The Effect of Heat Sterilization on Key Filtration Performance Parameters of a Commercial Polymeric (PVDF) Hollow-Fiber Ultrafiltration Membrane
Abstract
1. Introduction
2. Materials and Methods
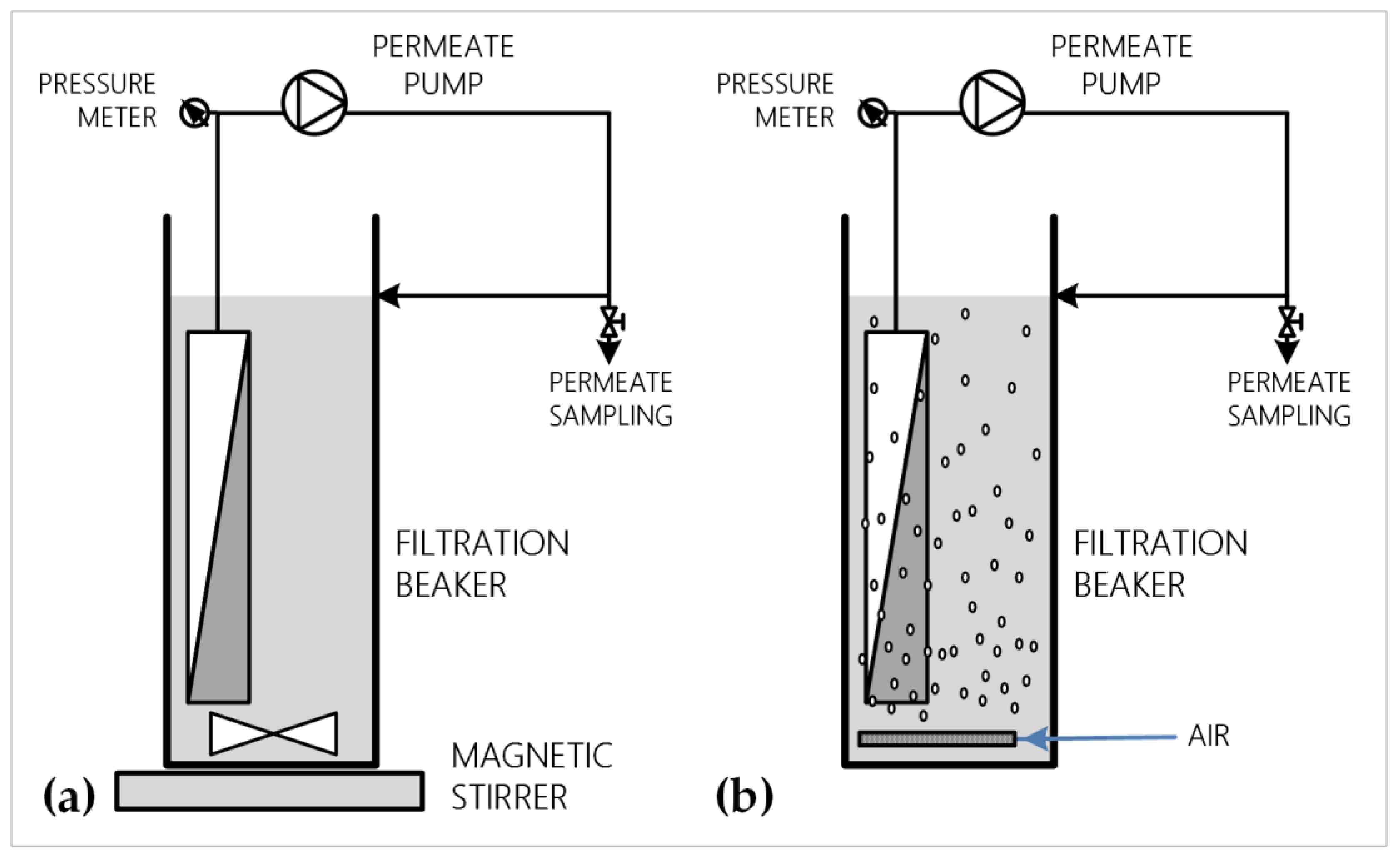
2.1. Experimental Set-Up/Analytical Methods
2.1.1. Membranes
2.1.2. Filtration Set-Up
2.1.3. Strain and Media
2.1.4. Dextrans
2.1.5. High Performance Liquid Chromatography (HPLC)
2.1.6. Total Organic Carbon (TOC)
2.1.7. Optical Density (OD600) and Cell Dry Weight (CDW)
2.1.8. Scanning Electron Microscope (SEM) Images
2.2. Experimental Procedures
2.2.1. Membrane Permeance Assessment
2.2.2. Membrane Typical Pore Size
2.2.3. Fermentation/Membrane Filtration Tests
3. Results and Discussion
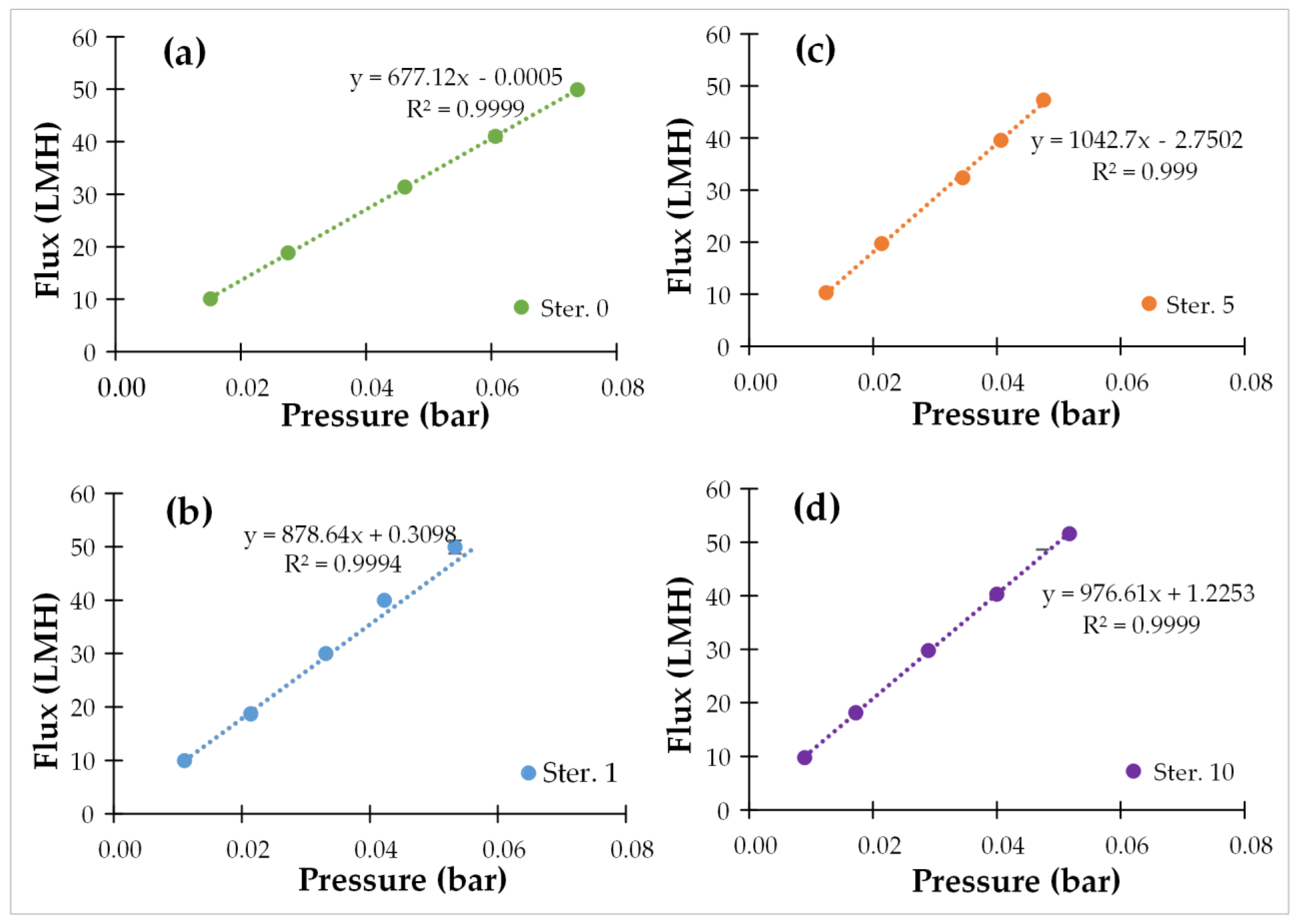
3.1. Membrane Permeance
3.2. Membrane Rejection
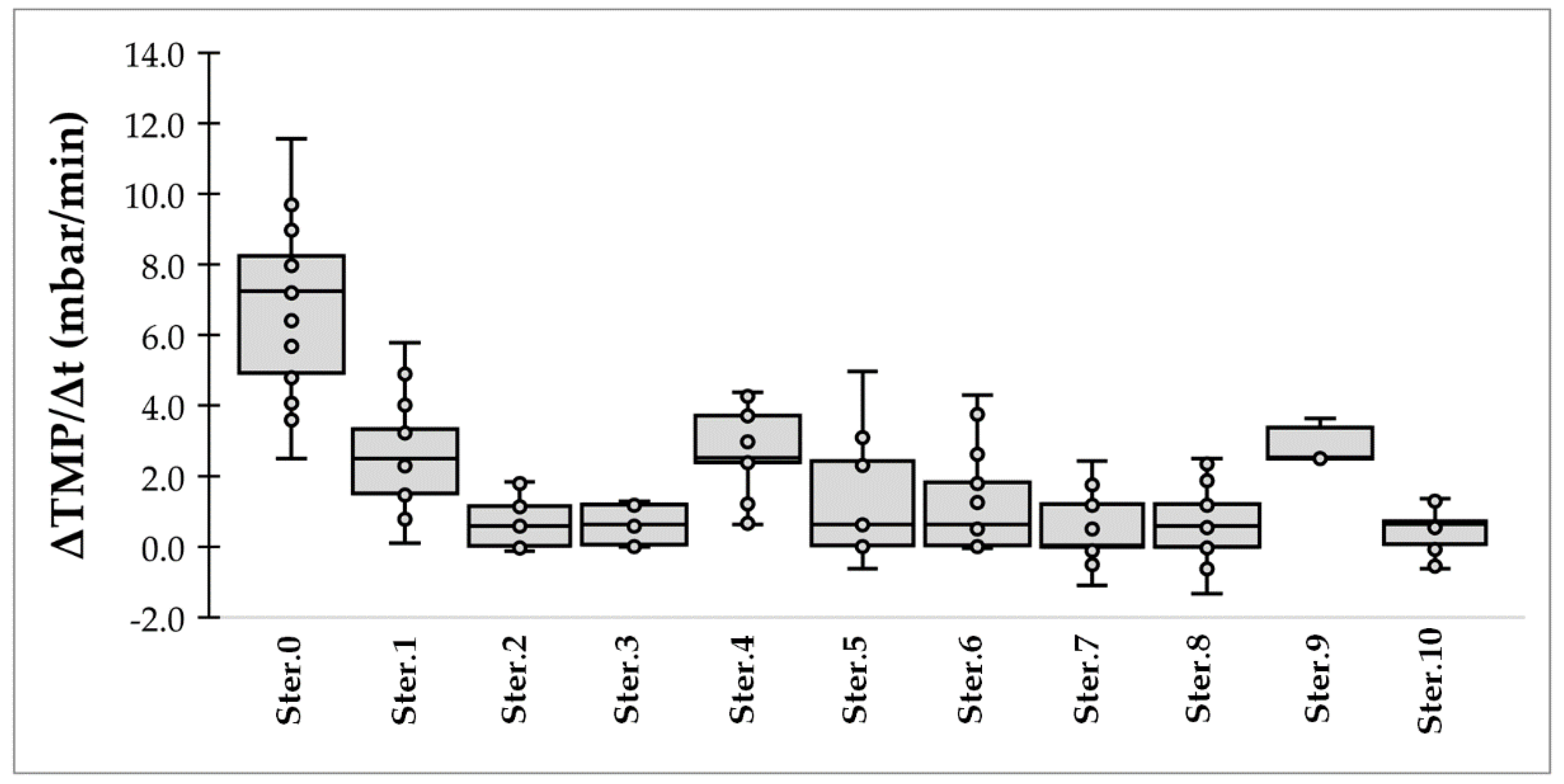
3.3. Membrane Fouling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Armenante, P.M.; Akiti, O. Sterilization processes in the pharmaceutical industry. In Chemical Engineering in the Pharmaceutical Industry: Drug Product Design, Development, and Modeling; Wiley Online Library: Hoboken, NJ, USA, 2019; pp. 311–379. [Google Scholar]
- Berovic, M. Sterilisation in biotechnology. Biotechnol. Annu. Rev. 2005, 11, 257–279. [Google Scholar] [PubMed]
- Berovic, M. Comprehensive Biotechnology, 2nd ed.; Moo-Young, M., Ed.; Academic Press: Cambridge, MA, USA, 2011; pp. 135–150. [Google Scholar]
- Richards, J.W. Introduction to industrial sterilization. In Introduction to Industrial Sterilization; Academic Press: New York, NY, USA, 1968. [Google Scholar]
- Li, G.; Chen, K.; Wei, Y.; Zeng, J.; Yang, Y.; He, F.; Li, H.; Ouyang, P. Mass transfer, gas holdup, and kinetic models of batch and continuous fermentation in a novel tectangular dynamic membrane airlift bioreactor. Engineering 2021, 27. [Google Scholar] [CrossRef]
- López-Gómez, J.P.; Alexandri, M.; Schneider, R.; Venus, J. A review on the current developments in continuous lactic acid fermentations and case studies utilising inexpensive raw materials. Process Biochem. 2019, 79, 1–10. [Google Scholar] [CrossRef]
- Lu, Z.; Wei, M.; Yu, L. Enhancement of pilot scale production of l(+)-lactic acid by fermentation coupled with separation using membrane bioreactor. Process Biochem. 2012, 47, 410–415. [Google Scholar] [CrossRef]
- Restaino, O.F.; Cimini, D.; De Rosa, M.; Catapano, A.; Schiraldi, C. High cell density cultivation of Escherichia coli K4 in a microfiltration bioreactor: A step towards improvement of chondroitin precursor production. Microb. Cell Factories 2011, 10, 10. [Google Scholar] [CrossRef]
- Ko, C.H.; Chiu, P.C.; Yang, C.L.; Chang, K.H. Xylitol conversion by fermentation using five yeast strains and polyelectrolyte-assisted ultrafiltration. Biotechnol. Lett. 2008, 30, 81–86. [Google Scholar] [CrossRef]
- Schiraldi, C.; Martino, A.; Acone, M.; Di Lernia, I.; Di Lazzaro, A.; Marulli, F.; Generoso, M.; Cartenì, M.; De Rosa, M. Effective production of a thermostable α-glucosidase from Sulfolobus solfataricus in Escherichia coli exploiting a microfiltration bioreactor. Biotechnol. Bioeng. 2000, 70, 670–676. [Google Scholar] [CrossRef]
- Wang, C.; Li, Q.; Tang, H.; Zhou, W.; Yan, D.; Xing, J.; Wan, Y. Clarification of succinic acid fermentation broth by ultrafiltration in succinic acid bio-refinery. J. Chem. Technol. Biotechnol. 2013, 88, 444–448. [Google Scholar] [CrossRef]
- Waszak, M.; Gryta, M. The ultrafiltration ceramic membrane used for broth separation in membrane bioreactor. Chem. Eng. J. 2016, 305, 129–135. [Google Scholar] [CrossRef]
- Krige, A.; Nicol, W. Continuous succinic acid fermentation by Escherichia coli KJ122 with cell recycle. Process Biochem. 2015, 50, 2004–2011. [Google Scholar] [CrossRef][Green Version]
- Brunetti, A.; Zito, P.F.; Giorno, L.; Drioli, E.; Barbieri, G. Membrane reactors for low temperature applications: An overview. Chem. Eng. Process. Process Intensif. 2018, 124, 282–307. [Google Scholar] [CrossRef]
- Baker, R.W. Membrane Technology and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Zydney, A.L.; Xenopoulos, A. Improving dextran tests for ultrafiltration membranes: Effect of device format. J. Membr. Sci. 2007, 291, 180–190. [Google Scholar] [CrossRef]
- Lorain, O.; Marcellino, S.; Deratani, A.; Gassara, S.; Duchemin, I.; Espenan, J.-M. New ultrafiltration (UF) membrane made with a new polymer material for long-lasting rejection performance, Neophil®. Water Pract. Technol. 2020, 15, 356–364. [Google Scholar] [CrossRef]
- Sandhya Rani, S.L.; Kumar, R.V. Insights on applications of low-cost ceramic membranes in wastewater treatment: A mini-review. Case Stud. Chem. Environ. Eng. 2021, 4, 100149. [Google Scholar] [CrossRef]
- Park, T.H.; Kim, I.H. Hollow-fibre fermenter using ultrafiltration. Appl. Microbiol. Biotechnol. 1985, 22, 190–194. [Google Scholar] [CrossRef]
- Schiraldi, C.; Marulli, F.; Di Lernia, I.; Martino, A.; De Rosa, M. A microfiltration bioreactor to achieve high cell density in Sulfolobus solfataricus fermentation. Extremophiles 1999, 3, 199–204. [Google Scholar] [CrossRef]
- Mimitsuka, T.; Sawai, K.; Kobayashi, K.; Tsukada, T.; Takeuchi, N.; Yamada, K.; Ogino, H.; Yonehara, T. Production of D-lactic acid in a continuous membrane integrated fermentation reactor by genetically modified Saccharomyces cerevisiae: Enhancement in D-lactic acid carbon yield. J Biosci. Bioeng. 2015, 119, 65–71. [Google Scholar] [CrossRef]
- Ramchandran, L.; Sanciolo, P.; Vasiljevic, T.; Broome, M.; Powell, I.; Duke, M. Improving cell yield and lactic acid production of Lactococcus lactis ssp. cremoris by a novel submerged membrane fermentation process. J. Membr. Sci. 2012, 403–404, 179–187. [Google Scholar] [CrossRef]
- Wang, C.; Li, Q.; Wang, D.; Xing, J. Improving the lactic acid production of Actinobacillus succinogenes by using a novel fermentation and separation integration system. Process Biochem. 2014, 49, 1245–1250. [Google Scholar] [CrossRef]
- Mimitsuka, T.; Na, K.; Morita, K.; Sawai, H.; Minegishi, S.; Henmi, M.; Yamada, K.; Shimizu, S.; Yonehara, T. A membrane-integrated fermentation reactor system: Its effects in reducing the amount of sub-raw materials for D-lactic acid continuous fermentation by Sporolactobacillus laevolacticus. Biosci. Biotechnol. Biochem. 2012, 76, 67–72. [Google Scholar] [CrossRef]
- Wang, C.; Ming, W.; Yan, D.; Zhang, C.; Yang, M.; Liu, Y.; Zhang, Y.; Guo, B.; Wan, Y.; Xing, J. Novel membrane-based biotechnological alternative process for succinic acid production and chemical synthesis of bio-based poly (butylene succinate). Bioresour. Technol. 2014, 156, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Sawai, H.; Mimitsuka, T.; Minegishi, S.; Henmi, M.; Yamada, K.; Shimizu, S.; Yonehara, T. A novel membrane-integrated fermentation reactor system: Application to pyruvic acid production in continuous culture by Torulopsis glabrata. Bioprocess Biosyst. Eng. 2011, 34, 721–725. [Google Scholar] [CrossRef] [PubMed]
- Sawai, H.; Na, K.; Sasaki, N.; Mimitsuka, T.; Minegishi, S.; Henmi, M.; Yamada, K.; Shimizu, S.; Yonehara, T. Membrane-integrated fermentation system for improving the optical purity of D-lactic acid produced during continuous fermentation. Biosci. Biotechnol. Biochem. 2011, 75, 2326–2332. [Google Scholar] [CrossRef]
- Luo, R.; Qin, Z.; Zhou, D.; Wang, D.; Hu, G.; Su, Z.; Zhang, S. Coupling the fermentation and membrane separation process for polyamides monomer cadaverine production from feedstock lysine. Eng. Life Sci. 2021, 21, 623–629. [Google Scholar] [CrossRef]
- Lee, R.K.; Ryu, H.W.; Oh, H.; Kim, M.; Wee, Y.J. Cell-recycle continuous fermentation of Enterococcus faecalis RKY1 for economical production of lactic acid by reduction of yeast extract supplementation. J. Microbiol. Biotechnol. 2014, 24, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsov, A.; Beloded, A.; Derunets, A.; Grosheva, V.; Vakar, L.; Kozlovskiy, R.; Shvets, V. Biosynthesis of lactic acid in a membrane bioreactor for cleaner technology of polylactide production. Clean Technol. Environ. Policy 2016, 19, 869–882. [Google Scholar] [CrossRef]
- Dey, P.; Pal, P. Direct production of l (+) lactic acid in a continuous and fully membrane-integrated hybrid reactor system under non-neutralizing conditions. J. Membr. Sci. 2012, 389, 355–362. [Google Scholar] [CrossRef]
- Bove, D.; Merello, S.; Frumento, D.; Arni, S.A.; Aliakbarian, B.; Converti, A. A Critical Review of Biological Processes and Technologies for Landfill Leachate Treatment. Chem. Eng. Technol. 2015, 38, 2115–2126. [Google Scholar] [CrossRef]
- Kang, G.-D.; Cao, Y.-M. Application and modification of poly(vinylidene fluoride) (PVDF) membranes—A review. J. Membr. Sci. 2014, 463, 145–165. [Google Scholar] [CrossRef]
- Judd, S. The MBR Book: Principles and Applications of Membrane Bioreactors for Water and Wastewater Treatment; Elsevier: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Haponska, M.; Trojanowska, A.; Nogalska, A.; Jastrzab, R.; Gumi, T.; Tylkowski, B. PVDF Membrane Morphology-Influence of Polymer Molecular Weight and Preparation Temperature. Polymers 2017, 9, 718. [Google Scholar] [CrossRef]
- Li, N.; Fu, Y.; Lu, Q.; Xiao, C. Microstructure and Performance of a Porous Polymer Membrane with a Copper Nano-Layer Using Vapor-Induced Phase Separation Combined with Magnetron Sputtering. Polymers 2017, 9, 524. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Hashim, N.A.; Liu, Y.; Abed, M.R.M.; Li, K. Progress in the production and modification of PVDF membranes. J. Membr. Sci. 2011, 375, 1–27. [Google Scholar] [CrossRef]
- Bastiurea, M.; Rodeanu, M.; Dima, D.; Murarescu, M.; Andrei, G. Thermal and Mechanical Properties of polyester composites with graphene oxide and graphite. Dig. J. Nanomater. Biostruct. (DJNB) 2015, 10, 521–533. [Google Scholar]
- Low, L.F.; Bakar, A.A. Mechanical, thermal and water absorption behavior of hollow epoxy particle–filled polyester composites. J. Compos. Mater. 2013, 48, 1725–1733. [Google Scholar] [CrossRef]
- Cobetter Filtration. Available online: https://www.cobetterfiltration.com/Industries/Pharmafiltration/Membrane/PVDF-Membrane/ (accessed on 24 March 2022).
- Rahimpour, A.; Madaeni, S.S.; Amirinejad, M.; Mansourpanah, Y.; Zereshki, S. The effect of heat treatment of PES and PVDF ultrafiltration membranes on morphology and performance for milk filtration. J. Membr. Sci. 2009, 330, 189–204. [Google Scholar] [CrossRef]
- Schock, G.; Miquel, A.; Birkenberger, R. Characterization of ultrafiltration membranes: Cut-off determination by gel permeation chromatography. J. Membr. Sci. 1989, 41, 55–67. [Google Scholar] [CrossRef]
- Pellegrin, B.; Mezzari, F.; Hanafi, Y.; Szymczyk, A.; Remigy, J.-C.; Causserand, C. Filtration performance and pore size distribution of hypochlorite aged PES/PVP ultrafiltration membranes. J. Membr. Sci. 2015, 474, 175–186. [Google Scholar] [CrossRef]
- Tkacik, G.; Michaels, S. A rejection profile test for ultrafiltration membranes & devices. Bio/technology 1991, 9, 941–946. [Google Scholar]
- Joss, A.; Böhler, M.; Wedi, D.; Siegrist, H. Proposing a method for online permeability monitoring in membrane bioreactors. Water Sci. Technol. 2009, 60, 497–506. [Google Scholar] [CrossRef]
- Filtersafe. Available online: https://filtersafe.net/blog/blog-desalination/how-does-filtration-purify-water-filtration-spectrum/ (accessed on 10 June 2022).



| Membrane Type and Configuration | Membrane Material | Membrane Sterilization Method | Bioreactor/Media Sterilization Method | Reference |
|---|---|---|---|---|
| Hollow fiber sidestream | Polysulfone (PS) | Autoclave sterilization (121 °C, 40 min) | Autoclave sterilization (121 °C, 40 min) | [13] |
| Microfiltration (MF) hollow fiber sidestream | - | 200 ppm NaClO solution | Autoclave sterilization (121 °C, 20 min) | [25] |
| MF hollow fiber sidestream | Polyethersulfone (PES) | 0.2 g/L NaClO solution | Autoclave sterilization (121 °C, 20 min) | [23] |
| Ultrafiltration (UF) sidestream | Cellulose acetate (CA) | - | Autoclave sterilization (115 °C, 30 min) | [11] |
| Hollow fiber sidestream | - | - | Autoclave sterilization (121 °C, 15 or 30 min) | [6] |
| Flat sheet submerged | Polyvinylidene Difluoride (PVDF) | Autoclave sterilization (121 °C, 20 min) | Autoclave sterilization (121 °C, 20 min) | [21,24,26,27] |
| MF hollow fiber submerged | Polypropylene (PP) PES | In situ sterilization * | Sterilization * | [10,20] |
| MF hollow fiber sidestream | - | 0.2 g/L NaClO solution | Autoclave sterilization (121 °C) | [28] |
| UF hollow fiber sidestream | PS | 0.1 M NaOH and 200 ppm NaOCl | Autoclave sterilization (121 °C, 15 min) | [29] |
| hollow fiber sidestream | PS | - | Sterilization * | [30] |
| UF sidestream | Ceramic | - | Sterilization * | [12] |
| UF hollow fiber submerged | PVDF | 1% NaOCl for 18 h | Sterilization * | [22] |
| MF sidestream | Ceramic | Steam sterilization (121 °C) | Steam sterilization (121 °C) | [7] |
| UF sidestream | Organic | 200 ppm NaClO solution | Steam sterilization (121 °C) | [7] |
| MF flat sheet sidestream | PVDF | 200 ppm NaClO solution + ultrapure water | Autoclave sterilization (121 °C, 15 min) | [31] |
| UF hollow fiber submerged | Polyamide | 5% formaldehyde for 24 h | Sterilization * | [19] |
| Property | Value |
|---|---|
| Melting point (°C) | 140–170 |
| Glass transition temperature (°C) | −41/−38 |
| Thermal stability, 1% mass loss, in air (°C) | 375 |
| Linear thermal expansion coefficient (10−6/°C) | 50–103 or 120–140 |
| Property | Value |
|---|---|
| Membrane Chemistry | Proprietary PVDF |
| Nominal Pore Size | 0.03 μm |
| Outside Fiber Diameter | 2.6 mm |
| Temperature Range | 5–40 °C |
| pH Range for Cleaning | 2.0–10.5 |
| Maximum Filtration Trans-membrane Pressure (TMP) | 0.6 bar |
| No of Ster. | Permeance (LMH/Bar) | MWCO (kDa) | ΔTMP/Δt (Mbar/Min) |
|---|---|---|---|
| 0 (initial) | 677 | 42.0 | 7.0 |
| 1 | 878 | 98.0 | 2.7 |
| 2 | 916 | 43.7 | 0.7 |
| 3 | 874 | 44.0 | 0.7 |
| 4 | 913 | 31.5 | 3.0 |
| 5 | 993 | 36.2 | 1.2 |
| 6 | 888 | 49.1 | 1.0 |
| 7 | 829 | 79.2 | 0.7 |
| 8 | 968 | 76.1 | 0.8 |
| 9 | 941 | 86.0 | 1.1 |
| 10 | 848 | 40.3 | 0.5 |
| Average (1–10) | 906 ± 49 | 58.4 ± 22.1 | 1.3 ± 1.4 |
| Parameter | Feed | Permeate |
|---|---|---|
| Oxalic Acid (g/L) | 0.00 ± 0.00 | 0.02 ± 0.01 |
| Citric Acid (g/L) | 6.44 ± 0.92 * | 3.73 ± 0.54 * |
| Malic Acid (g/L) | 0.97 ± 0.12 | 0.89 ± 0.08 |
| Acetic Acid (g/L) | 0.79 ± 0.10 | 0.54 ± 0.74 |
| Fumaric Acid (g/L) | 0.06 ± 0.01 | 0.03 ± 0.11 |
| OD (600 nm) | 18.11 ± 0.80 | 0.06 ± 0.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nastouli, A.; Tsirigka, A.; Harasek, M.; Karabelas, A.J.; Patsios, S.I. The Effect of Heat Sterilization on Key Filtration Performance Parameters of a Commercial Polymeric (PVDF) Hollow-Fiber Ultrafiltration Membrane. Membranes 2022, 12, 725. https://doi.org/10.3390/membranes12080725
Nastouli A, Tsirigka A, Harasek M, Karabelas AJ, Patsios SI. The Effect of Heat Sterilization on Key Filtration Performance Parameters of a Commercial Polymeric (PVDF) Hollow-Fiber Ultrafiltration Membrane. Membranes. 2022; 12(8):725. https://doi.org/10.3390/membranes12080725
Chicago/Turabian StyleNastouli, Alexandra, Asimina Tsirigka, Michael Harasek, Anastasios J. Karabelas, and Sotiris I. Patsios. 2022. "The Effect of Heat Sterilization on Key Filtration Performance Parameters of a Commercial Polymeric (PVDF) Hollow-Fiber Ultrafiltration Membrane" Membranes 12, no. 8: 725. https://doi.org/10.3390/membranes12080725
APA StyleNastouli, A., Tsirigka, A., Harasek, M., Karabelas, A. J., & Patsios, S. I. (2022). The Effect of Heat Sterilization on Key Filtration Performance Parameters of a Commercial Polymeric (PVDF) Hollow-Fiber Ultrafiltration Membrane. Membranes, 12(8), 725. https://doi.org/10.3390/membranes12080725









