Development of High Flux Nanocomposite Polyphenylsulfone/Oxidized Multiwalled Carbon Nanotubes Membranes for Ultrafiltration Using the Systems with Critical Solution Temperatures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Oxidized Multiwalled Carbon Nanotubes (O-MWCNT)
2.3. Preparation of O-MWCNT Dispersions in NMP
2.4. Characterization of O-MWCNT Dispersions
2.4.1. Determination of the Concentration of Dispersed O-MWCNT
2.4.2. Determination of the Average Diameter and Particle Size Distribution
2.5. Preparation of Casting Solutions
2.6. Characterization of PPSU-PEG-PVP-O-MWCNT-NMP Systems
2.7. Preparation of Membranes
2.8. Studies of Membrane Performance in Ultrafiltration
2.9. Studies of Membrane Performance in Ultrafiltration of Humic Acid (HAs) Aqueous Solution
2.10. Studies of Membrane Cross-Section Morphologies
2.11. Study of the Selective Layer Surface by Atomic Force Microscopy
2.12. Determination of Water Contact Angle
3. Results
3.1. Study of Dispersions of O-MWCNT
3.2. Study of Phase State and Viscosity of Polymer Systems PPSU-PEG-PVP-O-MWCNTs-NMP
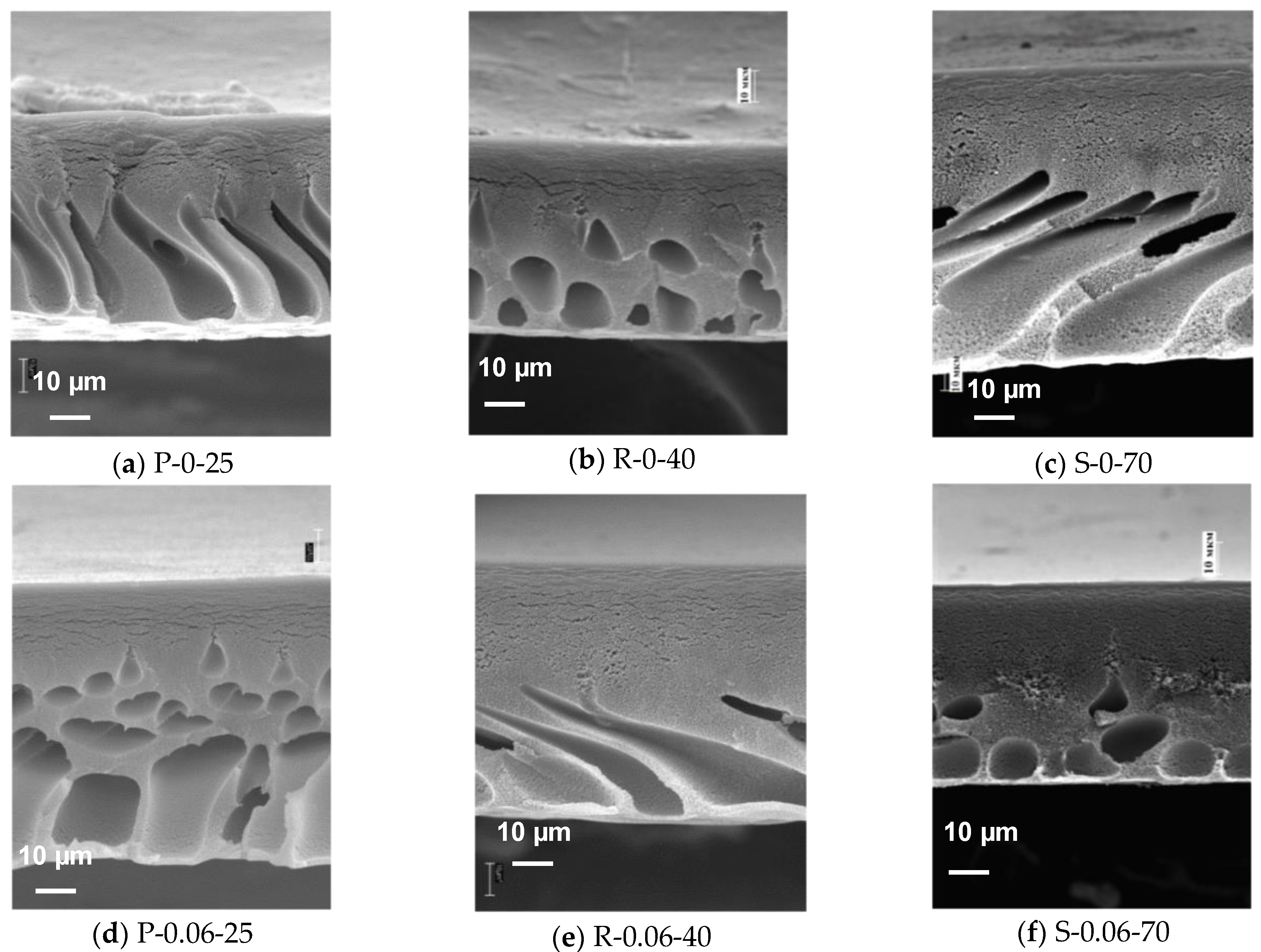
3.3. Study of the Effect of O-MWCNT Addition on Membrane Structure at Different Coagulation Bath Temperatures
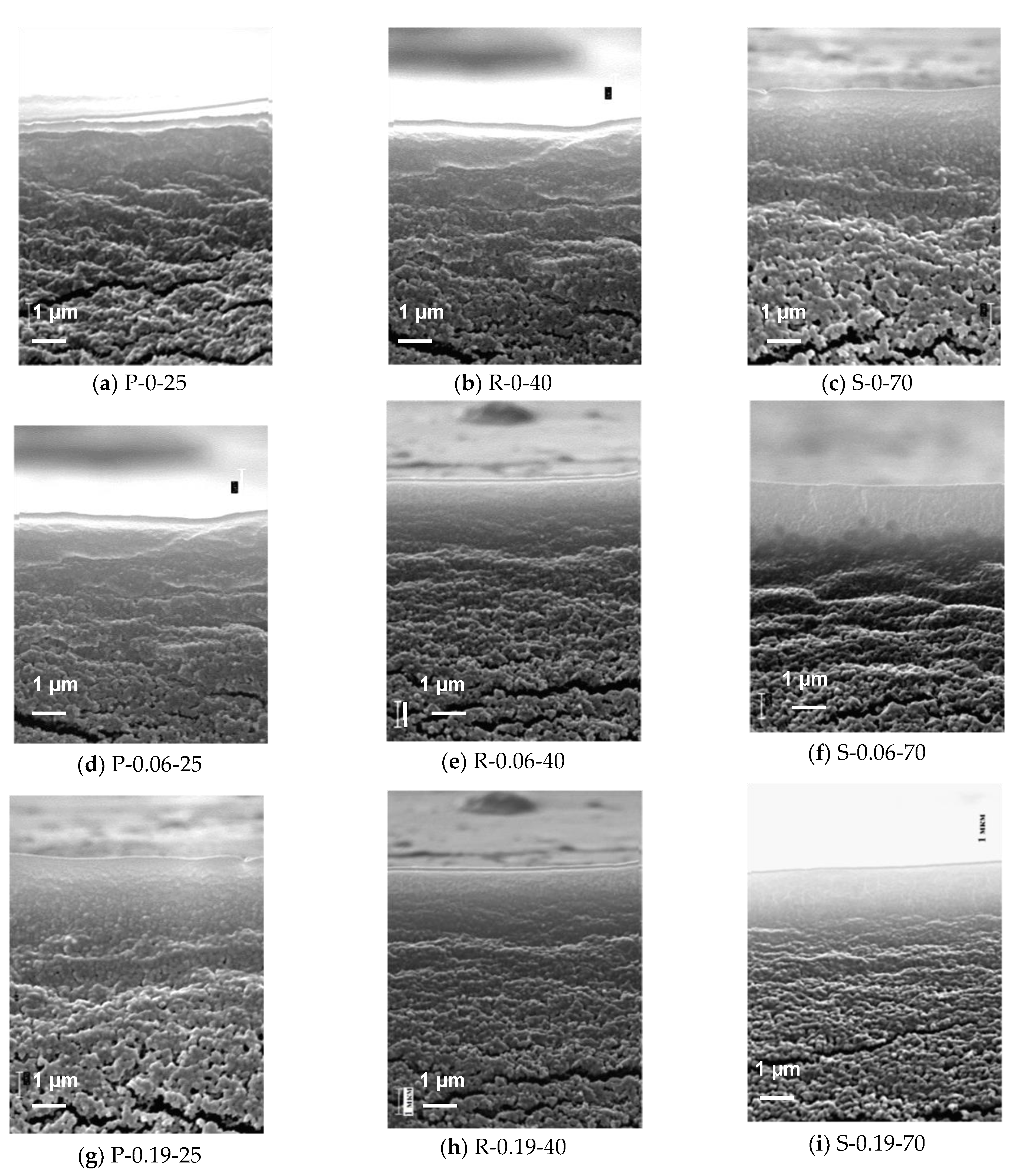
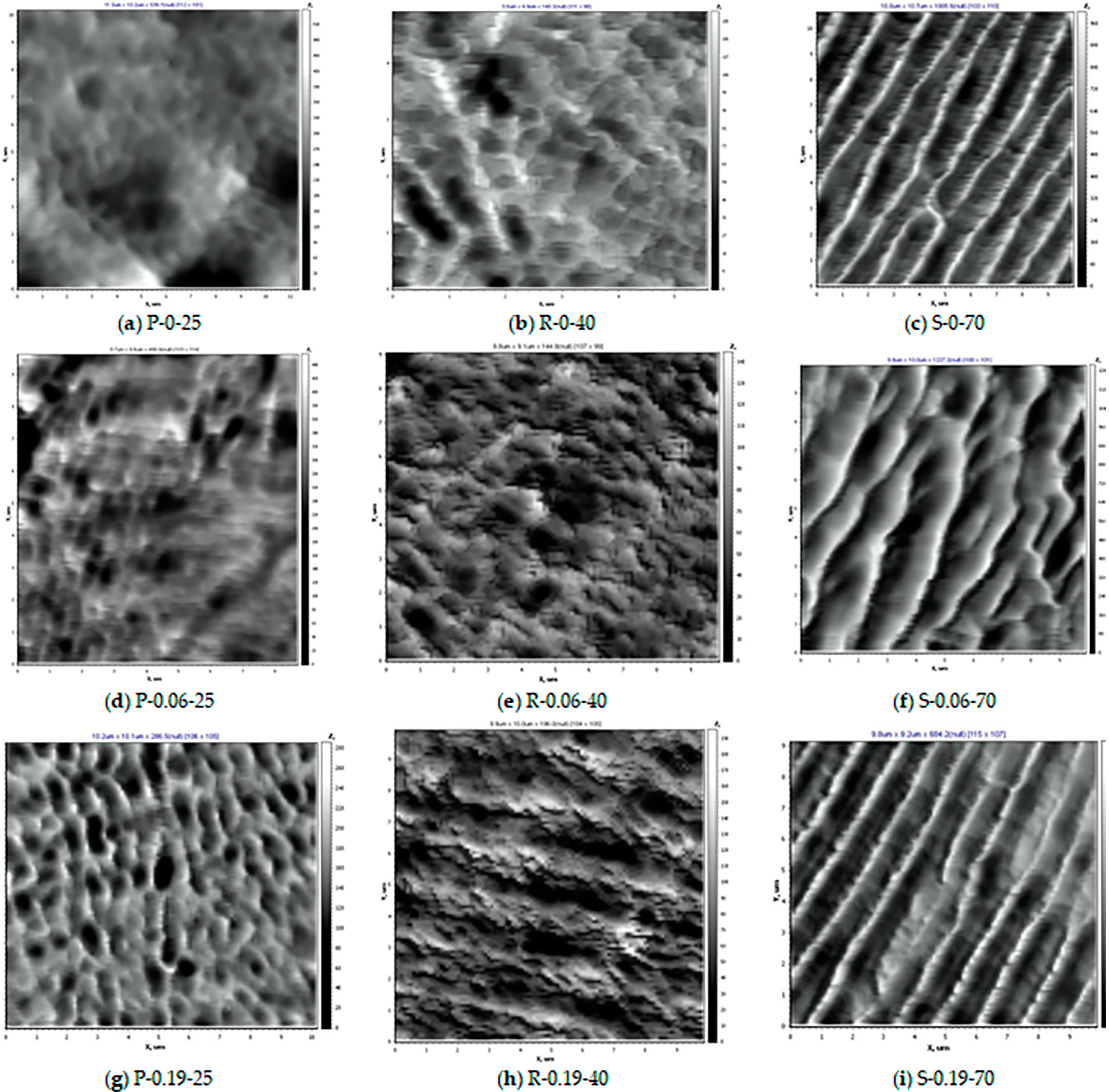
3.4. AFM Studies of the Selective Layer Surface of PPSU Membranes
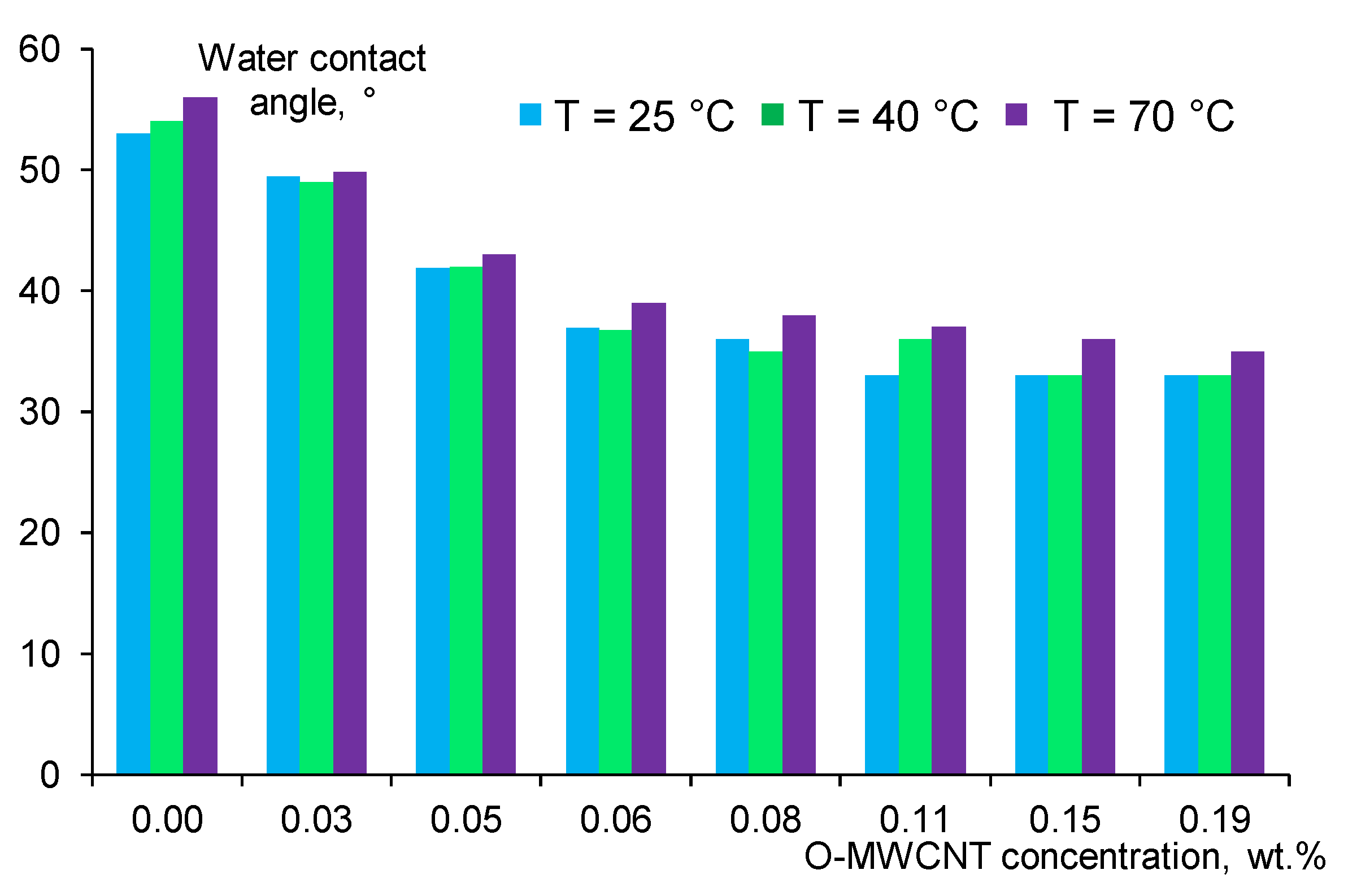
3.5. Effect of O-MWCNTs Content on Water Contact Angle of Membrane Selective Layer
3.6. Study of the PPSU and Nanocomposite PPSU/O-MWCNT Membranes Separation Performance
3.7. Study of Antifouling Perfromance during Humic Acid Solution Ultrafiltration
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rong, G.; Zhou, D.; Pang, J. Preparation of high-performance antifouling polyphenylsulfone ultrafiltration membrane by the addition of sulfonated polyaniline. J. Polym. Res. 2018, 25, 66. [Google Scholar] [CrossRef]
- Arockiasamy, D.L.; Alhoshan, M.; Alam, J.; Muthumareeswaran; Figoli, A.; Kumar, S.A. Separation of proteins and antifouling properties of polyphenylsulfone based mixed matrix hollow fiber membranes. Sep. Purif. Technol. 2017, 174, 529–543. [Google Scholar] [CrossRef]
- Isloor, A.M.; Nayak, M.C.; Inamuddin; Prabhu, B.; Ismail, N.; Ismail, A.; Asiri, A.M. Novel polyphenylsulfone (PPSU)/nano tin oxide (SnO2) mixed matrix ultrafiltration hollow fiber membranes: Fabrication, characterization and toxic dyes removal from aqueous solutions. React. Funct. Polym. 2019, 139, 170–180. [Google Scholar] [CrossRef]
- Plisko, T.; Karslyan, Y.; Bildyukevich, A. Effect of Polyphenylsulfone and Polysulfone Incompatibility on the Structure and Performance of Blend Membranes for Ultrafiltration. Materials 2021, 14, 5740. [Google Scholar] [CrossRef]
- Çalhan, A.; Deniz, S.; Kujawski, W.; Kujawa, J.; Knozowska, K.; Hasanoğlu, A. Silica Filled Polyphenylsulfone/Polydimethylsiloxane Composite Membranes for Pervaporation Separation of Biobutanol from ABE Mixtures. Chem. Eng. Process. Process Intensif. 2020, 156, 108099. [Google Scholar] [CrossRef]
- Plisko, T.V.; Bildyukevich, A.V.; Karslyan, Y.A.; Ovcharova, A.A.; Volkov, V.V. Development of high flux ultrafiltration polyphenylsulfone membranes applying the systems with upper and lower critical solution temperatures: Effect of polyethylene glycol molecular weight and coagulation bath temperature. J. Membr. Sci. 2018, 565, 266–280. [Google Scholar] [CrossRef]
- Mahmoudian, M.; Balkanloo, P.G. Clay-hyperbranched epoxy/polyphenylsulfone nanocomposite membranes. Iran. Polym. J. 2017, 26, 711–720. [Google Scholar] [CrossRef]
- Liu, Z.; Xiang, J.; Hu, X.; Cheng, P.; Zhang, L.; Du, W.; Wang, S.; Tang, N. Effects of coagulation-bath conditions on polyphenylsulfone ultrafiltration membranes. Chin. J. Chem. Eng. 2021, 34, 332–340. [Google Scholar] [CrossRef]
- Kumar, M.; Isloor, A.M.; Rao, T.S.; Ismail, A.F.; Farnood, R.; Nambissan, P. Removal of toxic arsenic from aqueous media using polyphenylsulfone/cellulose acetate hollow fiber membranes containing zirconium oxide. Chem. Eng. J. 2020, 393, 124367. [Google Scholar] [CrossRef]
- Shukla, A.K.; Alam, J.; Alhoshan, M.; Dass, L.A.; Ali, F.A.A.; Mishra, U.; Ansari, M.A. Removal of heavy metal ions using a carboxylated graphene oxide-incorporated polyphenylsulfone nanofiltration membrane. Environ. Sci. Water Res. Technol. 2018, 4, 438–448. [Google Scholar] [CrossRef]
- Tashvigh, A.A.; Luo, L.; Chung, T.-S.; Weber, M.; Maletzko, C. Performance enhancement in organic solvent nanofiltration by double crosslinking technique using sulfonated polyphenylsulfone (sPPSU) and polybenzimidazole (PBI). J. Membr. Sci. 2018, 551, 204–213. [Google Scholar] [CrossRef]
- Tashvigh, A.A.; Luo, L.; Chung, T.-S.; Weber, M.; Maletzko, C. A novel ionically cross-linked sulfonated polyphenylsulfone (sPPSU) membrane for organic solvent nanofiltration (OSN). J. Membr. Sci. 2018, 545, 221–228. [Google Scholar] [CrossRef]
- Liu, Q.; Wu, X.; Xie, Z.; Zhang, K. Construction of PPSU-MoS2/PA-MIL-101(Cr) Membrane with Highly Enhanced Permeance and Stability for Organic Solvent Nanofiltration. Membranes 2022, 12, 639. [Google Scholar] [CrossRef]
- Jaafer, M.J.; Al-Najar, J.A.; Alsalhy, Q.F. Poly(phenyl sulfone) hollow fiber forward osmosis membrane for saline water desalination. Chem. Eng. Process. Process Intensif. 2020, 157, 108119. [Google Scholar] [CrossRef]
- Jullok, N.; Martínez, R.; Wouters, C.; Luis, P.; Sanz, M.T.; van der Bruggen, B. A Biologically Inspired Hydrophobic Membrane for Application in Pervaporation. Langmuir 2013, 29, 1510–1516. [Google Scholar] [CrossRef]
- Yong, W.F.; Zhang, H. Recent advances in polymer blend membranes for gas separation and pervaporation. Prog. Mater. Sci. 2020, 116, 100713. [Google Scholar] [CrossRef]
- Rostami, S.B.; Saljoughi, E.; Mousavi, S.M.; Kiani, S. Preparation of polyphenylsulfone/graphene nanocomposite membrane for the pervaporation separation of cumene from water. Polym. Adv. Technol. 2022, 33, 340–352. [Google Scholar] [CrossRef]
- Kujawski, W.; Li, G.; van der Bruggen, B.; Pedišius, N.; Tonkonogij, J.; Tonkonogovas, A.; Stankevičius, A.; Šereika, J.; Jullok, N.; Kujawa, J. Preparation and Characterization of Polyphenylsulfone (PPSU) Membranes for Biogas Upgrading. Materials 2020, 13, 2847. [Google Scholar] [CrossRef]
- Feng, F.; Liang, C.-Z.; Wu, J.; Weber, M.; Maletzko, C.; Zhang, S.; Chung, T.-S. Polyphenylsulfone (PPSU)-Based Copolymeric Membranes: Effects of Chemical Structure and Content on Gas Permeation and Separation. Polymers 2021, 13, 2745. [Google Scholar] [CrossRef]
- Othman, N.H.; Noor, A.M.; Yusoff, M.S.A.; Goh, P.S.; Ismail, A.F. Effect of Ethylene Glycol as Pore Former on Polyphenylsulfone Hollow Fiber Membrane for Crude Palm Oil Deacidification through Membrane Contactor. J. Membr. Sci. Res. 2016, 3, 291–295. [Google Scholar] [CrossRef]
- Nozad, E.; Marjani, A.P.; Mahmoudian, M. A novel and facile semi-IPN system in fabrication of solvent resistant nano-filtration membranes for effective separation of dye contamination in water and organic solvents. Sep. Purif. Technol. 2022, 282, 120121. [Google Scholar] [CrossRef]
- Kiani, S.; Mousavi, S.M.; Shahtahmassebi, N.; Saljoughi, E. Hydrophilicity improvement in polyphenylsulfone nanofibrous filtration membranes through addition of polyethylene glycol. Appl. Surf. Sci. 2015, 359, 252–258. [Google Scholar] [CrossRef]
- Kiani, S.; Mousavi, S.M.; Saljoughi, E.; Shahtahmassebi, N. Preparation of amorphous polyphenylsulfone nanofiltration membrane via thermally-induced lamination. J. Non-Crystalline Solids 2021, 551, 120416. [Google Scholar] [CrossRef]
- Ershad, Z.S.; Shadjou, N.; Mahmoudian, M.; Ahour, F. Polyphenylsulfone membrane modified by novel dendritic fibrous nanosilica (KCC-1-nPr-NH-AcCys) toward water treatment. J. Environ. Chem. Eng. 2021, 9, 105329. [Google Scholar] [CrossRef]
- Arumugham, T.; Amimodu, R.G.; Kaleekkal, N.J.; Rana, D. Nano CuO/g-C3N4 sheets-based ultrafiltration membrane with enhanced interfacial affinity, antifouling and protein separation performances for water treatment application. J. Environ. Sci. 2019, 82, 57–69. [Google Scholar] [CrossRef]
- Ahmadi, R.; Sedighian, R.; Sanaeepur, H.; Amooghin, A.E.; Lak, S. Polyphenylsulfone/zinc ion-exchanged zeolite Y nanofiltration mixed matrix membrane for water desalination. J. Appl. Polym. Sci. 2022, 139, 52262. [Google Scholar] [CrossRef]
- Dai, J.; Li, S.; Liu, J.; He, J.; Li, J.; Wang, L.; Lei, J. Fabrication and characterization of a defect-free mixed matrix membrane by facile mixing PPSU with ZIF-8 core–shell microspheres for solvent-resistant nanofiltration. J. Membr. Sci. 2019, 589, 117261. [Google Scholar] [CrossRef]
- Alam, J.; Shukla, A.K.; Ansari, M.A.; Ali, F.A.A.; Alhoshan, M. Dye Separation and Antibacterial Activities of Polyaniline Thin Film-Coated Poly(phenyl sulfone) Membranes. Membranes 2020, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.K.; Alam, J.; Ali, F.A.A.; Alhoshan, M. Efficient soluble anionic dye removal and antimicrobial properties of ZnO embedded- Polyphenylsulfone membrane. Water Environ. J. 2021, 35, 670–684. [Google Scholar] [CrossRef]
- Nayak, M.C.; Isloor, A.M.; Moslehyani, A.; Ismail, A. Preparation and characterization of PPSU membranes with BiOCl nanowafers loaded on activated charcoal for oil in water separation. J. Taiwan Inst. Chem. Eng. 2017, 77, 293–301. [Google Scholar] [CrossRef]
- Nayak, M.C.; Isloor, A.M.; Moslehyani, A.; Ismail, N.; Ismail, A. Fabrication of novel PPSU/ZSM-5 ultrafiltration hollow fiber membranes for separation of proteins and hazardous reactive dyes. J. Taiwan Inst. Chem. Eng. 2018, 82, 342–350. [Google Scholar] [CrossRef]
- Arumugham, T.; Kaleekkal, N.J.; Rana, D.; Doraiswamy, M. Separation of oil/water emulsions using nano MgO anchored hybrid ultrafiltration membranes for environmental abatement. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Seshasayee, M.; Yu, Z.; Arthanareeswaran, G.; Das, D. Preparation of nanoclay embedded polymeric membranes for the filtration of natural organic matter (NOM) in a circular crossflow filtration system. J. Water Process Eng. 2020, 37, 101408. [Google Scholar] [CrossRef]
- Nayak, M.C.; Isloor, A.M.; Inamuddin; Lakshmi, B.; Marwani, H.M.; Khan, I. Polyphenylsulfone/multiwalled carbon nanotubes mixed ultrafiltration membranes: Fabrication, characterization and removal of heavy metals Pb2+, Hg2+, and Cd2+ from aqueous solutions. Arab. J. Chem. 2020, 13, 4661–4672. [Google Scholar] [CrossRef]
- Othman, N.H.; Noor, A.M.; Yusoff, M.S.A.; Goh, P.S.; Ismail, A.F.; Lau, W.J.; Ng, B.C.; Ismail, N. Effect Of Bore Fluid Compo-Sition On Structural And Performance Of Polyphenylsulfone Hollow Fiber Membrane Contactor For Deacdification Of Crude Palm Oil. Malays. J. Anal. Sci. 2017, 21, 633–642. [Google Scholar] [CrossRef]
- Golpour, M.; Pakizeh, M. Development of a new nanofiltration membrane for removal of kinetic hydrate inhibitor from water. Sep. Purif. Technol. 2017, 183, 237–248. [Google Scholar] [CrossRef] [Green Version]
- Golpour, M.; Pakizeh, M. Preparation and characterization of new PA-MOF/PPSU-GO membrane for the separation of KHI from water. Chem. Eng. J. 2018, 345, 221–232. [Google Scholar] [CrossRef]
- Kiani, S.; Mousavi, S.M.; Saljoughi, E.; Shahtahmassebi, N. Preparation and characterization of modified polyphenylsulfone membranes with hydrophilic property for filtration of aqueous media. Polym. Adv. Technol. 2018, 29, 1632–1648. [Google Scholar] [CrossRef]
- Hwang, L.-L.; Tseng, H.-H.; Chen, J.-C. Fabrication of polyphenylsulfone/polyetherimide blend membranes for ultrafiltration applications: The effects of blending ratio on membrane properties and humic acid removal performance. J. Membr. Sci. 2011, 384, 72–81. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Zhuang, G.-L.; Tasi, P.-F.; Tseng, H.-H. Removal of protein, histological dye and tetracycline from simulated bioindustrial wastewater with a dual pore size PPSU membrane. J. Hazard. Mater. 2022, 431, 128525. [Google Scholar] [CrossRef]
- Shukla, A.K.; Alam, J.; Alhoshan, M. Recent Advancements in Polyphenylsulfone Membrane Modification Methods for Separation Applications. Membranes 2022, 12, 247. [Google Scholar] [CrossRef]
- Yin, Q.; Zhang, Q.; Cui, Z.; Li, W.; Xing, W. Alkali resisting polyphenylsulfone ultrafiltration membrane with tailored microstructure. Polymer 2017, 124, 128–138. [Google Scholar] [CrossRef]
- Liu, J.; Zhong, Z.; Ma, R.; Zhang, W.; Li, J. Development of High-Antifouling PPSU Ultrafiltration Membrane by Using Compound Additives: Preparation, Morphologies, and Filtration Resistant Properties. Membranes 2016, 6, 35. [Google Scholar] [CrossRef] [Green Version]
- Bildyukevich, A.V.; Plisko, T.V.; Isaichykova, Y.A.; Ovcharova, A.A. Preparation of High-Flux Ultrafiltration Polyphenylsulfone Membranes. Pet. Chem. 2018, 58, 747–759. [Google Scholar] [CrossRef]
- Xiao, S.; Yu, S.; Yan, L.; Liu, Y.; Tan, X. Preparation and properties of PPSU/GO mixed matrix membrane. Chin. J. Chem. Eng. 2017, 25, 408–414. [Google Scholar] [CrossRef]
- Luo, L.; Chung, T.-S.; Weber, M.; Staudt, C.; Maletzko, C. Molecular interaction between acidic sPPSU and basic HPEI polymers and its effects on membrane formation for ultrafiltration. J. Membr. Sci. 2017, 524, 33–42. [Google Scholar] [CrossRef]
- Shukla, A.K.; Alam, J.; Ansari, M.A.; Alhoshan, M.; Alam, M.; Kaushik, A. Selective ion removal and antibacterial activity of silver-doped multi-walled carbon nanotube/polyphenylsulfone nanocomposite membranes. Mater. Chem. Phys. 2019, 233, 102–112. [Google Scholar] [CrossRef]
- Dehban, A.; Kargari, A.; Ashtiani, F.Z. Preparation and optimization of antifouling PPSU/PES/SiO2 nanocomposite ultrafiltration membranes by VIPS-NIPS technique. J. Ind. Eng. Chem. 2020, 88, 292–311. [Google Scholar] [CrossRef]
- Kumar, M.; Isloor, A.M.; Todeti, S.R.; Nagaraja, H.; Ismail, A.F.; Susanti, R. Effect of binary zinc-magnesium oxides on polyphenylsulfone/cellulose acetate derivatives hollow fiber membranes for the decontamination of arsenic from drinking water. Chem. Eng. J. 2020, 405, 126809. [Google Scholar] [CrossRef]
- Hwang, L.-L.; Chen, J.-C.; Wey, M.-Y. The properties and filtration efficiency of activated carbon polymer composite membranes for the removal of humic acid. Desalination 2013, 313, 166–175. [Google Scholar] [CrossRef]
- Ali, A.; Rashid, K.; Yahya, A.; Majdi, H.; Salih, I.; Yusoh, K.; Alsalhy, Q.; AbdulRazak, A.; Figoli, A. Fabrication of Gum Arabic-Graphene (GGA) Modified Polyphenylsulfone (PPSU) Mixed Matrix Membranes: A Systematic Evaluation Study for Ultrafiltration (UF) Applications. Membranes 2021, 11, 542. [Google Scholar] [CrossRef] [PubMed]
- Sani, N.A.A.; Lau, W.J.; Ismail, A.F. Polyphenylsulfone-based solvent resistant nanofiltration (SRNF) membrane incorporated with copper-1,3,5-benzenetricarboxylate (Cu-BTC) nanoparticles for methanol separation. RSC Adv. 2015, 5, 13000–13010. [Google Scholar] [CrossRef]
- Dehban, A.; Kargari, A.; Ashtiani, F.Z. Preparation and characterization of an antifouling poly (phenyl sulfone) ultrafiltration membrane by vapor-induced phase separation technique. Sep. Purif. Technol. 2019, 212, 986–1000. [Google Scholar] [CrossRef]
- Arockiasamy, D.L.; Alam, J.; Alhoshan, M. Carbon nanotubes-blended poly(phenylene sulfone) membranes for ultrafiltration applications. Appl. Water Sci. 2013, 3, 93–103. [Google Scholar] [CrossRef] [Green Version]
- Feng, Y.; Han, G.; Chung, T.-S.; Weber, M.; Widjojo, N.; Maletzko, C. Effects of polyethylene glycol on membrane formation and properties of hydrophilic sulfonated polyphenylenesulfone (sPPSU) membranes. J. Membr. Sci. 2017, 531, 27–35. [Google Scholar] [CrossRef]
- Sani, N.; Lau, W.; Nordin, N.; Ismail, A. Influence of organic solvents and operating conditions on the performance of polyphenylsulfone (PPSU)/copper-1,3,5-benzenetricarboxylate (Cu-BTC) solvent resistant nanofiltration (SRNF) membranes. Chem. Eng. Res. Des. 2016, 115, 66–76. [Google Scholar] [CrossRef]
- Yuan, X.-S.; Guo, Z.-Y.; Geng, H.-Z.; Rhen, D.S.; Wang, L.; Yuan, X.-T.; Li, J. Enhanced performance of conductive polysulfone/MWCNT/PANI ultrafiltration membrane in an online fouling monitoring application. J. Membr. Sci. 2019, 575, 160–169. [Google Scholar] [CrossRef]
- Wu, X.; Kang, D.; Liu, N.; Shao, H.; Dong, X.; Qin, S. Microstructure manipulation in PVDF/SMA/MWCNTs ultrafiltration membranes: Effects of hydrogen bonding and crystallization during the membrane formation. Sep. Purif. Technol. 2021, 278, 119523. [Google Scholar] [CrossRef]
- Arefi-Oskoui, S.; Khataee, A.; Behrouz, S.J.; Vatanpour, V.; Gharamaleki, S.H.; Orooji, Y.; Safarpour, M. Development of MoS2/O-MWCNTs/PES blended membrane for efficient removal of dyes, antibiotic, and protein. Sep. Purif. Technol. 2022, 280, 119822. [Google Scholar] [CrossRef]
- Ma, D.; Zou, X.; Zhao, Z.; Zhou, J.; Li, S.; Yin, H.; Wang, J. Hydrophilic PAA-g-MWCNT/TiO2@PES nano-matrix composite membranes: Anti-fouling, antibacterial and photocatalytic. Eur. Polym. J. 2022, 168, 111006. [Google Scholar] [CrossRef]
- Ajibade, T.F.; Tian, H.; Lasisi, K.H.; Xue, Q.; Yao, W.; Zhang, K. Multifunctional PAN UF membrane modified with 3D-MXene/O-MWCNT nanostructures for the removal of complex oil and dyes from industrial wastewater. Sep. Purif. Technol. 2021, 275, 119135. [Google Scholar] [CrossRef]
- Plisko, T.; Bildyukevich, A.; Zhao, L.; Huang, W.; Volkov, V.; Huang, Z. Formation of Polysulfone Hollow Fiber Membranes Using the Systems with Lower Critical Solution Temperature. Fibers 2021, 9, 28. [Google Scholar] [CrossRef]
- Plisko, T.V.; Bildyukevich, A.V.; Volkov, V.V.; Osipov, N.N. Formation of hollow fiber membranes doped with multiwalled carbon nanotube dispersions. Pet. Chem. 2015, 55, 318–332. [Google Scholar] [CrossRef]
- Plisko, T.V.; Bildyukevich, A.V. Debundling of multiwalled carbon nanotubes in N, N-dimethylacetamide by polymers. Colloid Polym. Sci. 2014, 292, 2571–2580. [Google Scholar] [CrossRef]
- Hasan, T.; Scardaci, V.; Tan, P.; Rozhin, A.G.; Milne, A.W.I.; Ferrari, A.C. Stabilization and “Debundling” of Single-Wall Carbon Nanotube Dispersions in N-Methyl-2-pyrrolidone (NMP) by Polyvinylpyrrolidone (PVP). J. Phys. Chem. C 2007, 111, 12594–12602. [Google Scholar] [CrossRef]
- Hasan, T.; Scardaci, V.; Tan, P.; Rozhin, A.; Milne, W.; Ferrari, A. Dispersibility and stability improvement of unfunctionalized nanotubes in amide solvents by polymer wrapping. Phys. E Low-Dimens. Syst. Nanostruct. 2008, 40, 2414–2418. [Google Scholar] [CrossRef]
- Rogovina, L.Z.; Vasil’Ev, V.G.; Braudo, E.E. Definition of the concept of polymer gel. Polym. Sci. Ser. C 2008, 50, 85–92. [Google Scholar] [CrossRef]
- Choi, J.-H.; Jegal, J.; Kim, W.-N. Fabrication and characterization of multi-walled carbon nanotubes/polymer blend membranes. J. Membr. Sci. 2006, 284, 406–415. [Google Scholar] [CrossRef]
- Kujawa, J.; Kujawski, W. Functionalization of Ceramic Metal Oxide Powders and Ceramic Membranes by Perfluoroalkylsilanes and Alkylsilanes Possessing Different Reactive Groups: Physicochemical and Tribological Properties. ACS Appl. Mater. Interfaces 2016, 8, 7509–7521. [Google Scholar] [CrossRef]
- Burts, K.S.; Plisko, T.V.; Sjölin, M.; Rodrigues, G.; Bildyukevich, A.V.; Lipnizki, F.; Ulbricht, M. Development of Antifouling Polysulfone Membranes by Synergistic Modification with Two Different Additives in Casting Solution and Coagulation Bath: Synperonic F108 and Polyacrylic Acid. Materials 2022, 15, 359. [Google Scholar] [CrossRef]
- Plisko, T.V.; Bildyukevich, A.V.; Burts, K.S.; Ermakov, S.S.; Penkova, A.V.; Kuzminova, A.I.; Dmitrenko, M.E.; Hliavitskaya, T.A.; Ulbricht, M. One-Step Preparation of Antifouling Polysulfone Ultrafiltration Membranes via Modification by a Cationic Polyelectrolyte Based on Polyacrylamide. Polymers 2020, 12, 1017. [Google Scholar] [CrossRef]
- Burts, K.; Plisko, T.; Bildyukevich, A.; Rodrigues, G.; Sjölin, M.; Lipnizki, F.; Ulbricht, M. Development of polysulfone ultrafiltration membranes with enhanced antifouling performance for the valorisation of side streams in the pulp and paper industry. Colloids Surfaces A Physicochem. Eng. Asp. 2022, 632, 127742. [Google Scholar] [CrossRef]
- Plisko, T.V.; Bildyukevich, A.V.; Burts, K.S.; Hliavitskaya, T.A.; Penkova, A.V.; Ermakov, S.S.; Ulbricht, M. Modification of Polysulfone Ultrafiltration Membranes via Addition of Anionic Polyelectrolyte Based on Acrylamide and Sodium Acrylate to the Coagulation Bath to Improve Antifouling Performance in Water Treatment. Membranes 2020, 10, 264. [Google Scholar] [CrossRef]

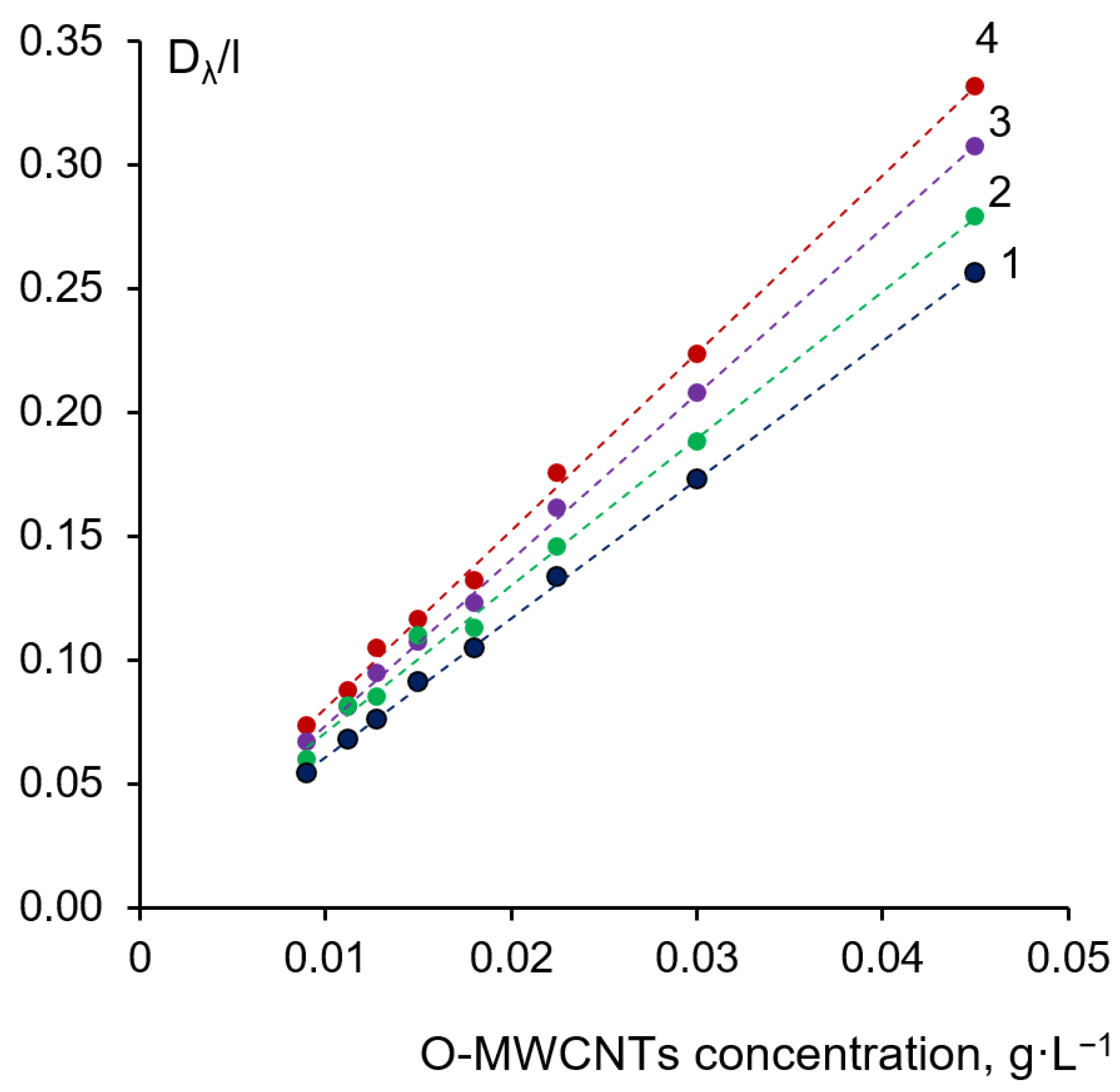
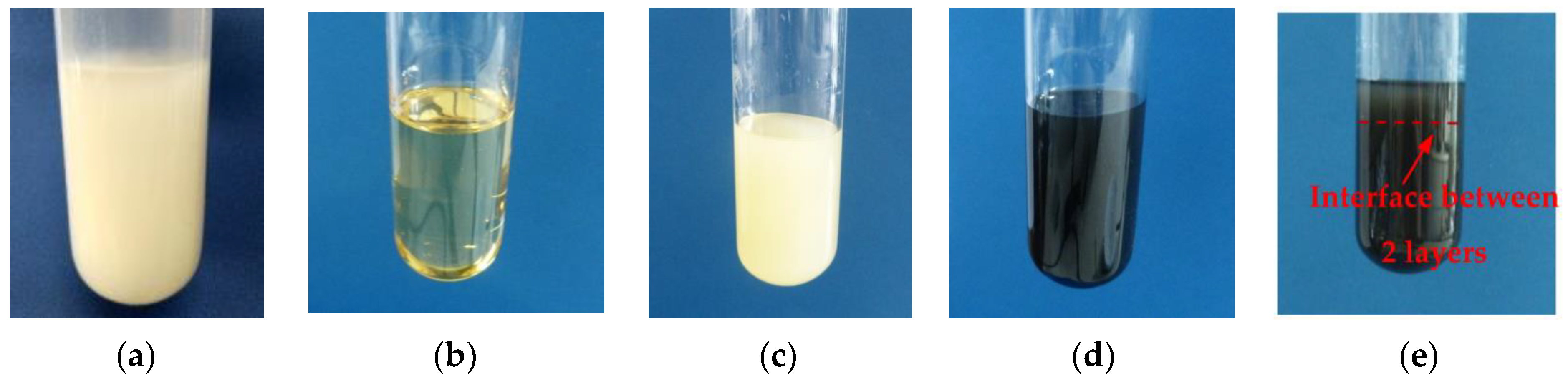
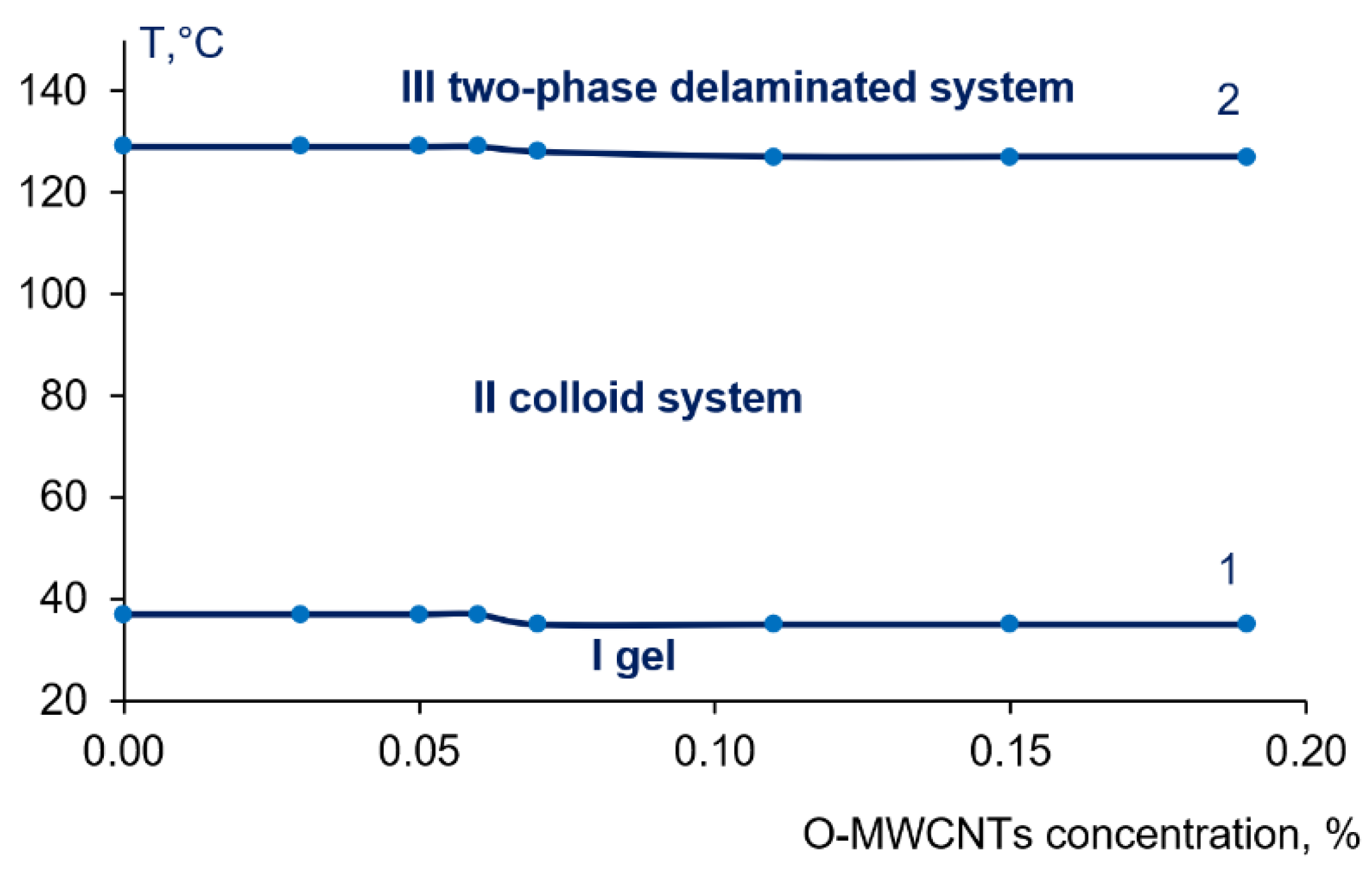
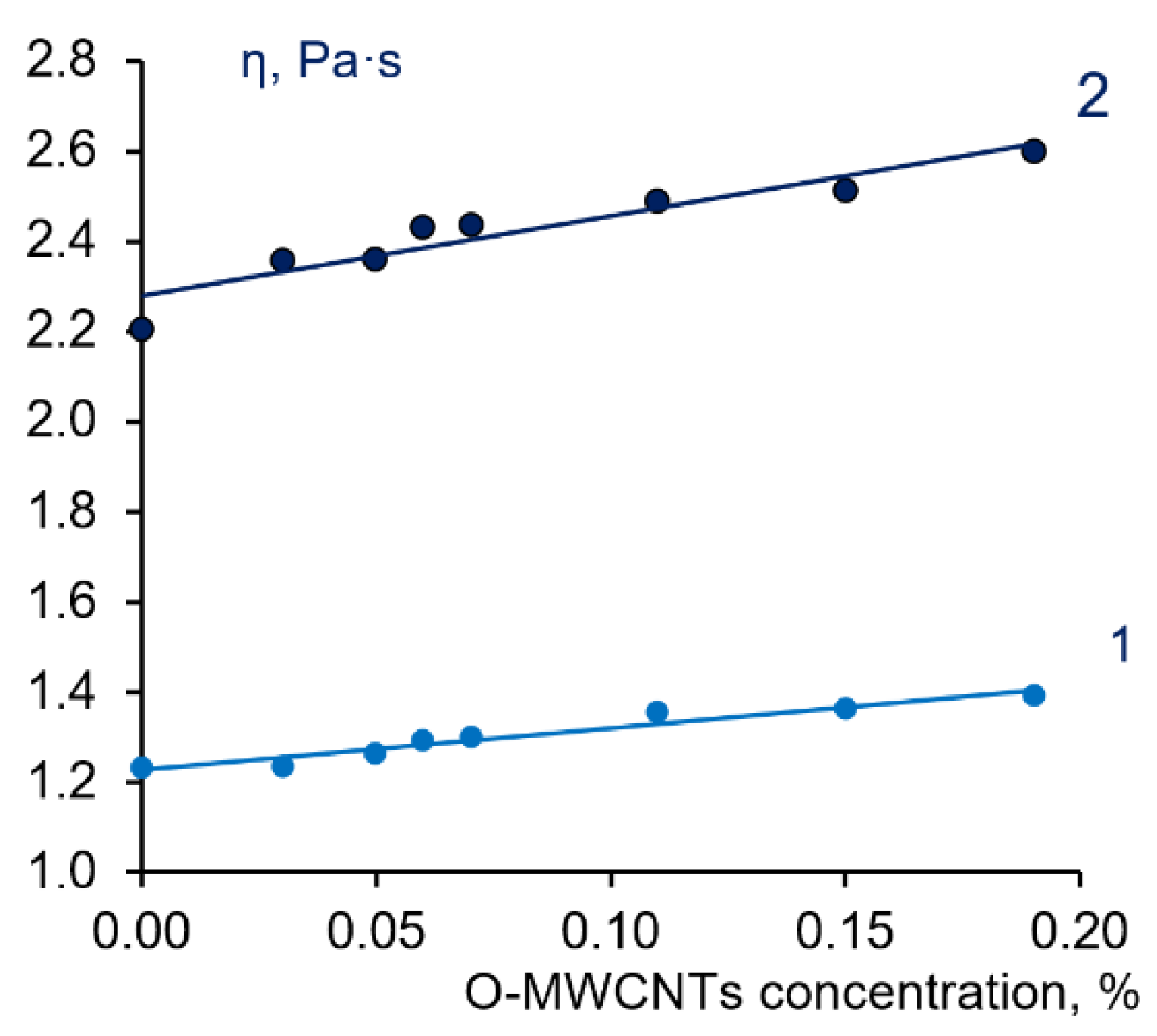



| Casting Solution | Nanofiller | Performance | Water Contact Angle, ° | FRR 2, % | Reference | ||
|---|---|---|---|---|---|---|---|
| Type | Content, wt.% | Pure Water Flux, L·m−2·h−1 | Rejection of BSA 1, % | ||||
| 17 wt.% PPSU in NMP | - | - | 177 at 0.3 MPa | - | - | - | [36] |
| GO | 0.05–0.4 | 174–211 at 0.3 MPa | - | 46–60 | - | ||
| PPSU:PVP 3:Tween-80:PG 4:NMP = 15:9:4.5:1.5:70 | - | - | 183.4 at 0.1 MPa | 70.1 | 54.4 | - | [43] |
| PPSU:PVP:Tween-80:PG:NMP = 15:6:3:1:75 | - | - | 148 at 0.1 MPa | 81 | 63 | - | |
| 22 wt.% PPSU-5 wt.% PEG-20,000 in NMP | - | - | 183 at. 0.5 MPa | 100 | 61.9 | 63.2 | [38] |
| 14 wt.% PPSU in NMP | - | - | 37.69 at 0.1 MPa | 52.69 | 49.38 | - | [53] |
| 18 wt.% PPSU-2 wt.% PVP 5 in NMP | Zeolite ZSM-5 | 0.4 | 113.98 at 0.3 MPa | 100 | 69 | 68.78 | [31] |
| 22 wt.% PPSU-3 wt.% PVP 6 in DMAc | - | - | 140 at 0.28 MPa | 99.9 | 68.2 | 85 | [40] |
| 16 wt.% PPSU-4 wt.% PVP in NMP | BiOCl on activated charcoal | 2 | 465 at 0.2 MPa | - | ~67 | 53.33 | [30] |
| 25 wt.% PPSU | TiO2 | 0.5 | 53 at 0.2 MPa | 95 | 46 | 60 | [2] |
| 15 wt.% PPSU-6 wt.% PEG-6000 in DMAc | GO | 1.5 | ~220 at 0.1 MPa | 94 | 65 | 86 | [45] |
| 20% wt.% PPSU-15 wt.% PEG-6000 in NMP | - | - | 486 at 0.1 MPa | 89 (HSA) | 76 | - | [6] |
| 15 wt.% PPSU in NMP | CuO/g-C3N4 | 0.5 | 202 at 0.069 MPa | 96 | 53 | 79 | [25] |
| 16 wt.% PPSU-4 wt.% PVP 5 in NMP | SnO2 | 0.4 | 362.9 at 0.2 MPa | - | 63.77 | - | [3] |
| 15 wt.% PPSU in NMP | Gum arabic-graphene | 0.15 | 119 at 0.4 MPa | 88 (sodium alginate) | 50 | - | [51] |
| 35 wt.%PPSU- 5 wt.%PEI-6 wt.% PEG | Activated carbon | 0.25 | 184 at 0.1 MPa | 80 (humic acids) | 116.6 | - | [50] |
| 10 wt.% PPSU-10 wt.% PES in NMP | SiO2 | 2.5 | 57 at 0.5 MPa | 80.49 | 52.57 | - | [48] |
| 17.5 wt.% PPSU in NMP | MWCNT | 0.5 | 46.6 at 0.0345 MPa | ~97 | 65 | - | [54] |
| O-MWCNT 8 | 0.5 | 56.7 at 0.0345 MPa | ~95 | 45 | - | ||
| 15 wt.% PPSU-15 wt.% PEG-20,000- 0.5 wt.% PVP 7 NMP | O-MWCNT | 0.06 | 544 at 0.1 MPa | 88 (HSA) 9 83 (humic acids) | 35 | 62 (humic acids) | This study |
| 0.19 | 983 at 0.1 MPa | 83 (HSA) 9 | 35 | ||||
| Membrane Code | Casting Solution Composition, wt.% | Preparation Conditions | |||||
|---|---|---|---|---|---|---|---|
| PPSU | PEG-20,000 | PVP K-30 | O-MWCNT | NMP | Temperature of Casting Solution, °C | Temperature of Coagulation Bath, °C | |
| P-0-25 | 15 | 15 | 0.5 | 0 | 69.50 | 40 | 25 |
| P-0.03-25 | 15 | 15 | 0.5 | 0.03 | 69.47 | 40 | 25 |
| P-0.05-25 | 15 | 15 | 0.5 | 0.05 | 69.45 | 40 | 25 |
| P-0.06-25 | 15 | 15 | 0.5 | 0.06 | 69.44 | 40 | 25 |
| P-0.07-25 | 15 | 15 | 0.5 | 0.07 | 69.43 | 40 | 25 |
| P-0.11-25 | 15 | 15 | 0.5 | 0.11 | 69.39 | 40 | 25 |
| P-0.15-25 | 15 | 15 | 0.5 | 0.15 | 69.35 | 40 | 25 |
| P-0.19-25 | 15 | 15 | 0.5 | 0.19 | 69.31 | 40 | 25 |
| R-0-40 | 15 | 15 | 0.5 | 0 | 69.50 | 40 | 40 |
| R-0.03-40 | 15 | 15 | 0.5 | 0.03 | 69.47 | 40 | 40 |
| R-0.05-40 | 15 | 15 | 0.5 | 0.05 | 69.45 | 40 | 40 |
| R-0.06-40 | 15 | 15 | 0.5 | 0.06 | 69.44 | 40 | 40 |
| R-0.07-40 | 15 | 15 | 0.5 | 0.07 | 69.43 | 40 | 40 |
| R-0.11-40 | 15 | 15 | 0.5 | 0.11 | 69.39 | 40 | 40 |
| R-0.15-40 | 15 | 15 | 0.5 | 0.15 | 69.35 | 40 | 40 |
| R-0.19-40 | 15 | 15 | 0.5 | 0.19 | 69.31 | 40 | 40 |
| S-0-70 | 15 | 15 | 0.5 | 0 | 69.50 | 40 | 70 |
| S-0.03-70 | 15 | 15 | 0.5 | 0.03 | 69.47 | 40 | 70 |
| S-0.05-70 | 15 | 15 | 0.5 | 0.05 | 69.45 | 40 | 70 |
| S-0.06-70 | 15 | 15 | 0.5 | 0.06 | 69.44 | 40 | 70 |
| S-0.07-70 | 15 | 15 | 0.5 | 0.07 | 69.43 | 40 | 70 |
| S-0.11-70 | 15 | 15 | 0.5 | 0.11 | 69.39 | 40 | 70 |
| S-0.15-70 | 15 | 15 | 0.5 | 0.15 | 69.35 | 40 | 70 |
| S-0.19-70 | 15 | 15 | 0.5 | 0.19 | 69.31 | 40 | 70 |
| PVP K-30 Concentration, g·L−1 | w, % | Actual Concentration of O-MWCNTs in the Dispersion, g·L−1 |
|---|---|---|
| 0.0 | 30 | 1.5 |
| 5.0 | 76 | 3.9 |
| 7.0 | 72 | 3.7 |
| 15.0 | 71 | 3.7 |
| 20.0 | 71 | 3.7 |
| 30.0 | 70 | 3.6 |
| 50.0 | 54 | 2.8 |
| Membrane Abbreviation | Roughness Parameters | |
|---|---|---|
| Ra [nm] | Rq [nm] | |
| P-0-25 | 2.9 | 3.8 |
| P-0.06-25 | 5.0 | 6.4 |
| P-0.19-25 | 5.4 | 6.9 |
| R-0-40 | 3.0 | 3.8 |
| R-0.06-40 | 3.7 | 4.9 |
| R-0.19-40 | 5.1 | 6.3 |
| S-0-70 | 6.0 | 8.1 |
| S-0.06-70 | 18.1 | 22.4 |
| S-0.19-70 | 19.7 | 24.3 |
| Membrane Abbreviation | JHAs [L m−2 h−1] | R, % | FRR [%] | DT [%] | Permeate Parameters | ||
|---|---|---|---|---|---|---|---|
| Color (λ = 400 nm) | pH | TOC [mg L−1] | |||||
| R-0-40 | 160 | 73 | 47 | 45 | 0 | 8.4 | 5.57 |
| R-0.06-40 | 145 | 83 | 62 | 37 | 0 | 8.3 | 3.66 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plisko, T.V.; Burts, K.S.; Bildyukevich, A.V. Development of High Flux Nanocomposite Polyphenylsulfone/Oxidized Multiwalled Carbon Nanotubes Membranes for Ultrafiltration Using the Systems with Critical Solution Temperatures. Membranes 2022, 12, 724. https://doi.org/10.3390/membranes12080724
Plisko TV, Burts KS, Bildyukevich AV. Development of High Flux Nanocomposite Polyphenylsulfone/Oxidized Multiwalled Carbon Nanotubes Membranes for Ultrafiltration Using the Systems with Critical Solution Temperatures. Membranes. 2022; 12(8):724. https://doi.org/10.3390/membranes12080724
Chicago/Turabian StylePlisko, Tatiana V., Katsiaryna S. Burts, and Alexandr V. Bildyukevich. 2022. "Development of High Flux Nanocomposite Polyphenylsulfone/Oxidized Multiwalled Carbon Nanotubes Membranes for Ultrafiltration Using the Systems with Critical Solution Temperatures" Membranes 12, no. 8: 724. https://doi.org/10.3390/membranes12080724
APA StylePlisko, T. V., Burts, K. S., & Bildyukevich, A. V. (2022). Development of High Flux Nanocomposite Polyphenylsulfone/Oxidized Multiwalled Carbon Nanotubes Membranes for Ultrafiltration Using the Systems with Critical Solution Temperatures. Membranes, 12(8), 724. https://doi.org/10.3390/membranes12080724








