Characterization of Electrospun Poly(ε-caprolactone) Nano/Micro Fibrous Membrane as Scaffolds in Tissue Engineering: Effects of the Type of Collector Used
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Solution
2.3. Electrospinning Process
2.4. Roughness of the Individual Fibers
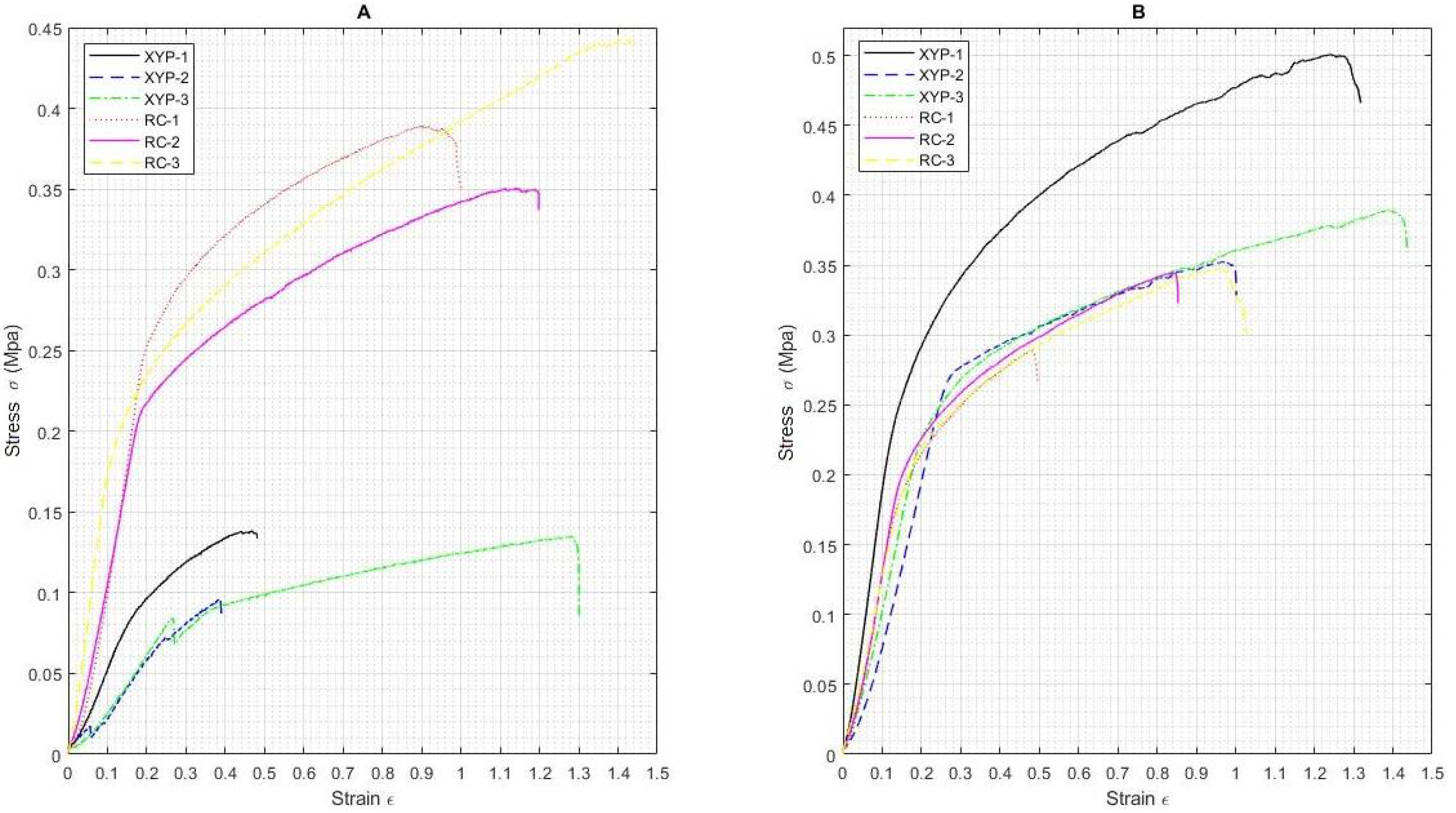
2.5. Mechanical Tests
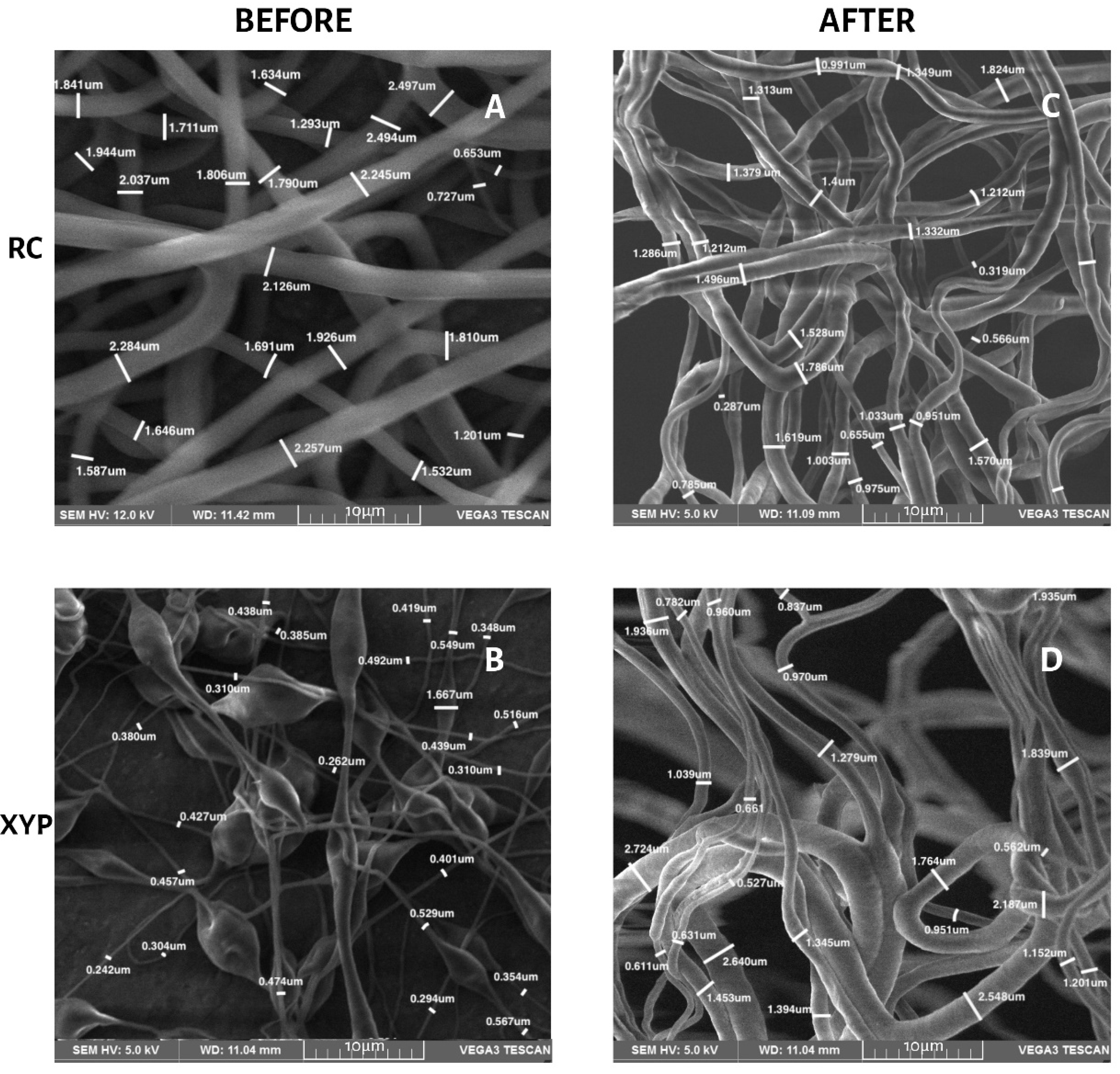
2.6. Morphology and Fibers Diameter
2.7. Contact Angle of the Scaffolds
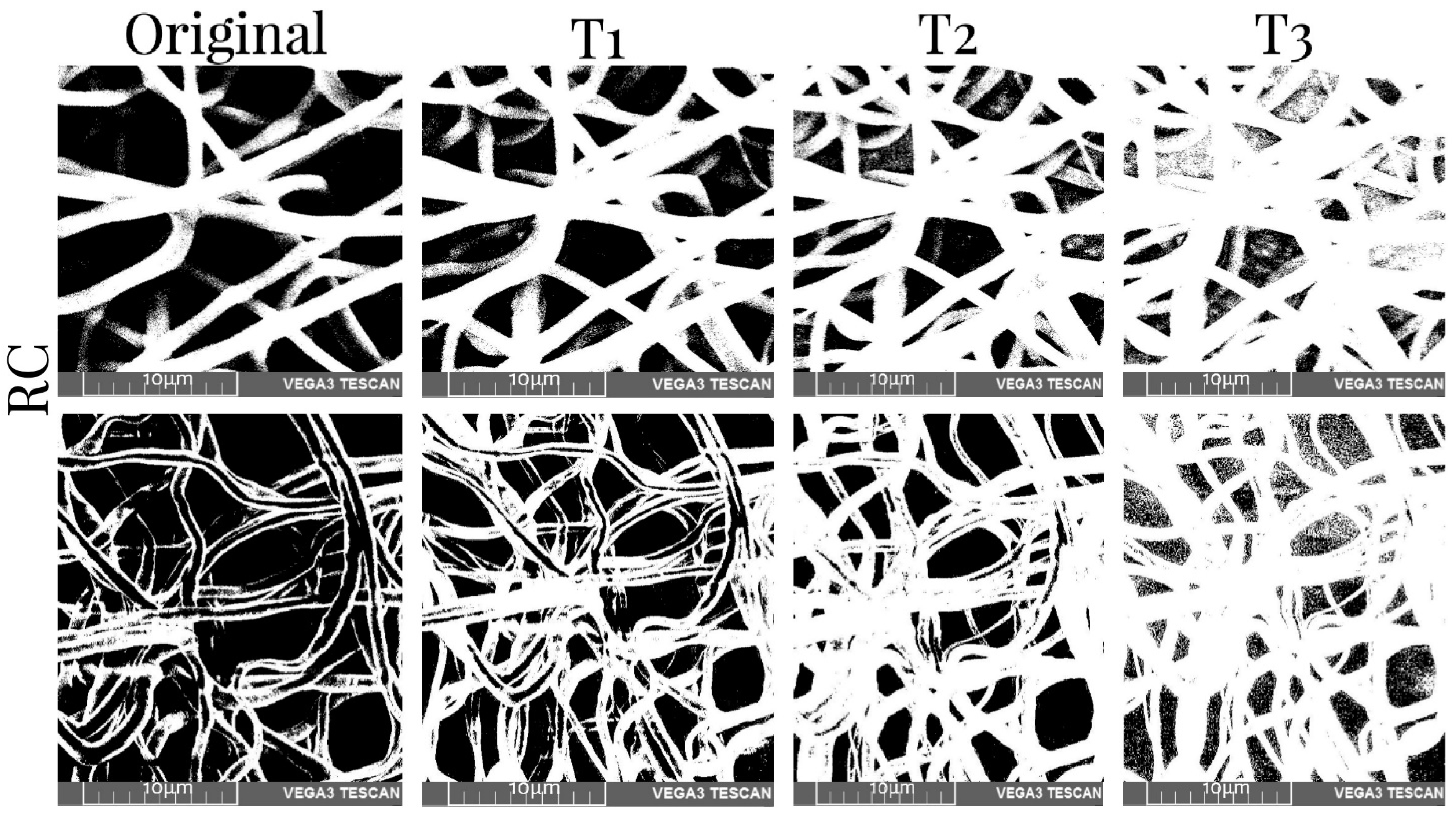
2.8. Porosity Measurement of the Scaffolds
2.9. Statistical Analysis
3. Results
3.1. Roughness of the Individual Fibers
3.2. Mechanical Tests
3.3. Morphology and Fiber Diameter
3.4. Contact Angle of the Scaffolds
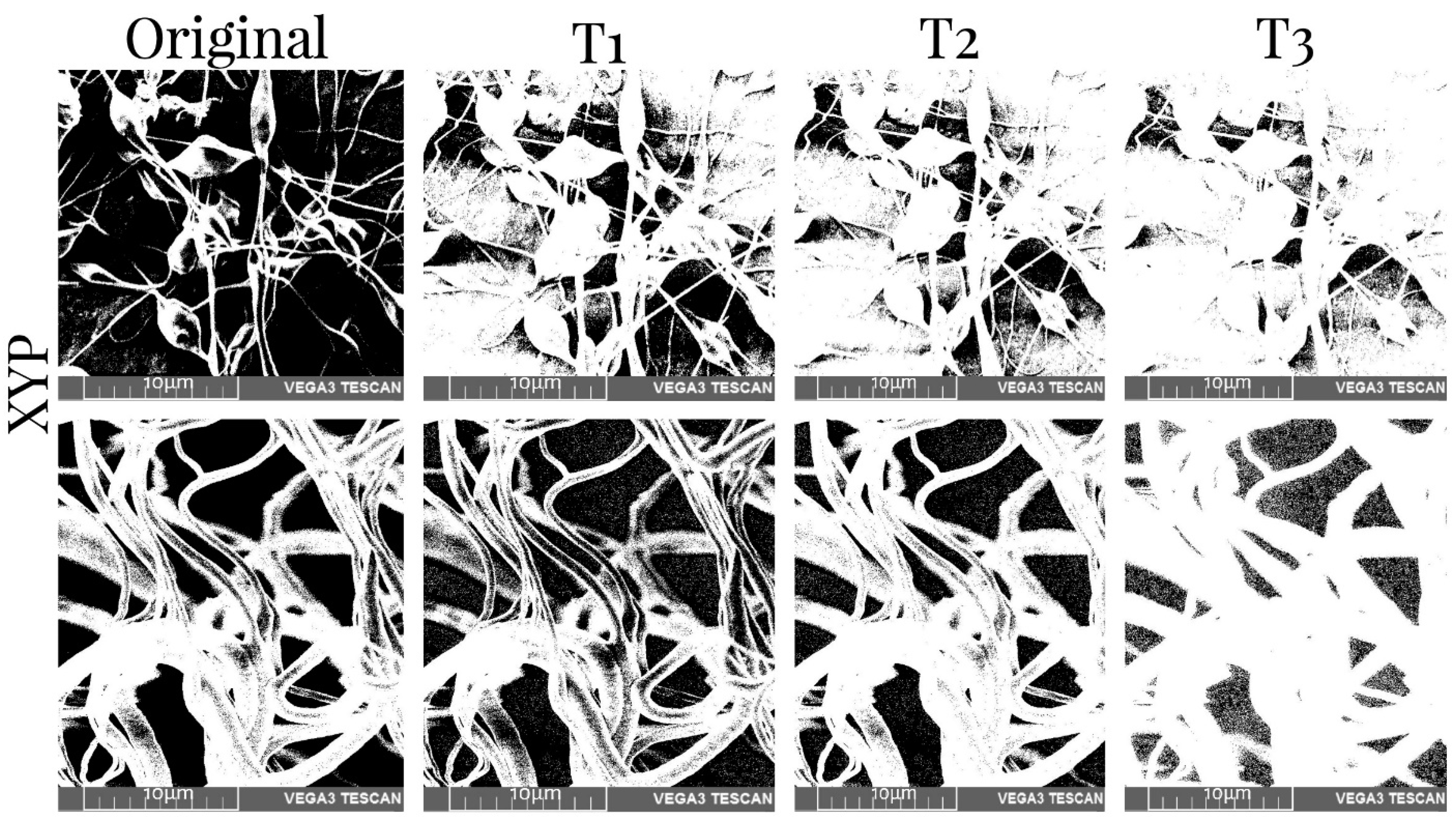
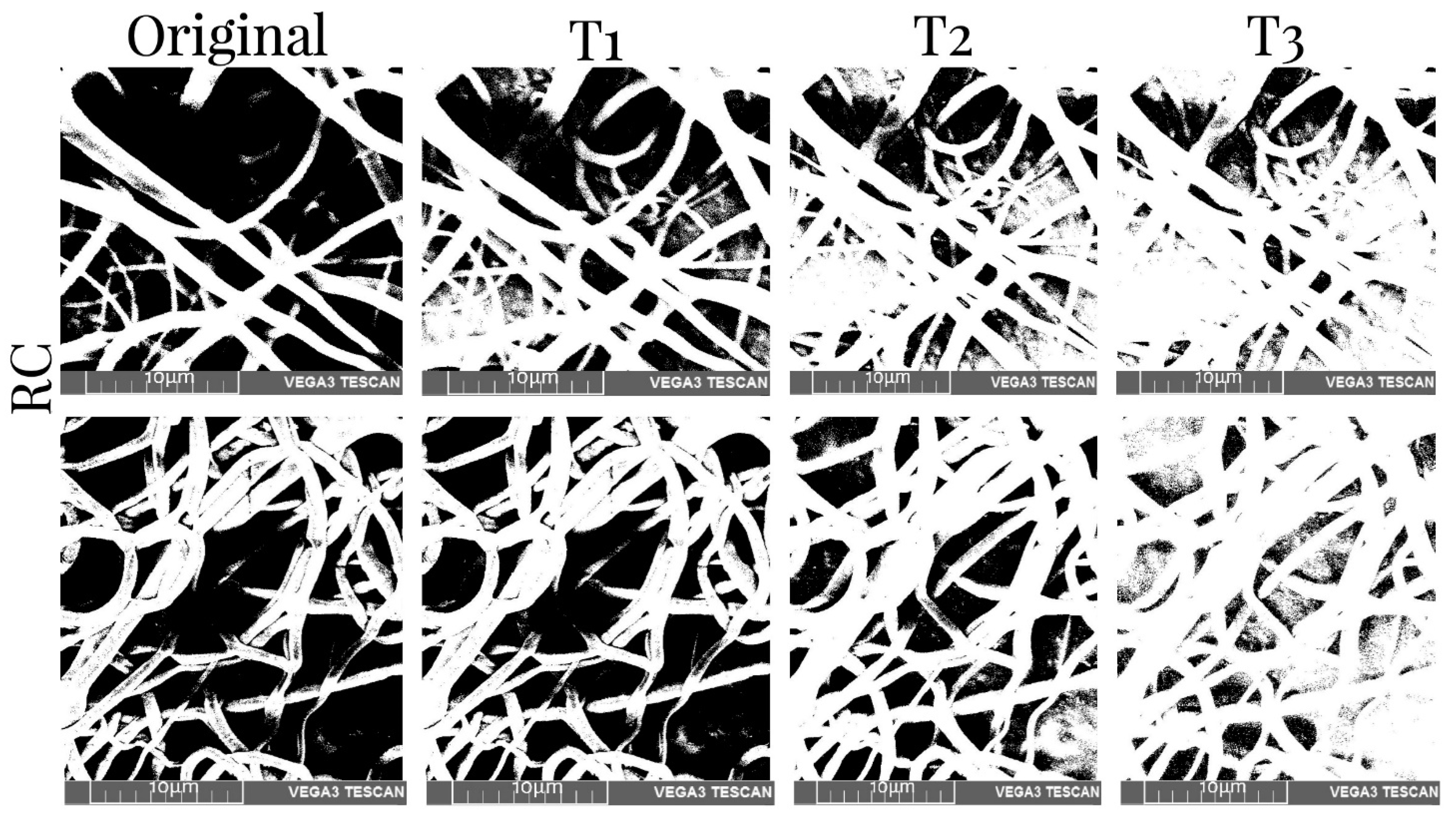
3.5. Porosity Measurement
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Williams, D.F. On the mechanisms of biocompatibility. Biomaterials 2008, 29, 2941–2953. [Google Scholar] [CrossRef] [PubMed]
- Ikada, Y. Challenges in tissue engineering. Journal of the Royal Society. Interface 2006, 3, 589–601. [Google Scholar] [PubMed]
- Marijanovic, I.; Antunovic, M.; Matic, I.; Panek, M.; Ivkovic, A. Bioreactor-based bone tissue engineering. In Advanced Techniques in Bone Regeneration; IntechOpen: Rijeka, Croatia, 2016. [Google Scholar]
- Dhandayuthapani, B.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Polymeric scaffolds in tissue engineering application: A review. Int. J. Polym. Sci. 2011, 2011, 290602. [Google Scholar] [CrossRef]
- Lee, J.; Cuddihy, M.J.; Kotov, N.A. Three-dimensional cell culture matrices: State of the art. Tissue Eng. Part B Rev. 2008, 14, 61–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hutmacher, D.W. Scaffolds in tissue engineering bone and cartilage. Biomaterials 2000, 21, 2529–2543. [Google Scholar] [CrossRef]
- Ratner, B.D.; Hoffman, A.S.; Schoen, F.J.; Lemons, J.E. Biomaterials Science: An Introduction to Materials in Medicine; Elsevier: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Tibbitt, M.W.; Rodell, C.B.; Burdick, J.A.; Anseth, K.S. Progress in material design for biomedical applications. Proc. Natl. Acad. Sci. USA 2015, 112, 14444–14451. [Google Scholar] [CrossRef] [Green Version]
- Ratner, B.D. A pore way to heal and regenerate: 21st century thinking on biocompatibility. Regen. Biomater. 2016, 3, 107–110. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.; Saini, M.; Dehiya, B.S.; Sindhu, A.; Kumar, V.; Kumar, R.; Lamberti, L.; Pruncu, C.I.; Thakur, R. Comprehensive survey on nanobiomaterials for bone tissue engineering applications. Nanomaterials 2020, 10, 2019. [Google Scholar] [CrossRef]
- Doostmohammadi, M.; Forootanfar, H.; Ramakrishna, S. Regenerative medicine and drug delivery: Progress via electrospun biomaterials. Mater. Sci. Eng. C 2020, 109, 110521. [Google Scholar] [CrossRef]
- Roseti, L.; Parisi, V.; Petretta, M.; Cavallo, C.; Desando, G.; Bartolotti, I.; Grigolo, B. Scaffolds for bone tissue engineering: State of the art and new perspectives. Mater. Sci. Eng. C 2017, 78, 1246–1262. [Google Scholar] [CrossRef]
- Turnbull, G.; Clarke, J.; Picard, F.; Riches, P.; Ja, L.; Han, F.; Li, B.; Shu, W. 3D bioactive composite scaffolds for bone tissue engineering. Bioact. Mater. 2018, 3, 278–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eltom, A.; Zhong, G.; Muhammad, A. Scaffold techniques and designs in tissue engineering functions and purposes: A review. Adv. Mater. Sci. Eng. 2019, 2019, 3429527. [Google Scholar] [CrossRef] [Green Version]
- Ozbolat, I.T.; Moncal, K.K.; Gudapati, H. Evaluation of bioprinter technologies. Addit. Manuf. 2017, 13, 179–200. [Google Scholar] [CrossRef] [Green Version]
- Barrero, A.; González Loscertales, I.; Márquez, M. Microchorros y Nanochorros. Investig. Cienc. 2005, 351, 44–51. [Google Scholar]
- Xie, J.; Jiang, J.; Davoodi, P.; Srinivasan, M.P.; Wang, C.-H. Electrohydrodynamic atomization: A two-decade effort to produce and process micro-/nanoparticulate materials. Chem. Eng. Sci. 2015, 125, 32–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahmati, M.; Mills, D.K.; Urbanska, A.M.; Saeb, R.M.; Venugopal, J.R.; Ramakrishna, S.; Mozafari, M. Electrospinning for tissue engineering applications. Prog. Mater. Sci. 2020, 117, 100721. [Google Scholar] [CrossRef]
- Wu, T.; Ding, M.; Shi, C.; Qiao, Y.; Wang, P.; Qiao, R.; Wang, X.; Zhong, J. Resorbable polymer electrospun nanofibers: History, shapes and application for tissue engineering. Chin. Chem. Lett. 2020, 31, 617–625. [Google Scholar] [CrossRef]
- Soares, R.M.D.; Siqueira, N.M.; Prabhakaram, M.P.; Ramakrishna, S. Electrospinning and electrospray of bio-based and natural polymers for biomaterials development. Mater. Sci. Eng. C 2018, 92, 969–982. [Google Scholar] [CrossRef]
- Calori, I.R.; Braga, G.; de Jesus, P.d.C.C.; Bi, H.; Tedesco, A.C. Polymer scaffolds as drug delivery systems. Eur. Polym. J. 2020, 129, 109621. [Google Scholar] [CrossRef]
- Ojha, S. Structure—Property relationship of electrospun fibers. In Electrospun Nanofibers; Elsevier: Amsterdam, The Netherlands, 2017; pp. 239–253. [Google Scholar]
- Munteanu, B.S.; Vasile, C. Electrospun Polymeric Nanostructures with Applications in Nanomedicine. In Polymeric Nanomaterials in Nanotherapeutics; Elsevier: Amsterdam, The Netherlands, 2019; pp. 261–297. [Google Scholar]
- Neves, N.M.; Campos, R.; Pedro, A.; Cunha, J.; Macedo, F.; Reis, R.L. Patterning of polymer nanofiber meshes by electrospinning for biomedical applications. Int. J. Nanomed. 2007, 2, 433. [Google Scholar]
- Kumar, P. Effect of Colletor on Electrospinning to Fabricate Aligned Nano Fiber. Ph.D. Thesis, National Institute of Technology, Rourkela, India, 2012. [Google Scholar]
- Yeo, M.; Kim, G. Micro/nano-hierarchical scaffold fabricated using a cell electrospinning/3D printing process for co-culturing myoblasts and HUVECs to induce myoblast alignment and differentiation. Acta Biomater. 2020, 107, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Ambrus, R.; Alshweiat, A.; Csóka, I.; Ovari, G.; Esmail, A.; Radacsi, N. 3D-printed electrospinning setup for the preparation of loratadine nanofibers with enhanced physicochemical properties. Int. J. Pharm. 2019, 567, 118455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.I.; Hwang, T.I.; Aguilar, L.E.; Park, C.H.; Kim, C.S. A controlled design of aligned and random nanofibers for 3D bi-functionalized nerve conduits fabricated via a novel electrospinning set-up. Sci. Rep. 2016, 6, 23761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joshi, M.K.; Shrestha, R.M.; Pant, H.R. 3D Nonwoven Fabrics for Biomedical Applications. In Generation, Development and Modifications of Natural Fibers; IntechOpen: Rijeka, Croatia, 2020. [Google Scholar]
- Clavijo-Grimaldo, D.; Ponce-Zapata, N.; Casadiego-Torrado, C. Control of Bacterial Proliferation and Formation of Biofilm in Membranes for Food Packaging Manufactured by Electrospinning. Chem. Eng. Trans. 2019, 75, 241–246. [Google Scholar]
- Ghasemi-Mobarakeh, L.; Semnani, D.; Morshed, M. A novel method for porosity measurement of various surface layers of nanofibers mat using image analysis for tissue engineering applications. J. Appl. Polym. Sci. 2007, 106, 2536–2542. [Google Scholar] [CrossRef]
- Vasita, R.; Katti, D.S. Nanofibers and their applications in tissue engineering. Int. J. Nanomed. 2006, 1, 15. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and electrospun nanofibers: Methods, materials and applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- Li, L.; LaBarbera, D.V. 3D High-content screening of organoids for drug discovery. Compr. Med. Chem. III 2017, 119, 388–415. [Google Scholar]
- Sánchez Cepeda, Á.P.; Vera-Graziano, R.; Muñoz-Prieto, E.d.J.; Gómez-Pachón, E.Y.; Bernad-Bernad, M.J.; Maciel-Cerda, A. Preparación y caracterización de membranas poliméricas electrohiladas de policaprolactona y quitosano para la liberación controlada de clorhidrato de tiamina. Cienc. Desarro. 2016, 7, 133–151. [Google Scholar] [CrossRef] [Green Version]
- Seidi, A.; Ramalingam, M.; Elloumi-Hannachi, I.; Ostrovidov, S.; Khademhosseini, A. Gradient biomaterials for soft-to-hard interface tissue engineering. Acta Biomater. 2011, 7, 1441–1451. [Google Scholar] [CrossRef]
- Greenfeld, I.; Rodricks, C.W.; Sui, X.; Wagner, H.D. Beaded fiber composites—Stiffness and strength modeling. J. Mech. Phys. Solids 2019, 125, 384–400. [Google Scholar] [CrossRef]
- Fujihara, K.; Kotaki, M.; Ramakrishna, S. Guided bone regeneration membrane made of polycaprolactone/calcium carbonate composite nano-fibers. Biomaterials 2005, 26, 4139–4147. [Google Scholar] [CrossRef] [PubMed]
- Karuppuswamy, P.; Venugopal, J.R.; Navaneethan, B.; Laiva, A.L.; Ramakrishna, S. Polycaprolactone nanofibers for the controlled release of tetracycline hydrochloride. Mater. Lett. 2015, 141, 180–186. [Google Scholar] [CrossRef]
- Gaumer, J.; Prasad, A.; Lee, D.; Lannutti, J. Structure—Function relationships and source-to-ground distance in electrospun polycaprolactone. Acta Biomater. 2009, 5, 1552–1561. [Google Scholar] [CrossRef] [PubMed]
- Cramariuc, B.; Cramariuc, R.; Scarlet, R.; Manea, L.R.; Lupu, I.G.; Cramariuc, O. Fiber diameter in electrospinning process. J. Electrost. 2013, 71, 189–198. [Google Scholar] [CrossRef]
- Fridrikh, S.V.; Jian, H.Y.; Brenner, M.P.; Rutledge, G.C. Controlling the fiber diameter during electrospinning. Phys. Rev. Lett. 2003, 90, 144502. [Google Scholar] [CrossRef] [Green Version]
- He, H.; Wang, Y.; Farkas, B.; Nagy, Z.K.; Molnar, K. Analysis and prediction of the diameter and orientation of AC electrospun nanofibers by response surface methodology. Mater. Des. 2020, 194, 108902. [Google Scholar] [CrossRef]
- El-Hadi, A.M.; Al-Jabri, F.Y. Influence of electrospinning parameters on fiber diameter and mechanical properties of poly (3-hydroxybutyrate) (PHB) and polyanilines (PANI) blends. Polymers 2016, 8, 97. [Google Scholar] [CrossRef] [Green Version]
- Moghadam, B.H.; Hasanzadeh, M. Predicting contact angle of electrospun PAN nanofiber mat using artificial neural network and response surface methodology. Adv. Polym. Technol. 2013, 32, 21365. [Google Scholar] [CrossRef]
- Szewczyk, P.K.; Ura, D.P.; Metwally, S.; Knapczyk-Korczak, J.; Gajek, M.; Marzec, M.M.; Bernasik, A.; Stachewicz, U. Roughness and fiber fraction dominated wetting of electrospun fiber-based porous meshes. Polymers 2019, 11, 34. [Google Scholar] [CrossRef] [Green Version]
- Dias, J.; Bártolo, P. Morphological char influence. Procedia CIRP 2013, 5, 216–221. [Google Scholar] [CrossRef]
- Alam, A.K.M.; Ewaldz, E.; Xiang, C.; Qu, W.; Bai, X. Tunable Wettability of Biodegradable Multilayer Sandwich-Structured Electrospun Nanofibrous Membranes. Polymers 2020, 12, 2092. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, A.P.; Joshi, M.K.; Lee, J.; Maharjan, B.; Ko, S.W.; Park, C.H.; Kim, C.S. Heterogeneous electrospun polycaprolactone/polyethylene glycol membranes with improved wettability, biocompatibility, and mineralization. Colloids Surf. A Physicochem. Eng. Asp. 2017, 520, 105–113. [Google Scholar] [CrossRef]
- Al-Amshawee, S.; Yunus, M.Y.B.M.; Lynam, J.G.; Lee, W.H.; Dai, F.; Dakhil, I.H. Roughness and wettability of biofilm carriers: A systematic review. Environ. Technol. Innov. 2020, 21, 101233. [Google Scholar] [CrossRef]

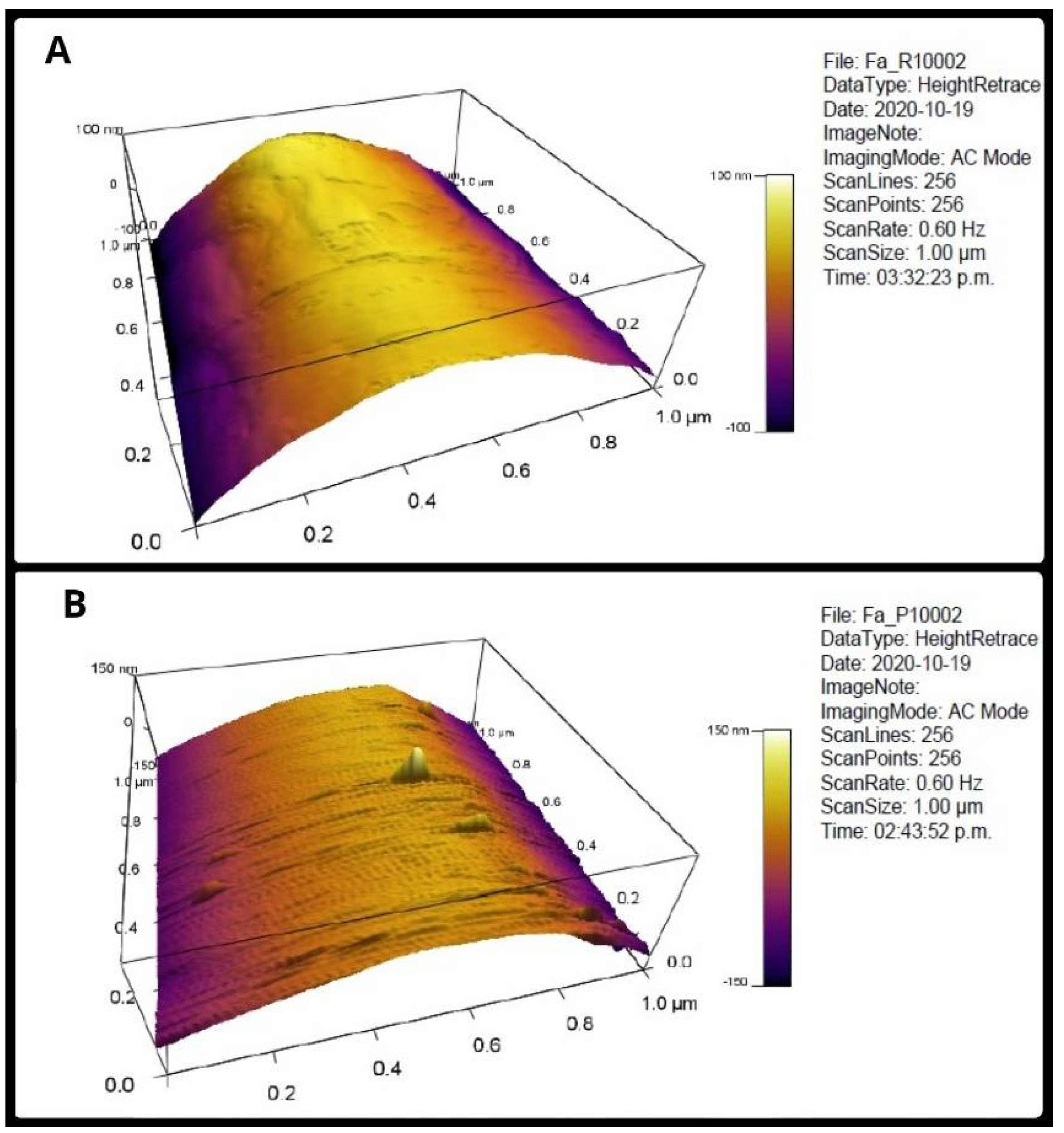


| Collector Type | Root Mean Square (Rms) (nm) | Arithmetical Average (Ra) (nm) | |
|---|---|---|---|
| RC | Mean (nm) | 51.47 | 42.72 |
| SD | 15.33 | 12.13 | |
| XYP | Mean (nm) | 36.94 | 38.25 |
| SD | 15.62 | 23.72 | |
| Collector Type | Deposition Time | Sample | Max_Force N | Max_Disp mm | Max_Stress N/mm2 MPa | Max_Strain % |
|---|---|---|---|---|---|---|
| RC | 45 min | Mean value | 0.18 | 105.06 | 0.39 | 114.91 |
| SD | 0.02 | 19.39 | 0.05 | 25.44 | ||
| 90 min | Mean value | 0.19 | 118.87 | 0.41 | 119.88 | |
| SD | 0.04 | 13.83 | 0.08 | 21.05 | ||
| XYP | 45 min | Mean value | 0.06 | 80.53 | 0.12 | 70.98 |
| SD | 0.01 | 32.99 | 0.02 | 49.14 | ||
| 90 min | Mean value | 0.15 | 77.47 | 0.33 | 75.87 | |
| SD | 0.02 | 24.51 | 0.03 | 24.95 |
| Collector Type | Deposition Time | Diameter (nm) | |||
|---|---|---|---|---|---|
| Mean (nm) | SD | ||||
| Before | After | Before | After | ||
| RC | RC45 | 1819 | 1147 | 549 | 424 |
| RC90 | 1386 | 1267 | 674 | 404 | |
| XYP | XYP45 | 548 | 1357 | 502 | 672 |
| XYP90 | 1472 | 1005 | 846 | 820 | |
| Deposition Time | Contact Angle | |
|---|---|---|
| RC45 | Mean | 51.95° |
| SD | 1.80 | |
| RC90 | Mean | 58.19° |
| SD | 6.66 | |
| XYP45 | Mean | 80.52° |
| SD | 3.87 | |
| XYP90 | Mean | 74.77° |
| SD | 15.63 | |
| Collector Type | Deposition Time | Sample Type | T1% | T2% | T3% |
|---|---|---|---|---|---|
| RC | 45 min | Before | 38.19 | 25.95 | 12.71 |
| After | 48.09 | 34.97 | 22.36 | ||
| 90 min | Before | 39.32 | 26.39 | 17.11 | |
| After | 29.29 | 19.88 | 15.68 | ||
| XYP | 45 min | Before | 28.06 | 18.89 | 9.26 |
| After | 47.80 | 31.42 | 15.45 | ||
| 90 min | Before | 29.42 | 19.47 | 9.66 | |
| After | 46.30 | 28.34 | 10.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clavijo-Grimaldo, D.; Casadiego-Torrado, C.A.; Villalobos-Elías, J.; Ocampo-Páramo, A.; Torres-Parada, M. Characterization of Electrospun Poly(ε-caprolactone) Nano/Micro Fibrous Membrane as Scaffolds in Tissue Engineering: Effects of the Type of Collector Used. Membranes 2022, 12, 563. https://doi.org/10.3390/membranes12060563
Clavijo-Grimaldo D, Casadiego-Torrado CA, Villalobos-Elías J, Ocampo-Páramo A, Torres-Parada M. Characterization of Electrospun Poly(ε-caprolactone) Nano/Micro Fibrous Membrane as Scaffolds in Tissue Engineering: Effects of the Type of Collector Used. Membranes. 2022; 12(6):563. https://doi.org/10.3390/membranes12060563
Chicago/Turabian StyleClavijo-Grimaldo, Dianney, Ciro Alfonso Casadiego-Torrado, Juan Villalobos-Elías, Adolfo Ocampo-Páramo, and Magreth Torres-Parada. 2022. "Characterization of Electrospun Poly(ε-caprolactone) Nano/Micro Fibrous Membrane as Scaffolds in Tissue Engineering: Effects of the Type of Collector Used" Membranes 12, no. 6: 563. https://doi.org/10.3390/membranes12060563
APA StyleClavijo-Grimaldo, D., Casadiego-Torrado, C. A., Villalobos-Elías, J., Ocampo-Páramo, A., & Torres-Parada, M. (2022). Characterization of Electrospun Poly(ε-caprolactone) Nano/Micro Fibrous Membrane as Scaffolds in Tissue Engineering: Effects of the Type of Collector Used. Membranes, 12(6), 563. https://doi.org/10.3390/membranes12060563









