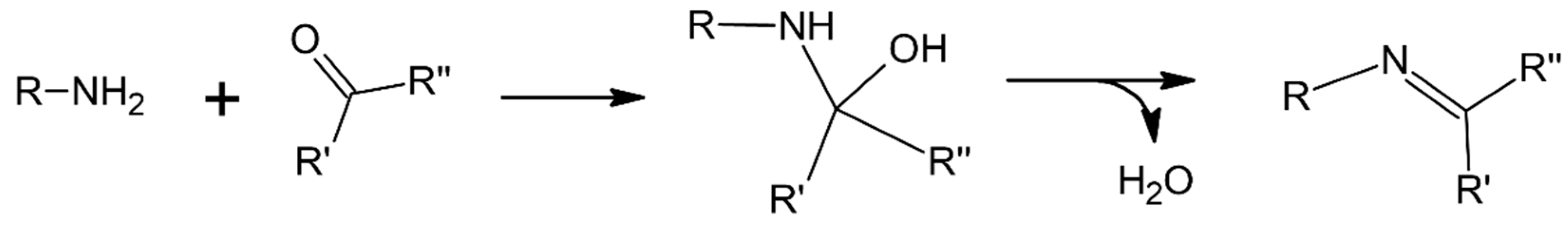
3.2.1. Thermal Properties and Chemical Structure
3.2.2. Mechanical Properties
3.2.3. Contact Angle and Surface Energy
3.2.4. Swelling Degree
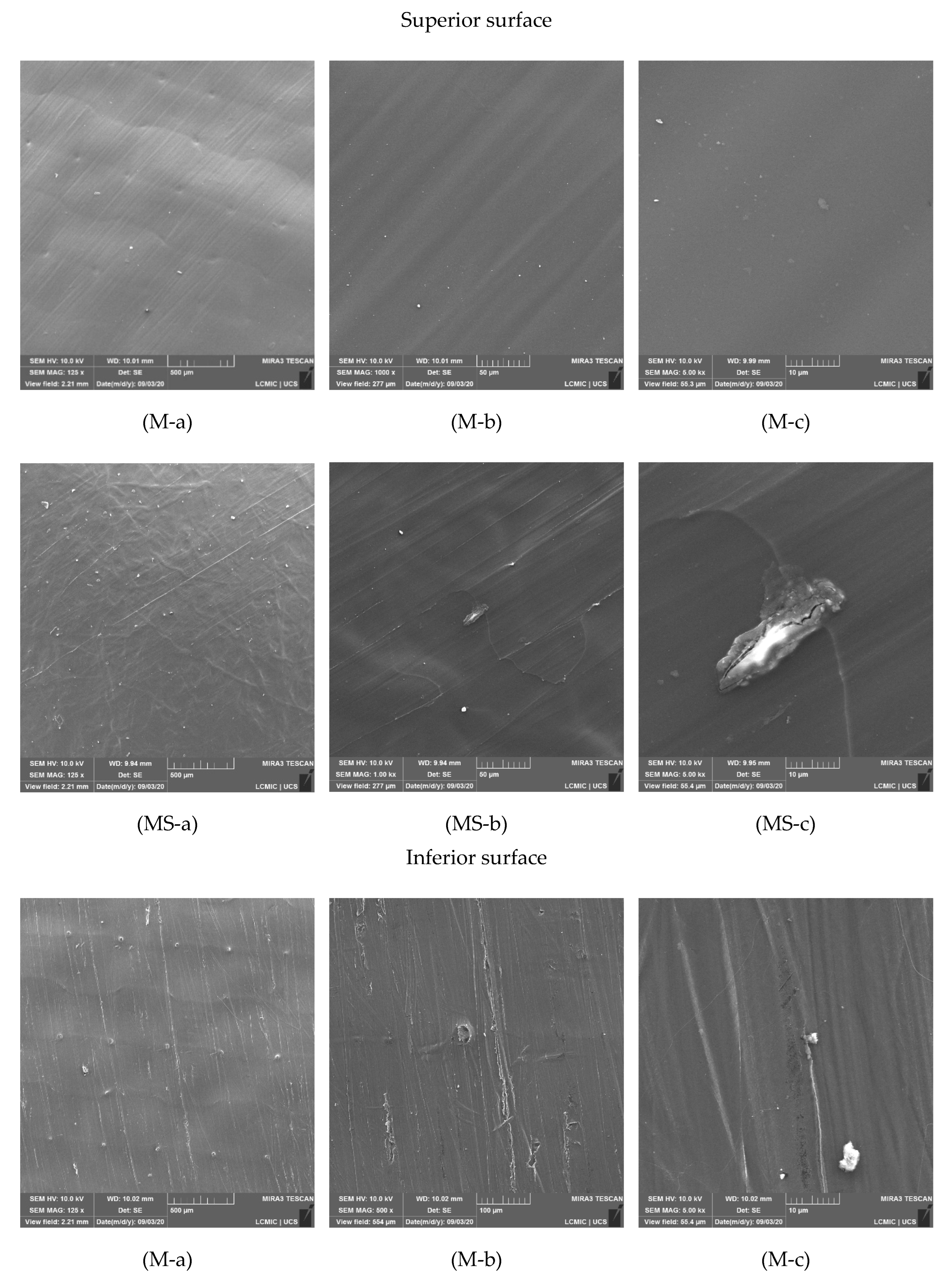
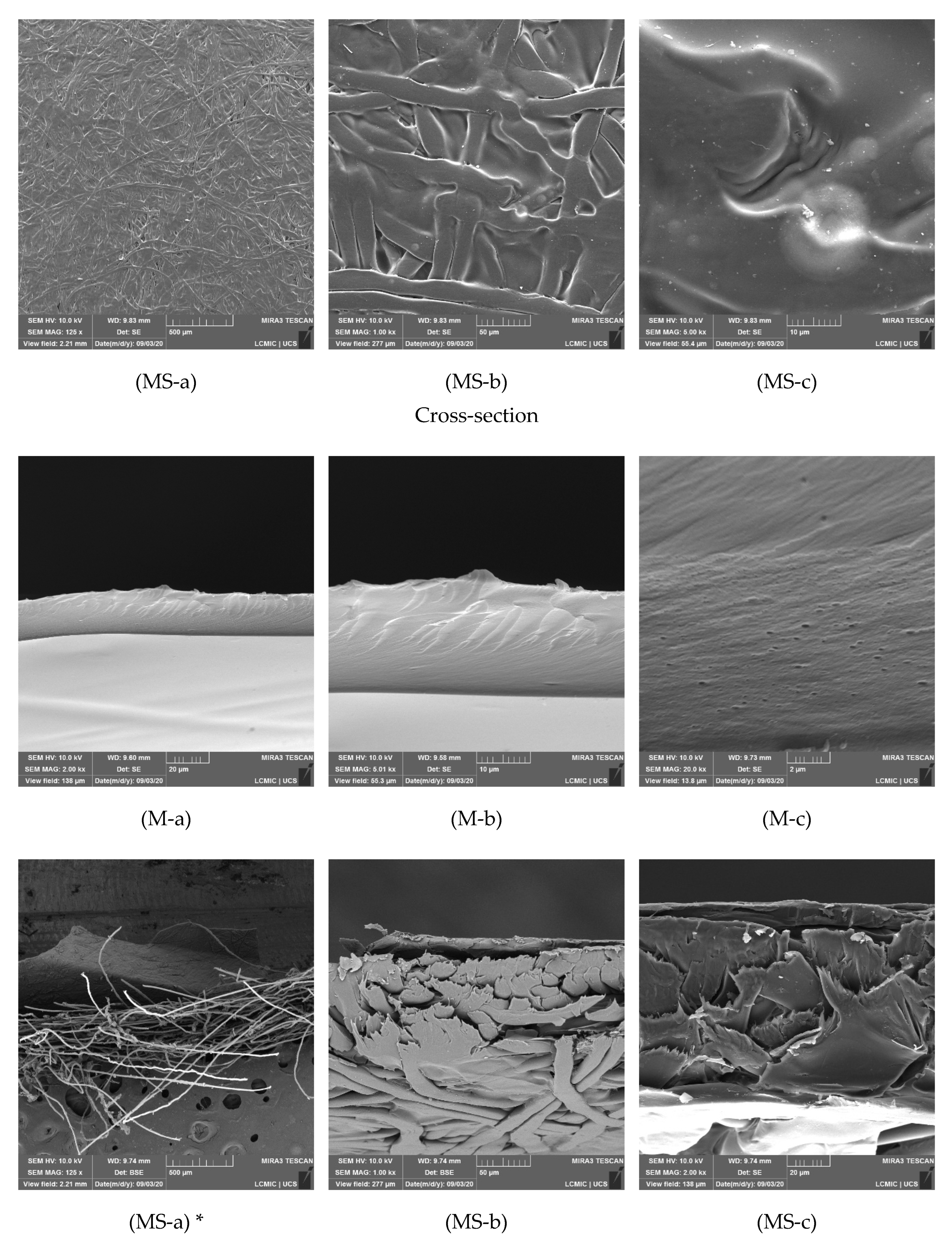
3.3. SEM Analysis and Membrane Microstructure
3.4. Pervaporation Tests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Silvestre, W.P.; Livinalli, N.F.; Baldasso, C.; Tessaro, I.C. Pervaporation in the Separation of Essential Oil Components: A Review. Trends Food Sci. Technol. 2019, 93, 42–52. [Google Scholar] [CrossRef]
- Isci, A.; Sahin, S.; Sumnu, G. Recovery of Strawberry Aroma Compounds by Pervaporation. J. Food Eng. 2006, 75, 36–42. [Google Scholar] [CrossRef]
- Dawiec-Liśniewska, A.; Szumny, A.; Podstawczyk, D.; Witek-Krowiak, A. Concentration of Natural Aroma Compounds from Fruit Juice Hydrolates by Pervaporation in Laboratory and Semi-Technical Scale. Part 1. Base Study. Food Chem. 2018, 258, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Aroujalian, A.; Raisi, A. Recovery of Volatile Aroma Components from Orange Juice by Pervaporation. J. Memb. Sci. 2007, 303, 154–161. [Google Scholar] [CrossRef]
- Sampranpiboon, P.; Jiraratananon, R.; Uttapap, D.; Feng, X.; Huang, R.Y.M. Separation of Aroma Compounds from Aqueous Solutions by Pervaporation Using Polyoctylmethyl Siloxane (POMS) and Polydimethyl Siloxane (PDMS) Membranes. J. Memb. Sci. 2000, 174, 55–65. [Google Scholar] [CrossRef]
- Castro-Muñoz, R. Pervaporation: The Emerging Technique for Extracting Aroma Compounds from Food Systems. J. Food Eng. 2019, 253, 27–39. [Google Scholar] [CrossRef]
- Mulder, M. Basic Principles of Membrane Technology, 2nd ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1996; ISBN 978-0-7923-4248-9. [Google Scholar]
- Nunes, S.P.; Peinemann, K. Membrane Technology in the Chemical Industry, 1st ed.; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2001; ISBN 3-527-28485-0. [Google Scholar]
- Cheng, X.; Pan, F.; Wang, M.; Li, W.; Song, Y.; Liu, G.; Yang, H.; Gao, B.; Wu, H.; Jiang, Z. Hybrid Membranes for Pervaporation Separations. J. Memb. Sci. 2017, 541, 329–346. [Google Scholar] [CrossRef]
- Figoli, A.; Santoro, S.; Galiano, F.; Basile, A. Pervaporation Membranes. In Pervaporation, Vapour Permeation and Membrane Distillation; Elsevier: Amsterdam, The Netherlands, 2015; pp. 19–63. ISBN 9781782422464. [Google Scholar]
- Smitha, B.; Suhanya, D.; Sridhar, S.; Ramakrishna, M. Separation of Organic-Organic Mixtures by Pervaporation—A Review. J. Memb. Sci. 2004, 241, 1–21. [Google Scholar] [CrossRef]
- Ulbricht, M. Advanced Functional Polymer Membranes. Polymer 2006, 47, 2217–2262. [Google Scholar] [CrossRef]
- Lalia, B.S.; Kochkodan, V.; Hashaikeh, R.; Hilal, N. A Review on Membrane Fabrication: Structure, Properties and Performance Relationship. Desalination 2013, 326, 77–95. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Wu, L.; Wei, G. Fabrication, Properties, Performances, and Separation Application of Polymeric Pervaporation Membranes: A Review. Polymers 2020, 12, 1466. [Google Scholar] [CrossRef] [PubMed]
- Pavoni, J.M.F. Desenvolvimento de Filmes Densos de Quitosana Para Aplicações Como Membranas e Embalagens. Ph.D. Thesis, Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil, 2018. [Google Scholar]
- Drikvand, H.N.; Golgoli, M.; Zargar, M.; Ulbricht, M.; Nejati, S.; Mansourpanah, Y. Thermo-Responsive Hydrophilic Support for Polyamide Thin-Film Composite Membranes with Competitive Nanofiltration Performance. Polymers 2022, 14, 3376. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi, H.; Moslehi, M. A New Thin Film Composite Nanofiltration Membrane Based on PET Nanofiber Support and Polyamide Top Layer: Preparation and Characterization. J. Polym. Res. 2016, 23, 257. [Google Scholar] [CrossRef]
- Fernandez-Saiz, P. Chitosan Polysaccharide in Food Packaging Applications. In Multifunctional and Nanoreinforced Polymers for Food Packaging; Woodhead Publishing Limited: Sawston, UK, 2011; pp. 571–593. ISBN 978-1-84569-738-9. [Google Scholar]
- Haghighi, H.; Biard, S.; Bigi, F.; De Leo, R.; Bedin, E.; Pfeifer, F.; Siesler, H.W.; Licciardello, F.; Pulvirenti, A. Comprehensive Characterization of Active Chitosan-Gelatin Blend Films Enriched with Different Essential Oils. Food Hydrocoll. 2019, 95, 33–42. [Google Scholar] [CrossRef]
- Sandri, G.; Rossi, S.; Bonferoni, M.C.; Miele, D.; Faccendini, A.; Del Favero, E.; Di Cola, E.; Icaro Cornaglia, A.; Boselli, C.; Luxbacher, T.; et al. Chitosan/Glycosaminoglycan Scaffolds for Skin Reparation. Carbohydr. Polym. 2019, 220, 219–227. [Google Scholar] [CrossRef]
- Naveed, M.; Phil, L.; Sohail, M.; Hasnat, M.; Baig, M.M.F.A.; Ihsan, A.U.; Shumzaid, M.; Kakar, M.U.; Mehmood Khan, T.; Akabar, M.D.; et al. Chitosan Oligosaccharide (COS): An Overview. Int. J. Biol. Macromol. 2019, 129, 827–843. [Google Scholar] [CrossRef]
- Sanjari, A.J.; Asghari, M. A Review on Chitosan Utilization in Membrane Synthesis. ChemBioEng Rev. 2016, 3, 134–158. [Google Scholar] [CrossRef]
- Struszczyk, M.H. Chitin and Chitosan Part II. Applications of Chitosan. Polimery/Polymers 2002, 47, 396–403. [Google Scholar] [CrossRef]
- Otvagina, K.; Penkova, A.; Dmitrenko, M.; Kuzminova, A.; Sazanova, T.; Vorotyntsev, A.; Vorotyntsev, I. Novel Composite Membranes Based on Chitosan Copolymers with Polyacrylonitrile and Polystyrene: Physicochemical Properties and Application for Pervaporation Dehydration of Tetrahydrofuran. Membranes 2019, 9, 38. [Google Scholar] [CrossRef]
- Ong, Y.K.; Shi, G.M.; Le, N.L.; Tang, Y.P.; Zuo, J.; Nunes, S.P.; Chung, T.S. Recent Membrane Development for Pervaporation Processes. Prog. Polym. Sci. 2016, 57, 1–31. [Google Scholar] [CrossRef]
- Chrzanowska, E.; Gierszewska, M.; Kujawa, J.; Raszkowska-Kaczor, A.; Kujawski, W. Development and Characterization of Polyamide- Supported Chitosan Nanocomposite Membranes for Hydrophilic Pervaporation. Polymers 2018, 10, 868. [Google Scholar] [CrossRef] [PubMed]
- Langari, S.; Saljoughi, E.; Mousavi, S.M. Chitosan/Polyvinyl Alcohol/Amino Functionalized Multiwalled Carbon Nanotube Pervaporation Membranes: Synthesis, Characterization, and Performance. Polym. Adv. Technol. 2018, 29, 84–94. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, M.; Ji, C.H.; Xu, X.R.; Ma, X.H.; Xu, Z.L. Multilayer Assembled CS-PSS/Ceramic Hollow Fiber Membranes for Pervaporation Dehydration. Sep. Purif. Technol. 2018, 203, 84–92. [Google Scholar] [CrossRef]
- Fini, M.N.; Soroush, S.; Montazer-Rahmati, M.M. Synthesis and Optimization of Chitosan Ceramic-Supported Membranes in Pervaporation Ethanol Dehydration. Membranes 2018, 8, 119. [Google Scholar] [CrossRef] [PubMed]
- Patil, M.B.; Aminabhavi, T.M. Pervaporation Separation of Toluene/Alcohol Mixtures Using Silicalite Zeolite Embedded Chitosan Mixed Matrix Membranes. Sep. Purif. Technol. 2008, 62, 128–136. [Google Scholar] [CrossRef]
- Huang, R.Y.M.; Moon, G.Y.; Pal, R. N-Acetylated Chitosan Membranes for the Pervaporation Separation of Alcohol/Toluene Mixtures. J. Memb. Sci. 2000, 176, 223–231. [Google Scholar] [CrossRef]
- Moulik, S.; Bukke, V.; Sajja, S.C.; Sridhar, S. Chitosan-Polytetrafluoroethylene Composite Membranes for Separation of Methanol and Toluene by Pervaporation. Carbohydr. Polym. 2018, 193, 28–38. [Google Scholar] [CrossRef]
- Silvestre, W.P.; Baldasso, C.; Tessaro, I.C. Potential of Chitosan-Based Membranes for the Separation of Essential Oil Components by Target-Organophilic Pervaporation. Carbohydr. Polym. 2020, 247, 116676. [Google Scholar] [CrossRef]
- Frick, J.M.; Ambrosi, A.; Pollo, L.D.; Tessaro, I.C. Influence of Glutaraldehyde Crosslinking and Alkaline Post-Treatment on the Properties of Chitosan-Based Films. J. Polym. Environ. 2018, 26, 2748–2757. [Google Scholar] [CrossRef]
- ASTM D2196-18e1; Standard Test Methods for Rheological Properties of Non-Newtonian Materials by Rotational Viscometer. ASTM: West Conshohocken, PA, USA, 2014; Volume 43, pp. 1–5.
- Rao, D.G. Studies on Viscosity-Molecular Weight Relationship of Chitosan Solutions. J. Food Sci. Technol. 1993, 30, 66–67. [Google Scholar]
- Czechowska-Biskup, R.; Wach, R.A.; Rosiak, J.M.; Ulański, P. Procedure for Determination of the Molecular Weight of Chitosan by Viscometry. Prog. Chem. Appl. Chitin. Deriv. 2018, 23, 45–54. [Google Scholar] [CrossRef]
- Duarte, J.; dos Santos, V.; Zeni, M. Comportamento Viscosimétrico Da Poliamida 66 Comercial Em Ácido Fórmico Y Ácido Clorídrico. Revista Iberoamericana de Polímeros 2016, 17, 293–303. [Google Scholar]
- Costa, C.N.; Teixeira, V.G.; Delpech, M.C.; Souza, J.V.S.; Costa, M.A.S. Viscometric Study of Chitosan Solutions in Acetic Acid/Sodium Acetate and Acetic Acid/Sodium Chloride. Carbohydr. Polym. 2015, 133, 245–250. [Google Scholar] [CrossRef] [PubMed]
- ASTM D882-18; Standard Test Method for Tensile Properties of Thin Plastic Sheeting. ASTM International: West Conshohocken, PA, USA, 2018; pp. 1–12.
- ASTM D7490-13; Standard Test Method for Measurement of the Surface Tension of Solid Coatings, Substrates and Pigments Using Contact Angle Measurements. ASTM International: West Conshohocken, PA, USA, 2013; pp. 1–5. [CrossRef]
- Fowkes, F.M. Attractive Forces at Interfaces. Ind. Eng. Chem. 1964, 56, 40–52. [Google Scholar] [CrossRef]
- Kozbial, A.; Li, Z.; Conaway, C.; McGinley, R.; Dhingra, S.; Vahdat, V.; Zhou, F.; D’Urso, B.; Liu, H.; Li, L. Study on the Surface Energy of Graphene by Contact Angle Measurements. Langmuir 2014, 30, 8598–8606. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.W.; Wijmans, J.G.G.; Huang, Y. Permeability, Permeance and Selectivity: A Preferred Way of Reporting Pervaporation Performance Data. J. Memb. Sci. 2010, 348, 346–352. [Google Scholar] [CrossRef]
- Teixeira, M.A.; Rodríguez, O.; Mota, F.L.; MacEdo, E.A.; Rodrigues, A.E. Evaluation of Group-Contribution Methods to Predict VLE and Odor Intensity of Fragrances. Ind. Eng. Chem. Res. 2011, 50, 9390–9402. [Google Scholar] [CrossRef]
- Dortmund Data Bank Property Estimation. Available online: http://www.ddbst.com/prp-estimate.html (accessed on 28 September 2021).
- De Souza, B.C.; Bossardi, F.F.; Furlan, G.R.; Folle, A.B.; Reginatto, C.; Polidoro, T.A.; Carra, S.; da Silveira, M.M.; Malvessi, E. Validated High-Performance Liquid Chromatographic (HPLC) Method for the Simultaneous Quantification of 2,3-Butanediol, Glycerol, Acetoin, Ethanol, and Phosphate in Microbial Cultivations. Anal. Lett. 2021, 54, 2395–2410. [Google Scholar] [CrossRef]
- Martínez-Mejía, G.; Vázquez-Torres, N.A.; Castell-Rodríguez, A.; del Río, J.M.; Corea, M.; Jiménez-Juárez, R. Synthesis of New Chitosan-Glutaraldehyde Scaffolds for Tissue Engineering Using Schiff Reactions. Colloids Surf. A Physicochem. Eng. Asp. 2019, 579, 123658. [Google Scholar] [CrossRef]
- Matet, M.; Heuzey, M.-C.; Pollet, E.; Ajji, A.; Avérous, L. Innovative Thermoplastic Chitosan Obtained by Thermo-Mechanical Mixing with Polyol Plasticizers. Carbohydr. Polym. 2013, 95, 241–251. [Google Scholar] [CrossRef]
- Fiori, A.P.S.D.M.; Gabiraba, V.P.; Praxedes, A.P.P.; Nunes, M.R.D.S.; Balliano, T.L.; da Silva, R.C.; Tonholo, J.; Ribeiro, A.S. Preparação e Caracterização de Nanocompósitos Poliméricos Baseados Em Quitosana e Argilo Minerais. Polímeros 2014, 24, 628–635. [Google Scholar] [CrossRef]
- Bach, C.; Dauchy, X.; Etienne, S. Characterization of Poly(Ethylene Terephthalate) Used in Commercial Bottled Water. IOP Conf. Ser. Mater. Sci. Eng. 2009, 5, 012005. [Google Scholar] [CrossRef]
- Ahani, M.; Khatibzadeh, M.; Mohseni, M. Preparation and Characterization of Poly(Ethylene Terephthalate)/Hyperbranched Polymer Nanocomposites by Melt Blending. Nanocomposites 2016, 2, 29–36. [Google Scholar] [CrossRef]
- Merck IR Spectrum Table & Chart | Sigma-Aldrich. Available online: https://www.sigmaaldrich.com/technical-documents/articles/biology/ir-spectrum-table.html (accessed on 21 February 2020).
- Donelli, I.; Freddi, G.; Nierstrasz, V.A.; Taddei, P. Surface Structure and Properties of Poly-(Ethylene Terephthalate) Hydrolyzed by Alkali and Cutinase. Polym. Degrad. Stab. 2010, 95, 1542–1550. [Google Scholar] [CrossRef]
- Pavoni, J.M.F.; dos Santos, N.Z.; May, I.C.; Pollo, L.D.; Tessaro, I.C. Impact of Acid Type and Glutaraldehyde Crosslinking in the Physicochemical and Mechanical Properties and Biodegradability of Chitosan Films. Polym. Bull. 2020, 78, 981–1000. [Google Scholar] [CrossRef]
- Beppu, M.M.; Vieira, R.S.; Aimoli, C.G.; Santana, C.C. Crosslinking of Chitosan Membranes Using Glutaraldehyde: Effect on Ion Permeability and Water Absorption. J. Memb. Sci. 2007, 301, 126–130. [Google Scholar] [CrossRef]
- Zhang, W.; Yu, Z.; Qian, Q.; Zhang, Z.; Wang, X. Improving the Pervaporation Performance of the Glutaraldehyde Crosslinked Chitosan Membrane by Simultaneously Changing Its Surface and Bulk Structure. J. Memb. Sci. 2010, 348, 213–223. [Google Scholar] [CrossRef]
- Liu, P.; Chen, M.; Ma, Y.; Hu, C.; Zhang, Q.; Zhu, A.; Liu, Q. A Hydrophobic Pervaporation Membrane with Hierarchical Microporosity for High-Efficient Dehydration of Alcohols. Chem. Eng. Sci. 2019, 206, 489–498. [Google Scholar] [CrossRef]
- Priyadarshi, R.; Sauraj; Kumar, B.; Negi, Y.S. Chitosan Film Incorporated with Citric Acid and Glycerol as an Active Packaging Material for Extension of Green Chilli Shelf Life. Carbohydr. Polym. 2018, 195, 329–338. [Google Scholar] [CrossRef]
- Mujtaba, M.; Morsi, R.E.; Kerch, G.; Elsabee, M.Z.; Kaya, M.; Labidi, J.; Khawar, K.M. Current Advancements in Chitosan-Based Film Production for Food Technology; A Review. Int. J. Biol. Macromol. 2019, 121, 889–904. [Google Scholar] [CrossRef]
- Clasen, C.; Wilhelms, T.; Kulicke, W.-M. Formation and Characterization of Chitosan Membranes. Biomacromolecules 2006, 7, 3210–3222. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cai, Z.; Sheng, L.; Ma, M.; Xu, Q.; Jin, Y. Structure-Property of Crosslinked Chitosan/Silica Composite Films Modified by Genipin and Glutaraldehyde under Alkaline Conditions. Carbohydr. Polym. 2019, 215, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Law, K.Y. Definitions for Hydrophilicity, Hydrophobicity, and Superhydrophobicity: Getting the Basics Right. J. Phys. Chem. Lett. 2014, 5, 686–688. [Google Scholar] [CrossRef] [PubMed]
- Prajitno, D.H.; Maulana, A.; Syarif, D.G. Effect of Surface Roughness on Contact Angle Measurement of Nanofluid on Surface of Stainless Steel 304 by Sessile Drop Method. J. Phys. Conf. Ser. 2016, 739, 012029. [Google Scholar] [CrossRef]
- Shenvi, S.S.; Rashid, S.A.; Ismail, A.F.; Kassim, M.A.; Isloor, A.M. Preparation and Characterization of PPEES/Chitosan Composite Nanofiltration Membrane. Desalination 2013, 315, 135–141. [Google Scholar] [CrossRef]
- Jasper, J.J. The Surface Tension of Pure Liquid Compounds. J. Phys. Chem. Ref. Data 1972, 1, 841–1010. [Google Scholar] [CrossRef]
- Jańczuk, B.; Białlopiotrowicz, T. Surface Free-Energy Components of Liquids and Low Energy Solids and Contact Angles. J. Colloid Interface Sci. 1989, 127, 189–204. [Google Scholar] [CrossRef]
- Kappert, E.J.; Raaijmakers, M.J.T.; Tempelman, K.; Cuperus, F.P.; Ogieglo, W.; Benes, N.E. Swelling of 9 Polymers Commonly Employed for Solvent-Resistant Nanofiltration Membranes: A Comprehensive Dataset. J. Memb. Sci. 2019, 569, 177–199. [Google Scholar] [CrossRef]
- Sahin, S. Principles of Pervaporation for the Recovery of Aroma Compounds and Applications in the Food and Beverage Industries; Woodhead Publishing Limited: Sawston, UK, 2010; ISBN 9781845696450. [Google Scholar]
- Al-Naamani, L.; Dobretsov, S.; Dutta, J. Chitosan-Zinc Oxide Nanoparticle Composite Coating for Active Food Packaging Applications. Innov. Food Sci. Emerg. Technol. 2016, 38, 231–237. [Google Scholar] [CrossRef]
- Shi, J.; Kang, H.; Li, N.; Teng, K.; Sun, W.; Xu, Z.; Qian, X.; Liu, Q. Chitosan Sub-Layer Binding and Bridging for Nanofiber-Based Composite Forward Osmosis Membrane. Appl. Surf. Sci. 2019, 478, 38–48. [Google Scholar] [CrossRef]
- Kazemi, M.; Jahanshahi, M.; Peyravi, M. Chitosan-Sodium Alginate Multilayer Membrane Developed by Fe0@WO3 Nanoparticles: Photocatalytic Removal of Hexavalent Chromium. Carbohydr. Polym. 2018, 198, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Terraza, C.A.; Martin, R.; Saldías, C.; González, M.; Leiva, Á.; Tundidor-Camba, A. Preparation of CuONPs@PVDF/Non-Woven Polyester Composite Membrane: Structural Influence of Nanoparticle Addition. Polymers 2018, 10, 862. [Google Scholar] [CrossRef] [PubMed]
- Lau, W.J.; Ismail, A.F.; Misdan, N.; Kassim, M.A. A Recent Progress in Thin Film Composite Membrane: A Review. Desalination 2012, 287, 190–199. [Google Scholar] [CrossRef]
- Guerrero, P.; Muxika, A.; Zarandona, I.; de la Caba, K. Crosslinking of Chitosan Films Processed by Compression Molding. Carbohydr. Polym. 2019, 206, 820–826. [Google Scholar] [CrossRef]
- Acosta, S.; Chiralt, A.; Santamarina, P.; Rosello, J.; González-Martínez, C.; Cháfer, M. Antifungal Films Based on Starch-Gelatin Blend, Containing Essential Oils. Food Hydrocoll. 2016, 61, 233–240. [Google Scholar] [CrossRef]
- Iranizadeh, S.T.; Chenar, M.P.; Mahboub, M.N.; Namaghi, H.A. Preparation and Characterization of Thin-Film Composite Reverse Osmosis Membrane on a Novel Aminosilane-Modified Polyvinyl Chloride Support. Braz. J. Chem. Eng. 2019, 36, 251–264. [Google Scholar] [CrossRef]
- Wang, S.; Li, T.; Chen, C.; Chen, S.; Liu, B.; Crittenden, J. Non-Woven PET Fabric Reinforced and Enhanced the Performance of Ultrafiltration Membranes Composed of PVDF Blended with PVDF-g-PEGMA for Industrial Applications. Appl. Surf. Sci. 2018, 435, 1072–1079. [Google Scholar] [CrossRef]
- Kononova, S.V.; Volod’ko, A.V.; Petrova, V.A.; Kruchinina, E.V.; Baklagina, Y.G.; Chusovitin, E.A.; Skorik, Y.A. Pervaporation Multilayer Membranes Based on a Polyelectrolyte Complex of λ-Carrageenan and Chitosan. Carbohydr. Polym. 2018, 181, 86–92. [Google Scholar] [CrossRef]
- Perry, R.H.; Green, D.W. Perry’s Chemical Engineers’ Handbook, 8th ed.; McGraw-Hill: New York, NY, USA, 2008; ISBN 978-0071422949. [Google Scholar]
- Zaitsau, D.H.; Verevkin, S.P.; Sazonova, A.Y. Vapor Pressures and Vaporization Enthalpies of 5-Nonanone, Linalool and 6-Methyl-5-Hepten-2-One. Data Evaluation. Fluid Phase Equilib. 2015, 386, 140–148. [Google Scholar] [CrossRef]
- Van der Bruggen, B.; Luis, P. Pervaporation; Elsevier Ltd.: Amsterdam, The Netherlands, 2014; ISBN 9780123983077. [Google Scholar]
- Smith, J.M.; van Ness, C.H.; Abbott, M.M. Introdução à Termodinâmica Da Engenharia Química, 7th ed.; LTC: São Paulo, Brazil, 2007; ISBN 978-8521615538. [Google Scholar]
- Jyoti, G.; Keshav, A.; Anandkumar, J. Review on Pervaporation: Theory, Membrane Performance, and Application to Intensification of Esterification Reaction. J. Eng. 2015, 2015, 927068. [Google Scholar] [CrossRef]
- Trifunović, O.; Trägårdh, G. The Influence of Support Layer on Mass Transport of Homologous Series of Alcohols and Esters through Composite Pervaporation Membranes. J. Memb. Sci. 2005, 259, 122–134. [Google Scholar] [CrossRef]
- Eljaddi, T.; Mendez, D.L.M.; Favre, E.; Roizard, D. Development of New Pervaporation Composite Membranes for Desalination: Theoretical and Experimental Investigations. Desalination 2021, 507, 115006. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, M.; Wu, H.; Yin, X.; Chen, J.; Jiang, Z. Mussel-Inspired Fabrication of Structurally Stable Chitosan/Polyacrylonitrile Composite Membrane for Pervaporation Dehydration. J. Memb. Sci. 2010, 348, 150–159. [Google Scholar] [CrossRef]
- Wang, X.-P.; Shen, Z.-Q.; Zhang, F.-Y.; Zhang, Y.-F. A Novel Composite Chitosan Membrane for the Separation of Alcohol-Water Mixtures. J. Memb. Sci. 1996, 119, 191–198. [Google Scholar] [CrossRef]
- Kang, C.-H.; Lin, Y.-F.; Huang, Y.-S.; Tung, K.-L.; Chang, K.-S.; Chen, J.-T.; Hung, W.-S.; Lee, K.-R.; Lai, J.-Y. Synthesis of ZIF-7/Chitosan Mixed-Matrix Membranes with Improved Separation Performance of Water/Ethanol Mixtures. J. Memb. Sci. 2013, 438, 105–111. [Google Scholar] [CrossRef]
- Dharupaneedi, S.P.; Anjanapura, R.V.; Han, J.M.; Aminabhavi, T.M. Functionalized Graphene Sheets Embedded in Chitosan Nanocomposite Membranes for Ethanol and Isopropanol Dehydration via Pervaporation. Ind. Eng. Chem. Res. 2014, 53, 14474–14484. [Google Scholar] [CrossRef]
- Dudek, G.; Gnus, M.; Turczyn, R.; Strzelewicz, A.; Krasowska, M. Pervaporation with Chitosan Membranes Containing Iron Oxide Nanoparticles. Sep. Purif. Technol. 2014, 133, 8–15. [Google Scholar] [CrossRef]
- Sunitha, K.; Satyanarayana, S.V.; Sridhar, S. Phosphorylated Chitosan Membranes for the Separation of Ethanol–Water Mixtures by Pervaporation. Carbohydr. Polym. 2012, 87, 1569–1574. [Google Scholar] [CrossRef]
- Zielińska, K.; Kujawski, W.; Chostenko, A.G. Chitosan Hydrogel Membranes for Pervaporative Dehydration of Alcohols. Sep. Purif. Technol. 2011, 83, 114–120. [Google Scholar] [CrossRef]
- Huang, R.Y.M.; Yeom, C.K. Pervaporation Separation of Aqueous Mixtures Using Crosslinked Polyvinyl Alcohol Membranes. III. Permeation of Acetic Acid-Water Mixtures. J. Memb. Sci. 1991, 58, 33–47. [Google Scholar] [CrossRef]
| Parameter | PS | M | MS | CV * (%) |
|---|---|---|---|---|
| Thickness (µm) | 91 ± 2 b | 20 ± 1 c | 120 ± 2 a | 2.5 |
| Young modulus (MPa) | 384 ± 40 a | 76 ± 7 b | 398 ± 18 a | 8.9 |
| Tensile strength (MPa) | 27 ± 9 a | 16 ± 1 b | 27 ± 3 a | 22.2 |
| Elongation at break (%) | 30 ± 4 a | 7 ± 1 b | 26 ± 3 a | 15.1 |
| Substance | Contact Angle (°) | ||
|---|---|---|---|
| M | MS | PS | |
| Distilled water | 85 ± 1 a | 88 ± 3 a | 63 ± 2 b |
| Diiodomethane | 36 ± 1 b | 44 ± 1 a | 27 ± 1 c |
| Ethanol 96% v/v | 33 ± 3 a | 31 ± 3 a | 0 ± 0 b |
| Linalool | 0 ± 0 b | 14 ± 1 a | 0 ± 0 b |
| Myrcene | 24 ± 3 a | 24 ± 3 a | 0 ± 0 b |
| Material | Surface Energy (mJ∙m−2) | ||
|---|---|---|---|
| Total (σ) | Dispersive (σD) | Polar (σP) | |
| M | 55 ± 1 | 42 ± 1 | 13 ± 1 |
| MS | 49 ± 1 | 38 ± 2 | 12 ± 1 |
| PS | 81 ± 3 | 45 ± 3 | 36 ± 3 |
| Liquid | Mass Swelling (%) | Volumetric Swelling (%) | ||
|---|---|---|---|---|
| M | MS | M | MS | |
| Distilled water | 318.1 ± 32.5 a | 53.3 ± 10.2 a | 229.3 ± 10.5 a | 37.3 ± 4.6 a |
| Ethanol 96% v/v | 1.0 ± 2.0 c | 0.0 ± 0.0 c | 4.6 ± 2.8 b | − 7.9 ± 2.2 c |
| Linalool | 19.3 ± 4.5 b | 16.9 ± 4.5 b | 8.4 ± 0.7 b | 6.1 ± 1.0 b |
| Myrcene | 14.1 ± 2.0 b | 13.0 ± 0.7 b | 8.8 ± 2.8 b | − 3.4 ± 1.8 c |
| Parameter | Unit | Non-Supported Membrane | PET-Supported Membrane | Coefficient of Variation (%) |
|---|---|---|---|---|
| Transmembrane flux (J) | kg·m−2·h−1 | 0.397 a | 0.121 b | 5.3 |
| Water flux (JW) | kg·m−2·h−1 | 0.378 a | 0.119 b | 4.7 |
| Ethanol flux (JE) | kg·m−2·h−1 | 0.019 a | 0.002 b | 8.4 |
| Water content in permeate (CW,P) | wt % | 95.2 b | 98.3 a | 0.2 |
| Ethanol content in permeate (CE,P) | wt % | 4.77 a | 1.74 b | 21.0 |
| Water enrichment factor (βW) | - | 3.17 b | 3.28 a | 3.1 |
| Ethanol enrichment factor (βE) | - | 0.07 a | 0.03 b | 10.3 |
| Water permeance (PW’) | kg∙m−2∙s−1∙Pa−1 | 5.61∙10−7 a | 1.76∙10−7 b | 19.0 |
| Ethanol permeance (PE’) | kg∙m−2∙s−1∙Pa−1 | 9.77·10−10 a | 1.13·10−10 b | 23.2 |
| Water permeability (PW) | kg·m−1·s−1·Pa−1 | 1.68∙10−11 a | 3.52∙10−12 b | 11.3 |
| Ethanol permeability (PE) | kg·m−1·s−1·Pa−1 | 2.93·10−14 a | 2.25·10−15 b | 18.3 |
| Selectivity (αW/E) | - | 611 b | 1974 a | 10.6 |
| PSI 1 | kg·m−2·h−1 | 228 a | 218 a | 11.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silvestre, W.P.; Duarte, J.; Tessaro, I.C.; Baldasso, C. Non-Supported and PET-Supported Chitosan Membranes for Pervaporation: Production, Characterization, and Performance. Membranes 2022, 12, 930. https://doi.org/10.3390/membranes12100930
Silvestre WP, Duarte J, Tessaro IC, Baldasso C. Non-Supported and PET-Supported Chitosan Membranes for Pervaporation: Production, Characterization, and Performance. Membranes. 2022; 12(10):930. https://doi.org/10.3390/membranes12100930
Chicago/Turabian StyleSilvestre, Wendel Paulo, Jocelei Duarte, Isabel Cristina Tessaro, and Camila Baldasso. 2022. "Non-Supported and PET-Supported Chitosan Membranes for Pervaporation: Production, Characterization, and Performance" Membranes 12, no. 10: 930. https://doi.org/10.3390/membranes12100930
APA StyleSilvestre, W. P., Duarte, J., Tessaro, I. C., & Baldasso, C. (2022). Non-Supported and PET-Supported Chitosan Membranes for Pervaporation: Production, Characterization, and Performance. Membranes, 12(10), 930. https://doi.org/10.3390/membranes12100930






