A Straightforward Method to Prepare MOF-Based Membranes via Direct Seeding of MOF-Polymer Hybrid Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Method
2.2.1. The Synthesis of Poly(Methacrylic Acid) (PMAA) and PISA Synthesis of Poly(Methacrylic Acid)–b-Poly(Methyl Methacrylate) Diblock Copolymer (PMAA-b-PMMA) NPs
2.2.2. Synthesis of UiO-PMAA-b-PMMA and UiO-NH2-PMAA-b-PMMA NPs
2.2.3. Synthesis of UiO-66 and UiO-66-NH2
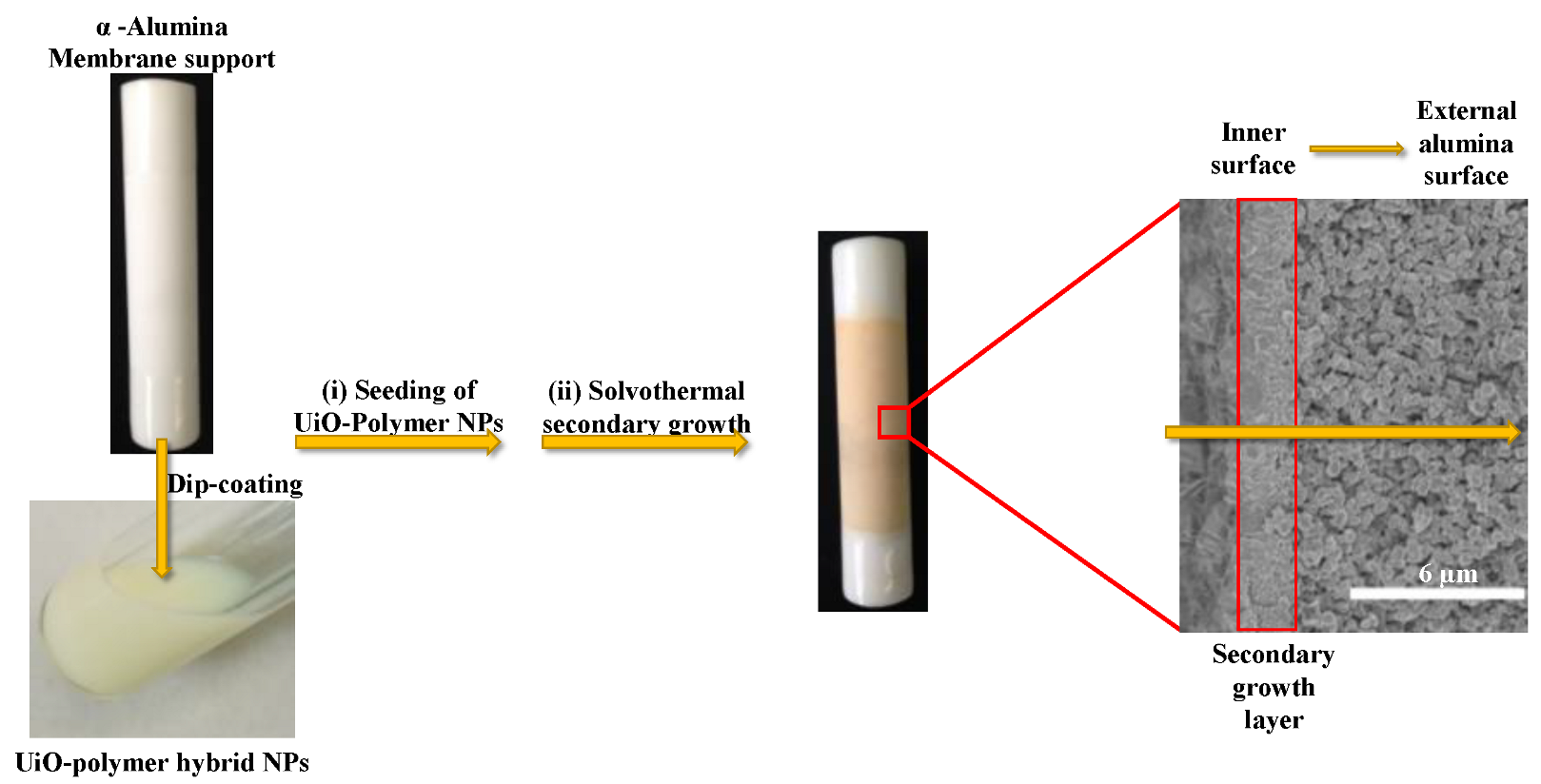
2.2.4. Membranes’ Preparation
2.2.5. Gas Permeation Experiments
2.2.6. Nanofiltration
2.3. Characterization
3. Results
3.1. Synthesis and Characterization of MOF-Polymer Hybrid NPs
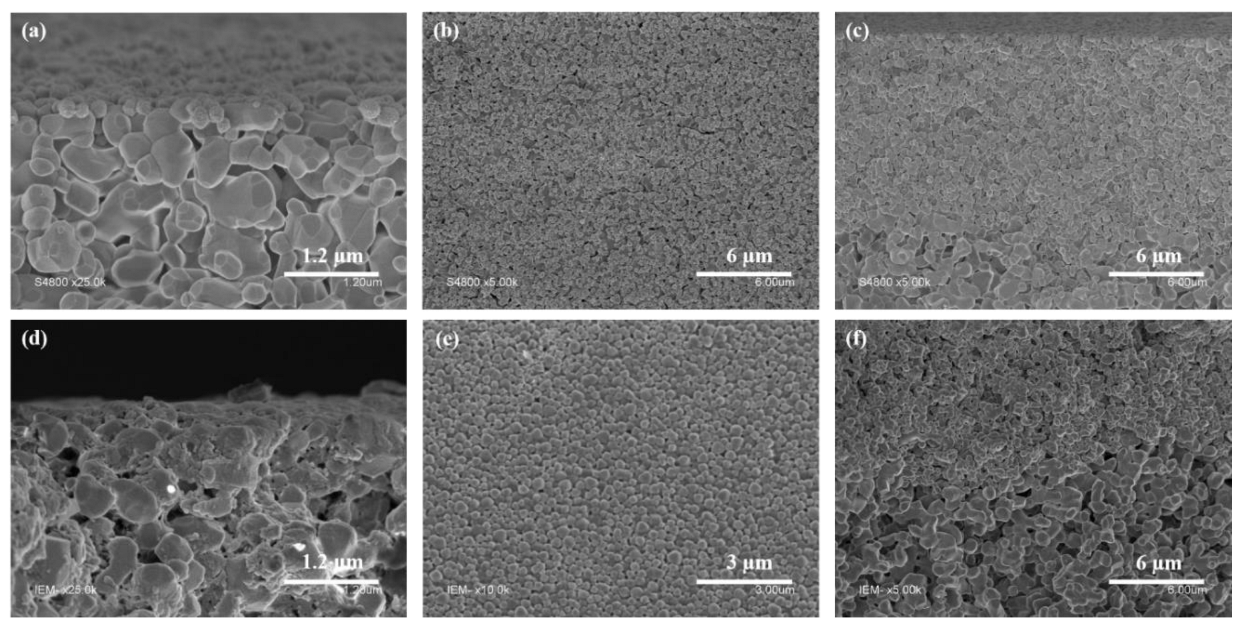
3.2. Membranes Seeding with UiO-Polymer Hybrid NPs
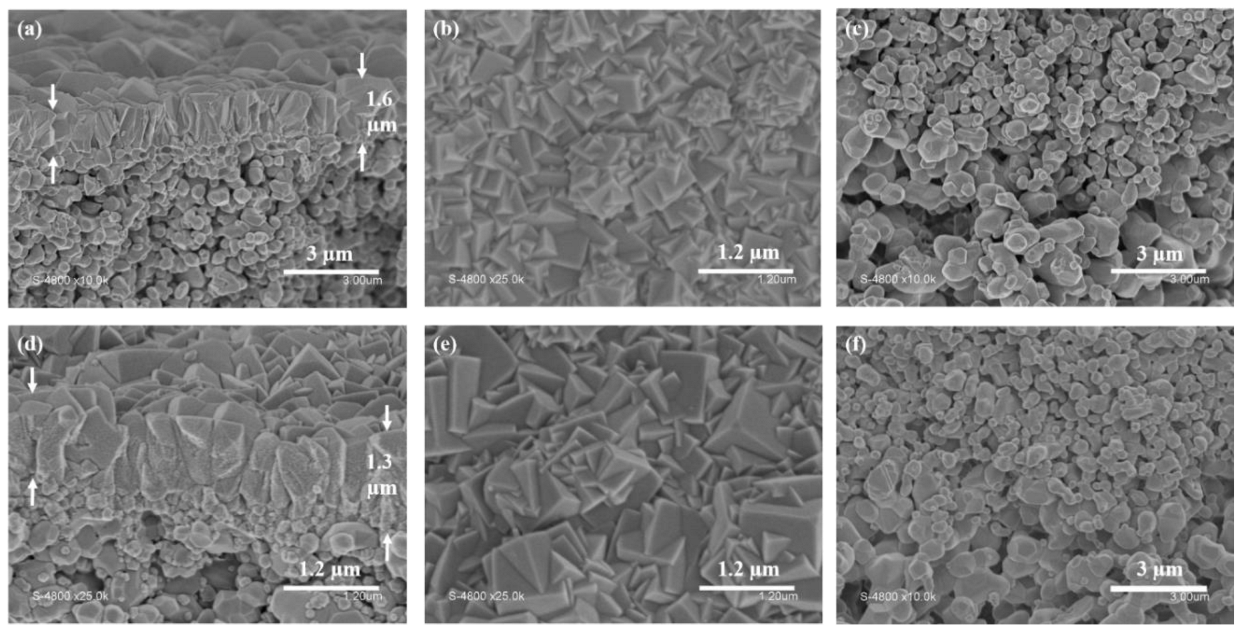
3.3. Secondary Growth
3.4. Single Gas Permeation Test
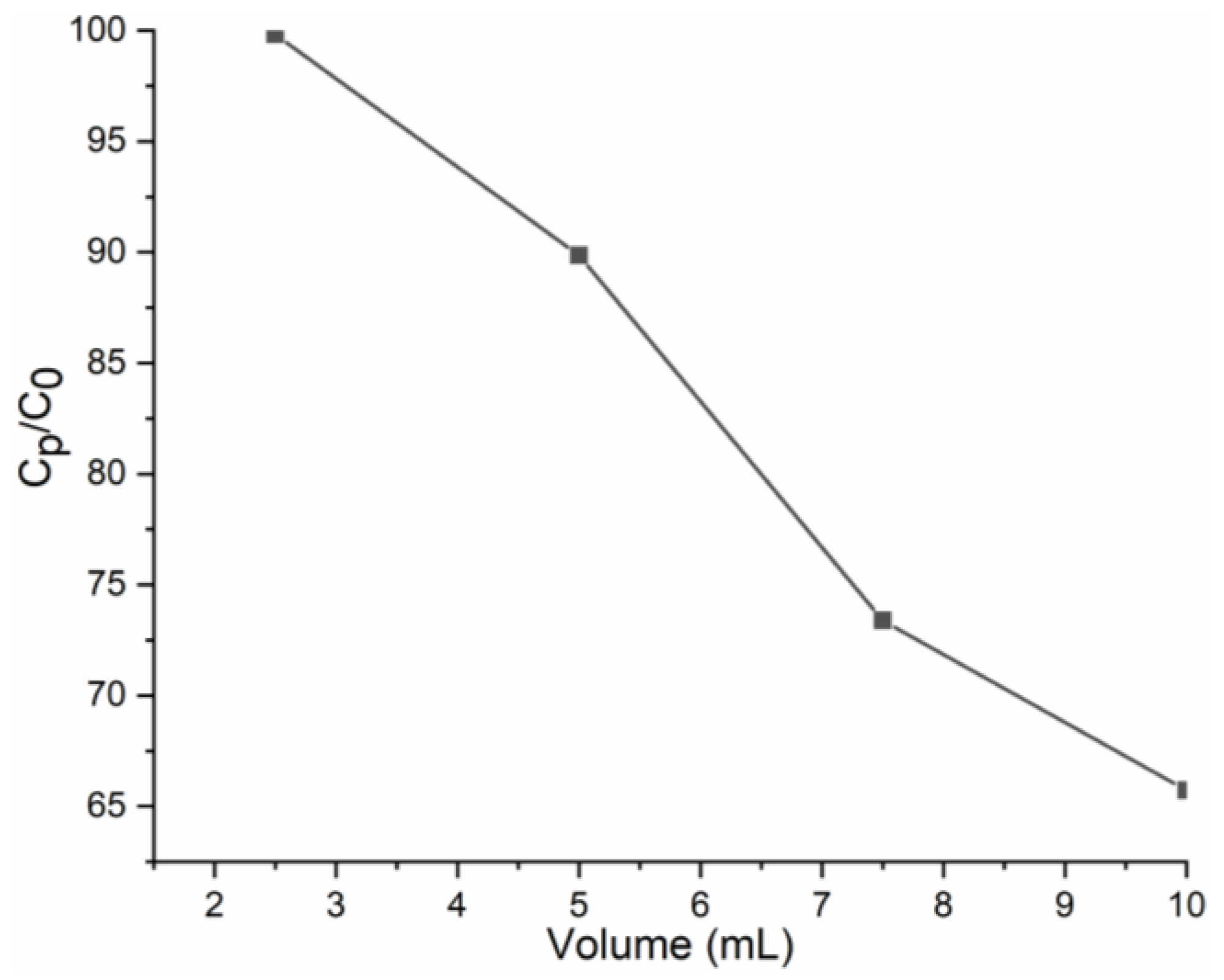
3.5. Nanofiltration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stock, N.; Biswas, S. Synthesis of metal-organic frameworks (MOFs): Routes to various MOF topologies, morphologies, and composites. Chem. Rev. 2012, 112, 933–969. [Google Scholar] [CrossRef] [PubMed]
- Yaghi, O.M. Reticular chemistry—Construction, properties, and precision reactions of frameworks. J. Am. Chem. Soc. 2016, 138, 15507–15509. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Liang, F.; Li, Y.; Wu, J.; Guan, S.; Wu, M.; Xie, S.; Luo, M.; Ma, D. A 2D Porous zinc-organic framework platform for loading of 5-fluorouracil. Inorganics 2022, 10, 202. [Google Scholar] [CrossRef]
- Qin, L.; Liang, F.; Li, Y.Y.; Wu, J.; Guan, S.; Wu, M.; Xie, S.; Luo, M.; Ma, D.; Liang, F.; et al. A microporous 2D cobalt-based MOF with pyridyl sites and open metal sites for selective adsorption of CO2. Colloids Surf. A Physicochem. Eng. Asp. 2022, 10, 202. [Google Scholar] [CrossRef]
- Jin, J.-C.; Wang, J.; Guo, J.; Yan, M.-H.; Wang, J.; Srivastava, D.; Kumar, A.; Sakiyama, H.; Muddassir, M.; Pan, Y. A 3D rare cubane-like tetramer Cu(II)-based MOF with 4-fold dia topology as an efficient photocatalyst for dye degradation. Colloids Surf. A Physicochem. Eng. Asp. 2023, 656, 130475. [Google Scholar] [CrossRef]
- Dong, X.; Li, D.; Li, Y.; Sakiyama, H.; Muddassir, M.; Pan, Y.; Srivastava, D.; Kumar, A. A 3,8-connected Cd(ii)-based metal-organic framework as an appropriate luminescent sensor for the antibiotic sulfasalazine. CrystEngComm 2022, 7157–7165. [Google Scholar] [CrossRef]
- Denny, M.S.; Moreton, J.C.; Benz, L.; Cohen, S.M. Metal-organic frameworks for membrane-based separations. Nat. Rev. Mater. 2016, 1, 16078–16095. [Google Scholar] [CrossRef]
- Qiu, S.; Xue, M.; Zhu, G. Metal-organic framework membranes: From synthesis to separation application. Chem. Soc. Rev. 2014, 43, 6116–6140. [Google Scholar] [CrossRef]
- Fang, M.; Montoro, C.; Semsarilar, M. Metal and covalent organic frameworks for membrane applications. Membranes 2020, 10, 107. [Google Scholar] [CrossRef]
- Xu, X.; Nikolaeva, D.; Hartanto, Y.; Luis, P. MOF-based membranes for pervaporation. Sep. Purif. Technol. 2021, 278, 119233–119264. [Google Scholar] [CrossRef]
- Zhang, R.; Ji, S.; Wang, N.; Wang, L.; Zhang, G.; Li, J.R. Coordination-driven in situ self-assembly strategy for the preparation of metal-organic framework hybrid membranes. Angew. Chem. Int. Ed. 2014, 53, 9775–9779. [Google Scholar] [CrossRef]
- Bux, H.; Liang, F.; Li, Y.; Cravillon, J.; Wiebcke, M.; Caro, J. Zeolitic imidazolate framework membrane with molecular sieving properties by microwave-assisted solvothermal synthesis. J. Am. Chem. Soc. 2009, 131, 16000–16001. [Google Scholar] [CrossRef]
- Kang, Z.; Xue, M.; Fan, L.; Ding, J.; Guo, L.; Gao, L.; Qiu, S. Single nickel source in situ fabrication of a stable homochiral MOF membrane with chiral resolution properties. Chem. Commun. 2013, 49, 10569–10571. [Google Scholar] [CrossRef]
- Wu, Y.-Q.; Xie, L.-H.; Qin, X.; Sun, Y.-X.; Xie, Y.-B.; Li, J.-R. Continuous crystalline membranes of a Ni(II)-based pillared-layer metal-organic framework in situ grown on nickel foam with two orientations. Crystals 2018, 8, 383. [Google Scholar] [CrossRef]
- Liu, X.; Demir, N.K.; Wu, Z.; Li, K. Highly water-stable zirconium metal-organic framework UiO-66 membranes supported on alumina hollow fibers for desalination. J. Am. Chem. Soc. 2015, 137, 6999–7002. [Google Scholar] [CrossRef]
- Huang, A.; Liu, Q.; Wang, N.; Caro, J. Highly hydrogen permselective ZIF-8 membranes supported on polydopamine functionalized macroporous stainless-steel-nets. J. Mater. Chem. A 2014, 2, 8246–8251. [Google Scholar] [CrossRef]
- Qiao, Z.; Wang, Z.; Zhang, C.; Yuan, S.; Zhu, Y.; Wang, J. Ceramic tubular MOF hybrid membrane fabricated through in situ layer-by-layer self-assembly for nanofiltration. AIChE J. 2016, 62, 538–546. [Google Scholar]
- Sun, Y.; Yang, F.; Wei, Q.; Wang, N.; Qin, X.; Zhang, S.; Wang, B.; Nie, Z.; Ji, S.; Yan, H.; et al. Oriented nano-microstructure-assisted controllable fabrication of metal-organic framework membranes on nickel foam. Adv. Mater. 2016, 28, 2374–2381. [Google Scholar] [CrossRef]
- Hu, Y.; Dong, X.; Nan, J.; Jin, W.; Ren, X.; Xu, N.; Lee, Y.M. Metal-organic framework membranes fabricated via reactive seeding. Chem. Commun. 2011, 47, 737–739. [Google Scholar] [CrossRef]
- Kwon, H.T.; Jeong, H.-K. Highly propylene-selective supported zeolite-imidazolate framework (ZIF-8) membranes synthesized by rapid microwave-assisted seeding and secondary growth. Chem. Commun. 2013, 49, 3854–3856. [Google Scholar] [CrossRef]
- Dong, X.; Lin, Y.S. Synthesis of an organophilic ZIF-71 membrane for pervaporation solvent separation. Chem. Commun. 2013, 49, 1196–1198. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Huang, K.; Liu, S.; Ren, R.; Jin, W.; Lin, Y.S. Synthesis of zeolitic imidazolate framework-78 molecular-sieve membrane: Defect formation and elimination. J. Mater. Chem. 2012, 22, 19222–19227. [Google Scholar] [CrossRef]
- Du, X.D.; Yi, X.H.; Wang, P.; Zheng, W.; Deng, J.; Wang, C.C. Robust photocatalytic reduction of Cr(VI) on UiO-66-NH2(Zr/Hf) metal-organic framework membrane under sunlight irradiation. Chem. Eng. J. 2019, 356, 393–399. [Google Scholar] [CrossRef]
- Drobek, M.; Bechelany, M.; Vallicari, C.; Abou Chaaya, A.; Charmette, C.; Salvador-Levehang, C.; Miele, P.; Julbe, A. An innovative approach for the preparation of confined ZIF-8 membranes by conversion of ZnO ALD layers. J. Memb. Sci. 2015, 475, 39–46. [Google Scholar] [CrossRef]
- Liu, Y.; Zeng, G.; Pan, Y.; Lai, Z. Synthesis of highly c -oriented ZIF-69 membranes by secondary growth and their gas permeation properties. J. Memb. Sci. 2011, 379, 46–51. [Google Scholar] [CrossRef]
- Friebe, S.; Geppert, B.; Steinbach, F.; Caro, J. Metal-organic framework UiO-66 layer: A highly oriented membrane with good selectivity and hydrogen permeance. ACS Appl. Mater. Interfaces 2017, 9, 12878–12885. [Google Scholar] [CrossRef]
- Li, Y.; Liang, F.; Bux, H.; Yang, W.; Caro, J. Zeolitic imidazolate framework ZIF-7 based molecular sieve membrane for hydrogen separation. J. Memb. Sci. 2010, 354, 48–54. [Google Scholar] [CrossRef]
- Li, Y.S.; Liang, F.Y.; Bux, H.; Feldhoff, A.; Yang, W.S.; Caro, J. Molecular sieve membrane: Supported metal-organic framework with high hydrogen selectivity. Angew. Chemie Int. Ed. 2010, 49, 548–551. [Google Scholar] [CrossRef]
- Cavka, J.H.; Jakobsen, S.; Olsbye, U.; Guillou, N.; Lamberti, C.; Bordiga, S.; Lillerud, K.P. A new zirconium inorganic building brick forming metal organic frameworks with exceptional stability. J. Am. Chem. Soc. 2008, 130, 13850–13851. [Google Scholar] [CrossRef]
- Bai, Y.; Dou, Y.; Xie, L.H.; Rutledge, W.; Li, J.R.; Zhou, H.C. Zr-based metal-organic frameworks: Design, synthesis, structure, and applications. Chem. Soc. Rev. 2016, 45, 2327–2367. [Google Scholar] [CrossRef]
- Hu, Z.; Zhao, D. De facto methodologies toward the synthesis and scale-up production of UiO-66-type metal-organic frameworks and membrane materials. Dalt. Trans. 2015, 44, 19018–19040. [Google Scholar] [CrossRef]
- Fang, M.; Cambedouzou, J.; Cot, D.; Montoro, C.; Semsarilar, M. Facile membrane preparation from colloidally stable metal-organic framework-polymer nanoparticles. J. Memb. Sci. 2022, 657, 120669–120679. [Google Scholar] [CrossRef]
- Liu, X. Metal-organic framework UiO-66 membranes. Front. Chem. Sci. Eng. 2020, 14, 216–232. [Google Scholar] [CrossRef]
- Kandiah, M.; Nilsen, M.H.; Usseglio, S.; Jakobsen, S.; Olsbye, U.; Tilset, M.; Larabi, C.; Quadrelli, E.A.; Bonino, F.; Lillerud, K.P. Synthesis and stability of tagged UiO-66 Zr-MOFs. Chem. Mater. 2010, 22, 6632–6640. [Google Scholar] [CrossRef]
- Schaate, A.; Roy, P.; Godt, A.; Lippke, J.; Waltz, F.; Wiebcke, M.; Behrens, P. Modulated synthesis of Zr-based metal-organic frameworks: From nano to single crystals. Chem. Eur. J. 2011, 17, 6643–6651. [Google Scholar] [CrossRef]
- Martí-Rujas, J. Structural elucidation of microcrystalline MOFs from powder X-ray diffraction. Dalt. Trans. 2020, 49, 13897–13916. [Google Scholar] [CrossRef]
- Harris, K.D.M. Powder diffraction crystallography of molecular solids. In Topics in Current Chemistry; Springer: Berlin/Heidelberg, Germany, 2012; Volume 315, pp. 133–177. [Google Scholar]
- Guo, H.; Liu, J.; Li, Y.; Caro, J.; Huang, A. Post-synthetic modification of highly stable UiO-66-NH2 membranes on porous ceramic tubes with enhanced H2 separation. Microporous Mesoporous Mater. 2021, 313, 110823–110831. [Google Scholar] [CrossRef]
- Øien, S.; Wragg, D.; Reinsch, H.; Svelle, S.; Bordiga, S.; Lamberti, C.; Lillerud, K.P. Detailed structure analysis of atomic positions and defects in zirconium metal-organic frameworks. Cryst. Growth Des. 2014, 14, 5370–5372. [Google Scholar] [CrossRef]


| Membrane | UiO-66 | UiO-66-NH2 | Knudsen Selectivity Values | |
|---|---|---|---|---|
| Gas | ||||
| He/N2 | 2.00 | 2.09 | 2.65 | |
| He/CO2 | 2.17 | 2.41 | 3.32 | |
| He/SF6 | 2.68 | 3.43 | 6.08 | |
| N2/CO2 | 1.01 | 1.15 | 1.25 | |
| N2/SF6 | 1.34 | 1.64 | 2.82 | |
| CO2/SF6 | 1.24 | 1.42 | 1.83 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, M.; Drobek, M.; Cot, D.; Montoro, C.; Semsarilar, M. A Straightforward Method to Prepare MOF-Based Membranes via Direct Seeding of MOF-Polymer Hybrid Nanoparticles. Membranes 2023, 13, 65. https://doi.org/10.3390/membranes13010065
Fang M, Drobek M, Cot D, Montoro C, Semsarilar M. A Straightforward Method to Prepare MOF-Based Membranes via Direct Seeding of MOF-Polymer Hybrid Nanoparticles. Membranes. 2023; 13(1):65. https://doi.org/10.3390/membranes13010065
Chicago/Turabian StyleFang, Mingyuan, Martin Drobek, Didier Cot, Carmen Montoro, and Mona Semsarilar. 2023. "A Straightforward Method to Prepare MOF-Based Membranes via Direct Seeding of MOF-Polymer Hybrid Nanoparticles" Membranes 13, no. 1: 65. https://doi.org/10.3390/membranes13010065
APA StyleFang, M., Drobek, M., Cot, D., Montoro, C., & Semsarilar, M. (2023). A Straightforward Method to Prepare MOF-Based Membranes via Direct Seeding of MOF-Polymer Hybrid Nanoparticles. Membranes, 13(1), 65. https://doi.org/10.3390/membranes13010065










