Sequential Membrane Filtration to Recover Polyphenols and Organic Acids from Red Wine Lees: The Antioxidant Properties of the Spray-Dried Concentrate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Laboratory-Scale Cross-Flow Filtration Pilot Unit
2.3. Spray-Drying of AFC30 Concentrate
2.4. Preparation of Food System for Testing Antioxidant Potential of Dried Concentrates
2.5. Analytical Methods
2.5.1. HPLC Determination of Organic Acids and Polyphenols
2.5.2. Antioxidant Potential Analyses
2.5.3. Total Phenols
2.5.4. TBARS
2.5.5. Oxymyoglobin Content
2.6. Statistical Analysis
3. Results and Discussion
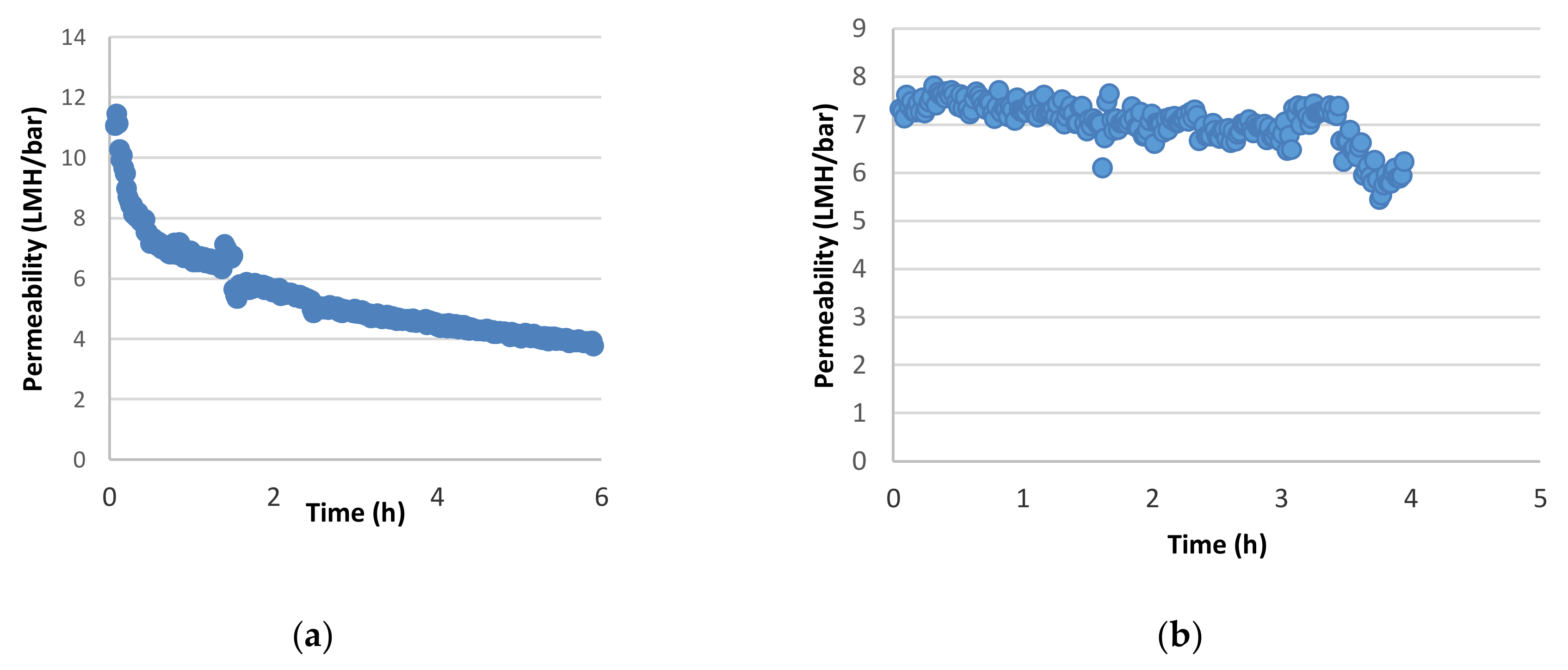
3.1. Membrane Filtration
3.2. Spray-Drying of Nanofiltration Condensate
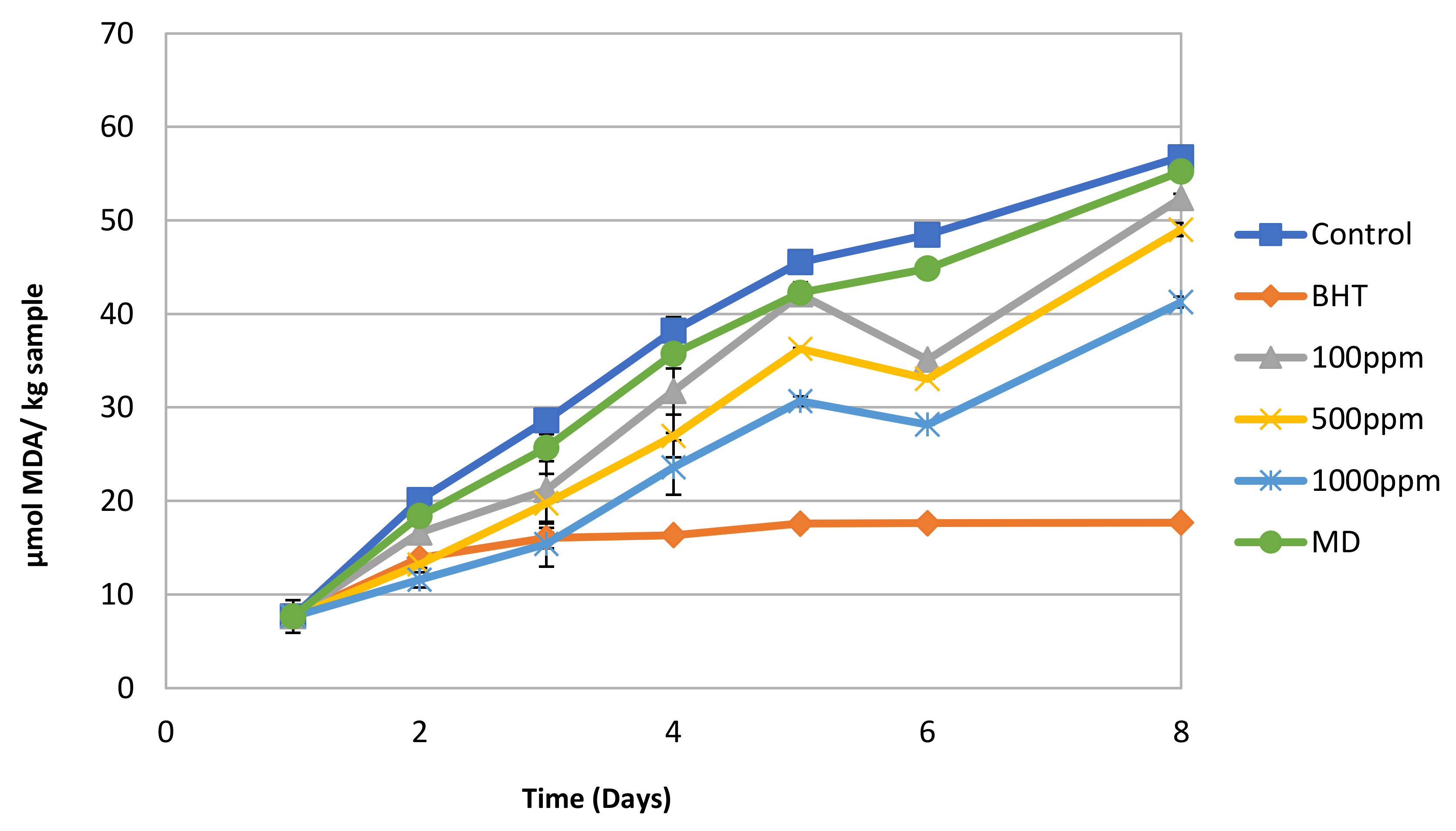
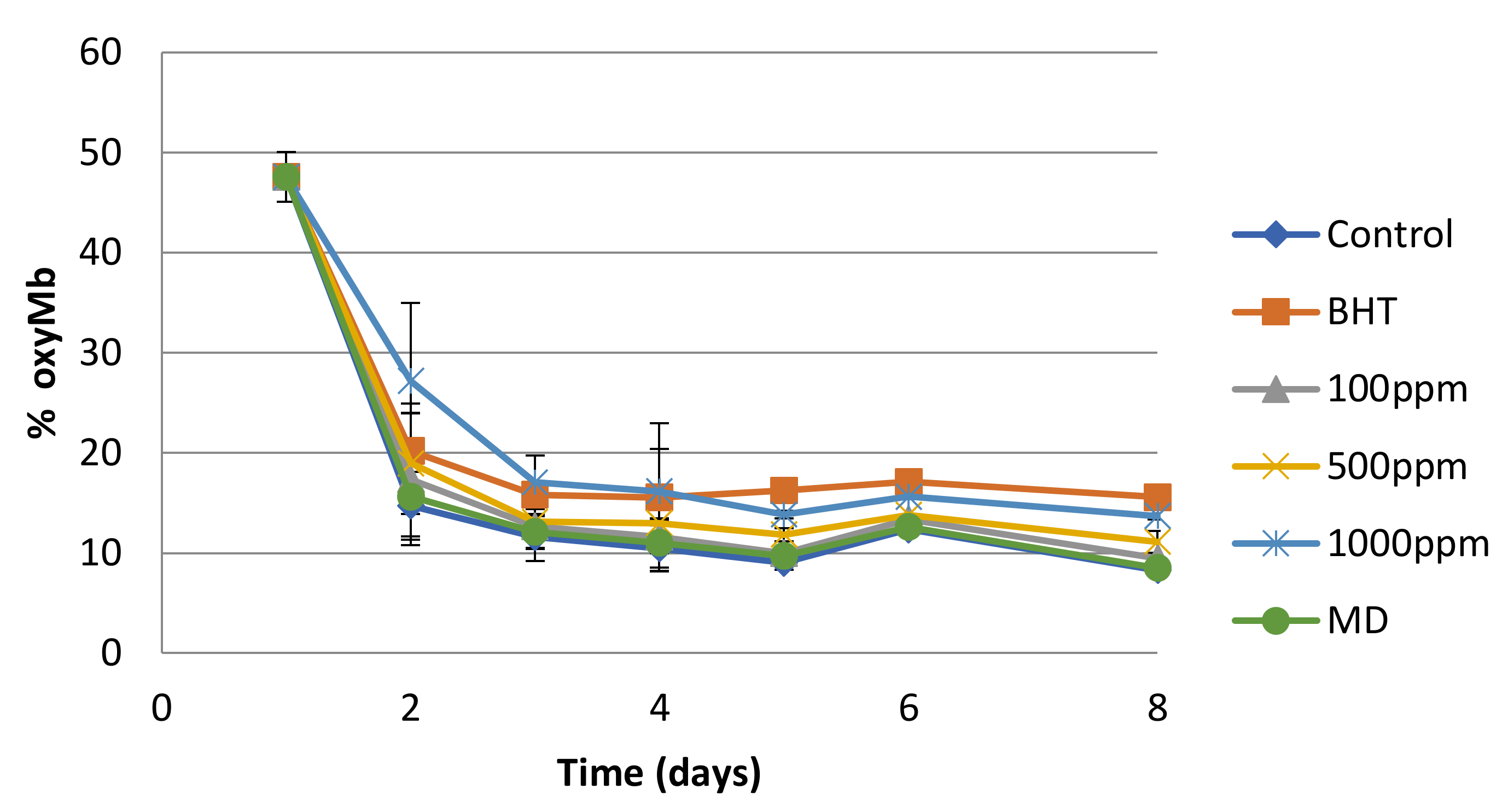
3.3. Antioxidant Potential of Spray-Dried Nanofiltration Powder
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Da Costa, D.C.F.; Rangel, L.P.; Quarti, J.; Santos, R.A.; Silva, J.L.; Fialho, E. Bioactive Compounds and Metabolites from Grapes and Red Wine in Breast Cancer Chemoprevention and Therapy. Molecules 2020, 25, 3531. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Xiao, L.; Wang, Y.; Li, H.; Huang, Z.; He, X. Health benefits of wine: Don’t expect resveratrol too much. Food Chem. 2014, 156, 258–263. [Google Scholar] [CrossRef]
- Leikert, J.F.; Räthel, T.R.; Wohlfart, P.; Cheynier, V.; Vollmar, A.M.; Dirsch, V.M. Red Wine Polyphenols Enhance Endothelial Nitric Oxide Synthase Expression and Subsequent Nitric Oxide Release from Endothelial Cells. Circulation 2002, 106, 1614–1617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- OIV. Statistical Report on World Vitiviniculture. Available online: https://www.oiv.int/en/the-international-organisation-of-vine-and-wine (accessed on 5 February 2022).
- Oliveira, M.; Duarte, E.D.A. Integrated approach to winery waste: Waste generation and data consolidation. Front. Environ. Sci. Eng. 2014, 10, 168–176. [Google Scholar] [CrossRef]
- Ruggieri, L.; Cadena, E.; Martínez-Blanco, J.; Gasol, C.M.; Rieradevall, J.; Gabarrell, X.; Gea, T.; Sort, X.; Sanchez, A. Recovery of organic wastes in the Spanish wine industry. Technical, economic and environmental analyses of the composting process. J. Clean. Prod. 2009, 17, 830–838. [Google Scholar] [CrossRef] [Green Version]
- Jara-Palacios, M.J. Wine Lees as a Source of Antioxidant Compounds. Antioxidants 2019, 8, 45. [Google Scholar] [CrossRef] [Green Version]
- Dimou, C.; Kopsahelis, N.; Papadaki, A.; Papanikolaou, S.; Kookos, I.K.; Mandala, I.; Koutinas, A.A. Wine lees valorization: Biorefinery development including production of a generic fermentation feedstock employed for poly(3-hydroxybutyrate) synthesis. Food Res. Int. 2015, 73, 81–87. [Google Scholar] [CrossRef]
- Barcia, M.T.; Pertuzatti, P.B.; Gómez-Alonso, S.; Godoy, H.T.; Hermosín-Gutiérrez, I. Phenolic composition of grape and winemaking by-products of Brazilian hybrid cultivars BRS Violeta and BRS Lorena. Food Chem. 2014, 159, 95–105. [Google Scholar] [CrossRef]
- Lee, J.; Rennaker, C. Antioxidant capacity and stilbene contents of wines produced in the Snake River Valley of Idaho. Food Chem. 2007, 105, 195–203. [Google Scholar] [CrossRef]
- Barbosa, J.; Borges, S.; Amorim, M.M.; Pereira, M.J.V.; Oliveira, A.L.S.; Pintado, M.M.; Teixeira, P. Comparison of spray drying, freeze drying and convective hot air drying for the production of a probiotic orange powder. J. Funct. Foods 2015, 17, 340–351. [Google Scholar] [CrossRef]
- Ricci, A.; Mejia, J.A.A.; Versari, A.; Chiarello, E.; Bordoni, A.; Parpinello, G.P. Microencapsulation of polyphenolic compounds recovered from red wine lees: Process optimization and nutraceutical study. Food Bioprod. Process. 2021, 132, 1–12. [Google Scholar] [CrossRef]
- Moschona, A.; Liakopoulou-Kyriakides, M. Encapsulation of biological active phenolic compounds extracted from wine wastes in alginate-chitosan microbeads. J. Microencapsul. 2018, 35, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Gaona, I.J.A.; Bater, C.; Zamora, M.C.; Chirife, J. Spray drying encapsulation of red wine: Stability of total monomeric anthocyanins and structural alterations upon storage. J. Food Process. Preserv. 2017, 42, e13457. [Google Scholar] [CrossRef]
- Louli, V.; Ragoussis, N.; Magoulas, K. Recovery of phenolic antioxidants from wine industry by-products. Bioresour. Technol. 2003, 92, 201–208. [Google Scholar] [CrossRef]
- Ignat, I.; Volf, I.; Popa, V.I. A critical review of methods for characterisation of polyphenolic compounds in fruits and vegetables. Food Chem. 2011, 126, 1821–1835. [Google Scholar] [CrossRef]
- De Iseppi, A.; Lomolino, G.; Marangon, M.; Curioni, A. Current and future strategies for wine yeast lees valorization. Food Res. Int. 2020, 137, 109352. [Google Scholar] [CrossRef]
- Cassano, A.; De Luca, G.; Conidi, C.; Drioli, E. Effect of polyphenols-membrane interactions on the performance of membrane-based processes. A review. Coord. Chem. Rev. 2017, 351, 45–75. [Google Scholar] [CrossRef]
- Giacobbo, A.; Prado, J.M.D.; Meneguzzi, A.; Bernardes, A.M.; de Pinho, M.N. Microfiltration for the recovery of polyphenols from winery effluents. Sep. Purif. Technol. 2015, 143, 12–18. [Google Scholar] [CrossRef]
- Giacobbo, A.; Bernardes, A.M.; de Pinho, M.N. Sequential pressure-driven membrane operations to recover and fractionate polyphenols and polysaccharides from second racking wine lees. Sep. Purif. Technol. 2017, 173, 49–54. [Google Scholar] [CrossRef]
- López-Borrell, A.; López-Pérez, M.-F.; Cardona, S.C.; Lora-García, J. Experimental Study and Mathematical Modeling of a Nanofiltration Membrane System for the Recovery of Polyphenols from Wine Lees. Membranes 2022, 12, 240. [Google Scholar] [CrossRef]
- Kontogiannopoulos, K.N.; I Patsios, S.; Mitrouli, S.T.; Karabelas, A.J. Tartaric acid and polyphenols recovery from winery waste lees using membrane separation processes. J. Chem. Technol. Biotechnol. 2017, 92, 2934–2943. [Google Scholar] [CrossRef]
- Romero-Díez, R.; Rodríguez-Rojo, S.; Cocero, M.J.; Duarte, C.M.M.; Matias, A.A.; Bronze, M.R. Phenolic characterization of aging wine lees: Correlation with antioxidant activities. Food Chem. 2018, 259, 188–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayes, J.; Canonico, I.; Allen, P. Effects of organic tomato pulp powder and nitrite level on the physicochemical, textural and sensory properties of pork luncheon roll. Meat Sci. 2013, 95, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Vareltzis, P.; Hultin, H.O.; Autio, W.R. Hemoglobin-mediated lipid oxidation of protein isolates obtained from cod and haddock white muscle as affected by citric acid, calcium chloride and pH. Food Chem. 2008, 108, 64–74. [Google Scholar] [CrossRef]
- Wang, T.; Jónsdóttir, R.; Kristinsson, H.G.; Thorkelsson, G.; Jacobsen, C.; Hamaguchi, P.Y.; Ólafsdóttir, G. Inhibition of haemoglobin-mediated lipid oxidation in washed cod muscle and cod protein isolates by Fucus vesiculosus extract and fractions. Food Chem. 2010, 123, 321–330. [Google Scholar] [CrossRef]
- Tang, S.; Ou, S.; Huang, X.; Li, W.; Kerry, J.; Buckley, D. Effects of added tea catechins on colour stability and lipid oxidation in minced beef patties held under aerobic and modified atmospheric packaging conditions. J. Food Eng. 2006, 77, 248–253. [Google Scholar] [CrossRef]
- Kalbasi, A.; Cisneros-Zevallos, L. Fractionation of Monomeric and Polymeric Anthocyanins from Concord Grape (Vitis labrusca L.) Juice by Membrane Ultrafiltration. J. Agric. Food Chem. 2007, 55, 7036–7042. [Google Scholar] [CrossRef]
- Cassano, A.; Conidi, C.; Giorno, L.; Drioli, E. Fractionation of olive mill wastewaters by membrane separation techniques. J. Hazard. Mater. 2013, 248–249, 185–193. [Google Scholar] [CrossRef]
- Conidi, C.; Drioli, E.; Cassano, A. Membrane-based agro-food production processes for polyphenol separation, purification and concentration. Curr. Opin. Food Sci. 2018, 23, 149–164. [Google Scholar] [CrossRef]
- Tao, Y.; Wu, D.; Zhang, Q.-A.; Sun, D.-W. Ultrasound-assisted extraction of phenolics from wine lees: Modeling, optimization and stability of extracts during storage. Ultrason. Sonochem. 2014, 21, 706–715. [Google Scholar] [CrossRef]
- Díaz-Reinoso, B.; Moure, A.; Domínguez, H.; Parajó, J.C. Ultra- and nanofiltration of aqueous extracts from distilled fermented grape pomace. J. Food Eng. 2009, 91, 587–593. [Google Scholar] [CrossRef]
- Mejia, J.A.A.; Ricci, A.; Figueiredo, A.S.; Versari, A.; Cassano, A.; Parpinello, G.P.; De Pinho, M.N. Recovery of Phenolic Compounds from Red Grape Pomace Extract through Nanofiltration Membranes. Foods 2020, 9, 1649. [Google Scholar] [CrossRef] [PubMed]
- Doan, H.; Lohi, A. Fouling in Membrane Filtration and Remediation Methods. In Mass Transfer—Advances in Sustainable Energy and Environment Oriented Numerical Modeling; IntechOpen: London, UK, 2013; pp. 195–219. [Google Scholar] [CrossRef] [Green Version]
- Piñón-Balderrama, C.I.; Leyva-Porras, C.; Terán-Figueroa, Y.; Espinosa-Solís, V.; Álvarez-Salas, C.; Saavedra-Leos, M.Z. Encapsulation of Active Ingredients in Food Industry by Spray-Drying and Nano Spray-Drying Technologies. Processes 2020, 8, 889. [Google Scholar] [CrossRef]
- Moreno, T.; de Paz, E.; Navarro, I.; Rodríguez-Rojo, S.; Matias, A.; Duarte, C.; Sanz-Buenhombre, M.; Cocero, M. Spray Drying Formulation of Polyphenols-Rich Grape Marc Extract: Evaluation of Operating Conditions and Different Natural Carriers. Food Bioprocess Technol. 2016, 9, 2046–2058. [Google Scholar] [CrossRef]
- Aguilera, J.M. The food matrix: Implications in processing, nutrition and health. Crit. Rev. Food Sci. Nutr. 2018, 59, 3612–3629. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, B.; Howes, T. Implication of glass transition for the drying and stability of dried foods. J. Food Eng. 1999, 40, 71–79. [Google Scholar] [CrossRef]
- Kha, T.C.; Nguyen, M.H.; Roach, P. Effects of spray drying conditions on the physicochemical and antioxidant properties of the Gac (Momordica cochinchinensis) fruit aril powder. J. Food Eng. 2010, 98, 385–392. [Google Scholar] [CrossRef]
- Ramanathan, R.; Suman, S.P.; Faustman, C. Biomolecular Interactions Governing Fresh Meat Color in Post-mortem Skeletal Muscle: A Review. J. Agric. Food Chem. 2020, 68, 12779–12787. [Google Scholar] [CrossRef]
- Wu, H.; Richards, M.P.; Undeland, I. Lipid oxidation and antioxidant delivery systems in muscle food. Compr. Rev. Food Sci. Food Saf. 2022. [Google Scholar] [CrossRef]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A Comprehensive Review on Lipid Oxidation in Meat and Meat Products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef] [Green Version]
- Chaijan, M. Review: Lipid and Myoglobin Oxidations in Muscle Foods. Songklanakarin J. Sci. Technol. 2008, 30, 47–53. [Google Scholar]
- Vareltzis, P.; Adamopoulos, K.G.; Hultin, H.O. Interactions between Hemoglobin and Cod Muscle Constituents following Treatment at Extreme pH Values. J. Food Sci. 2011, 76, C1003–C1009. [Google Scholar] [CrossRef] [PubMed]
| Property | Value |
|---|---|
| % moisture | 95.6 |
| Total solids (g/L) | 68.5 |
| Total dissolved solids (TDS) (g/L) | 23.7 |
| pH | 4.09 |
| Conductivity (mS/cm) | 2.06 |
| Membrane | Application | Material | pH Range | Max. Operating Pressure (bar) | Max. Temperature (°C) |
|---|---|---|---|---|---|
| FPA03 | Ultrafiltration (UF) | PVDF | 1.5–10.5 | 7 | 60 |
| AFC30 | Nanofiltration (NF) | Polyamide, TFC | 1.5–9.5 | 60 | 60 |
| Membrane Type | Stream | Volume (L) | VCF | pH | Turbidity (NTU) | %DPPH Inhibition | FRAP (μmol TPTZ/L) | Total Phenols (mg gallic acid/L) |
|---|---|---|---|---|---|---|---|---|
| UF(FPA03) | Feed | 10 | 4.09 | 150 | 61.52 ± 0.097 a | 15.2 ± 0.078 a | 315.27 ± 0.21 a | |
| Permeate | 6.2 | 2.6 | 4.86 | 0.6 | 83.48 ± 0.066 b | 22.2 ± 0.093 b | 403.46 ± 0.13 b | |
| Concentrate | 3.8 | 4.07 | 206 | 53.53 ± 0.117 c | 13.9 ± 0.115 a | 138.33 ± 0.10 c | ||
| NF(AFC30) | Feed | 6.2 | 4.86 | 0.6 | 83.48 ± 0.066 a | 22.2 ± 0.093 b | 403.46 ± 0.153 b | |
| Permeate | 4.7 | 4.1 | 4.05 | 0.05 | 18.19 ± 0.058 d | 3.78 ± 0.034 c | 58.74 ± 0. 111 d | |
| Concentrate | 1.5 | 3.9 | 0.5 | 92.85 ± 0.070 e | 36.3 ± 0.119 d | 1351.15 ± 0.063 e |
| Substance (ppm) | Feed | Permeate UF (FPA03) | Concentrate UF (FPA03) | Permeate NF (AFC30) | Concentrate NF (AFC30) |
|---|---|---|---|---|---|
| Tartaric acid | 1630 | 1660 | 1060 | 820 | 3645 |
| DL-malic acid | 420 | 455 | 335 | 310 | 800 |
| DL-lactic acid | 1520 | 1240 | 1560 | 795 | 1550 |
| Citric acid | 410 | 460 | 300 | 320 | 840 |
| Total acids (mg) | 39,800 | 23,653 | 12,369 | 10,551.5 | 10,252.5 |
| Ferulic acid | 51 | 60 | 32 | 23 | 155 |
| Epicatechin | 100 | 116 | 43 | 34 | 360 |
| Quercetin | 17.5 | 19.5 | 13 | 15 | 38 |
| Total phenols (mg) | 1685 | 1212.1 | 334.4 | 338.4 | 829.5 |
| Compound | Separation Factors (SFAB) | |
|---|---|---|
| UF (FPA03) | NF (AFC30) | |
| L-tartaric acid | 0.54 | 5.17 |
| DL- malic acid | 0.47 | 8.95 |
| DL- lactic acid | 0.27 | 9.18 |
| Citric acid | 0.53 | 8.79 |
| Total acids | 0.40 | 5.76 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filippou, P.; Mitrouli, S.T.; Vareltzis, P. Sequential Membrane Filtration to Recover Polyphenols and Organic Acids from Red Wine Lees: The Antioxidant Properties of the Spray-Dried Concentrate. Membranes 2022, 12, 353. https://doi.org/10.3390/membranes12040353
Filippou P, Mitrouli ST, Vareltzis P. Sequential Membrane Filtration to Recover Polyphenols and Organic Acids from Red Wine Lees: The Antioxidant Properties of the Spray-Dried Concentrate. Membranes. 2022; 12(4):353. https://doi.org/10.3390/membranes12040353
Chicago/Turabian StyleFilippou, Polychronis, Soultana T. Mitrouli, and Patroklos Vareltzis. 2022. "Sequential Membrane Filtration to Recover Polyphenols and Organic Acids from Red Wine Lees: The Antioxidant Properties of the Spray-Dried Concentrate" Membranes 12, no. 4: 353. https://doi.org/10.3390/membranes12040353
APA StyleFilippou, P., Mitrouli, S. T., & Vareltzis, P. (2022). Sequential Membrane Filtration to Recover Polyphenols and Organic Acids from Red Wine Lees: The Antioxidant Properties of the Spray-Dried Concentrate. Membranes, 12(4), 353. https://doi.org/10.3390/membranes12040353






