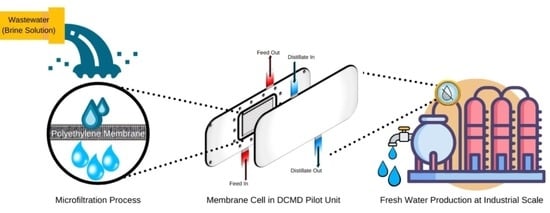
Long-Term Treatment of Highly Saline Brine in a Direct Contact Membrane Distillation (DCMD) Pilot Unit Using Polyethylene Membranes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Polyethylene Membranes
2.2. Preparation of Feed
2.3. Operation of the Direct Contact Membrane Distillation (DCMD) Module
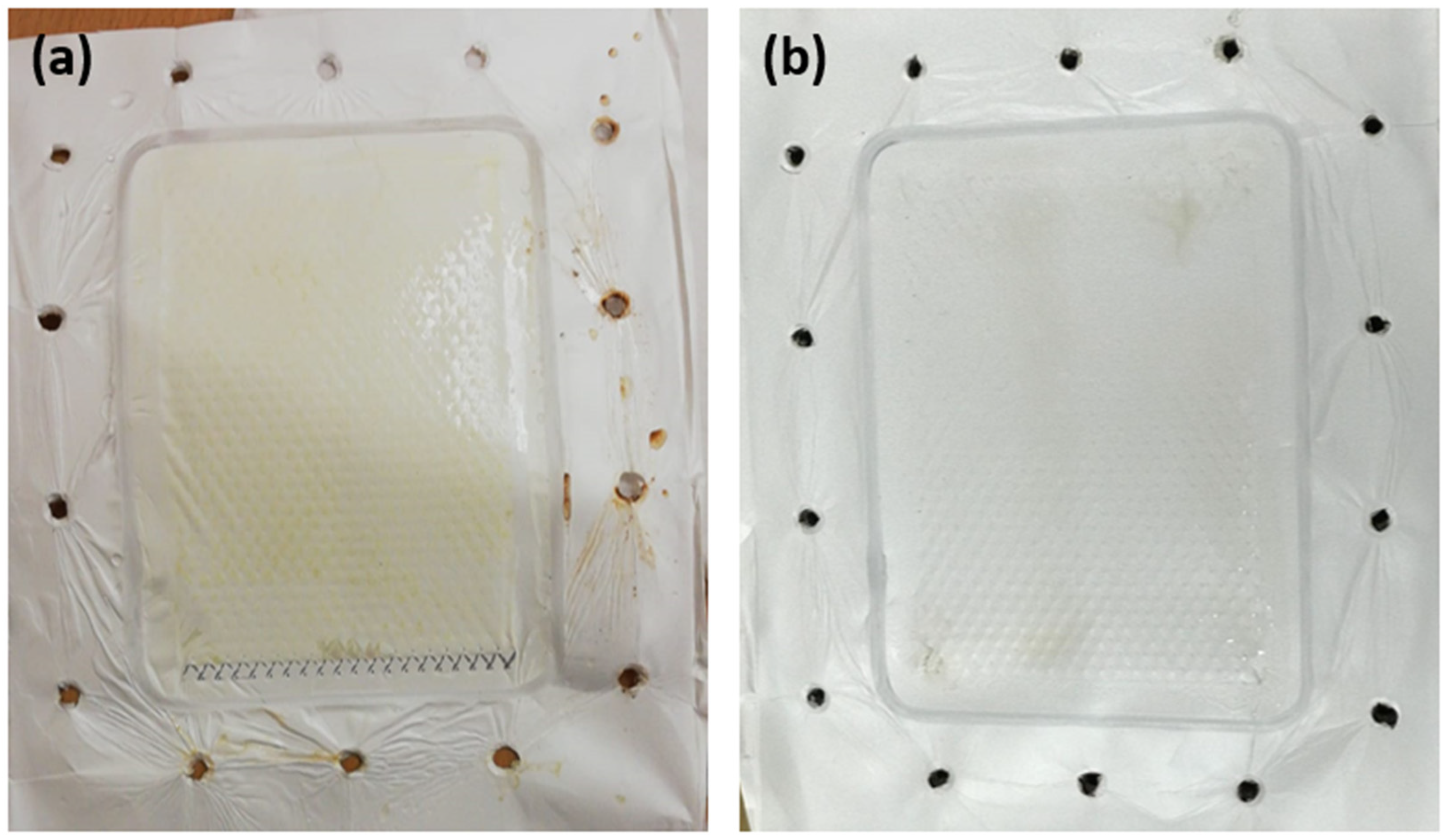
2.4. Fouling Analysis for DCMD Pilot Unit
2.5. MD Performance Evaluation
2.5.1. Salt Rejection and Flux Calculations
2.5.2. Membrane Fouling Experiments
2.5.3. Energy Consumption Analysis
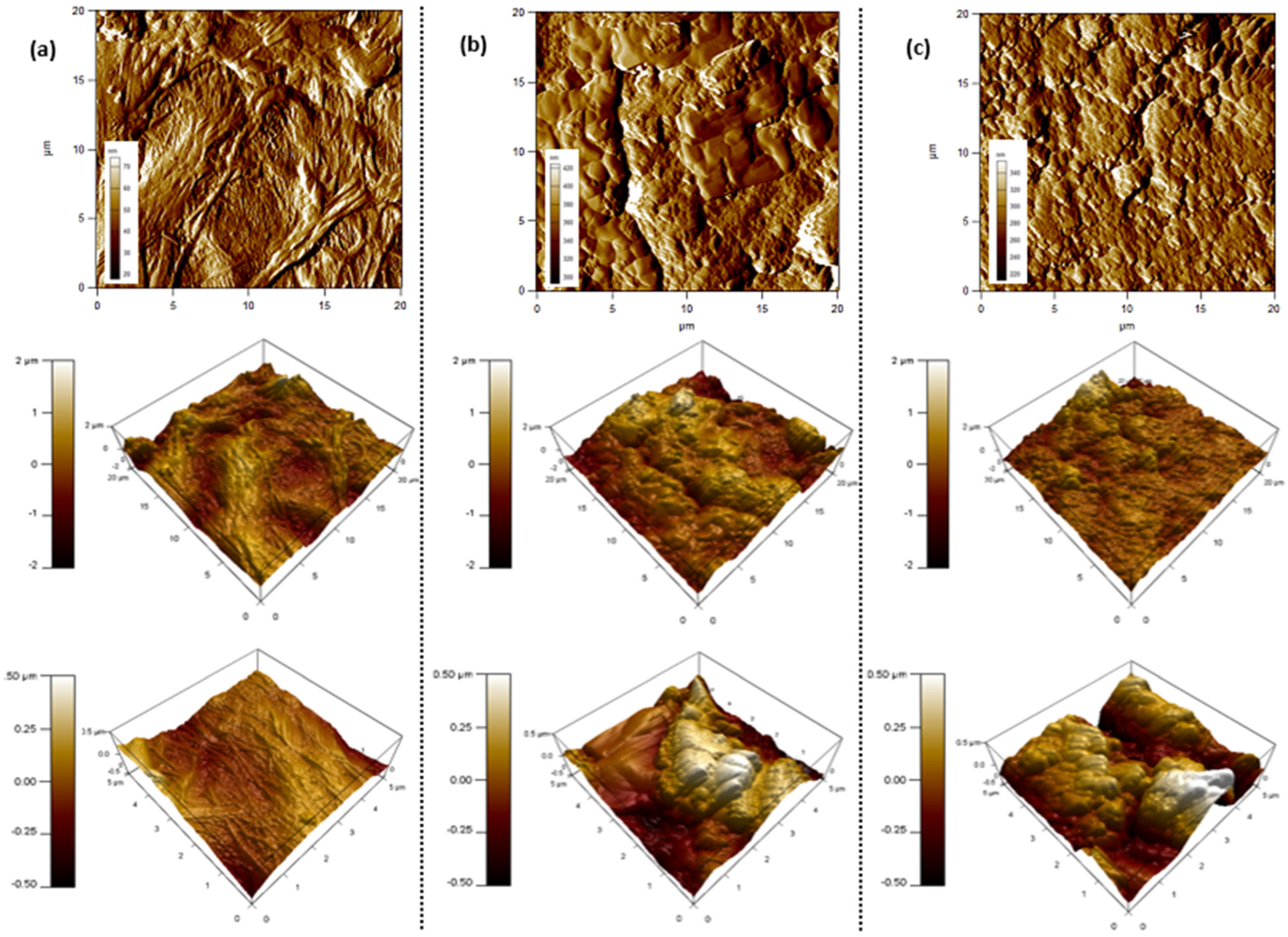
2.6. Membrane Characterization
3. Results and Discussion
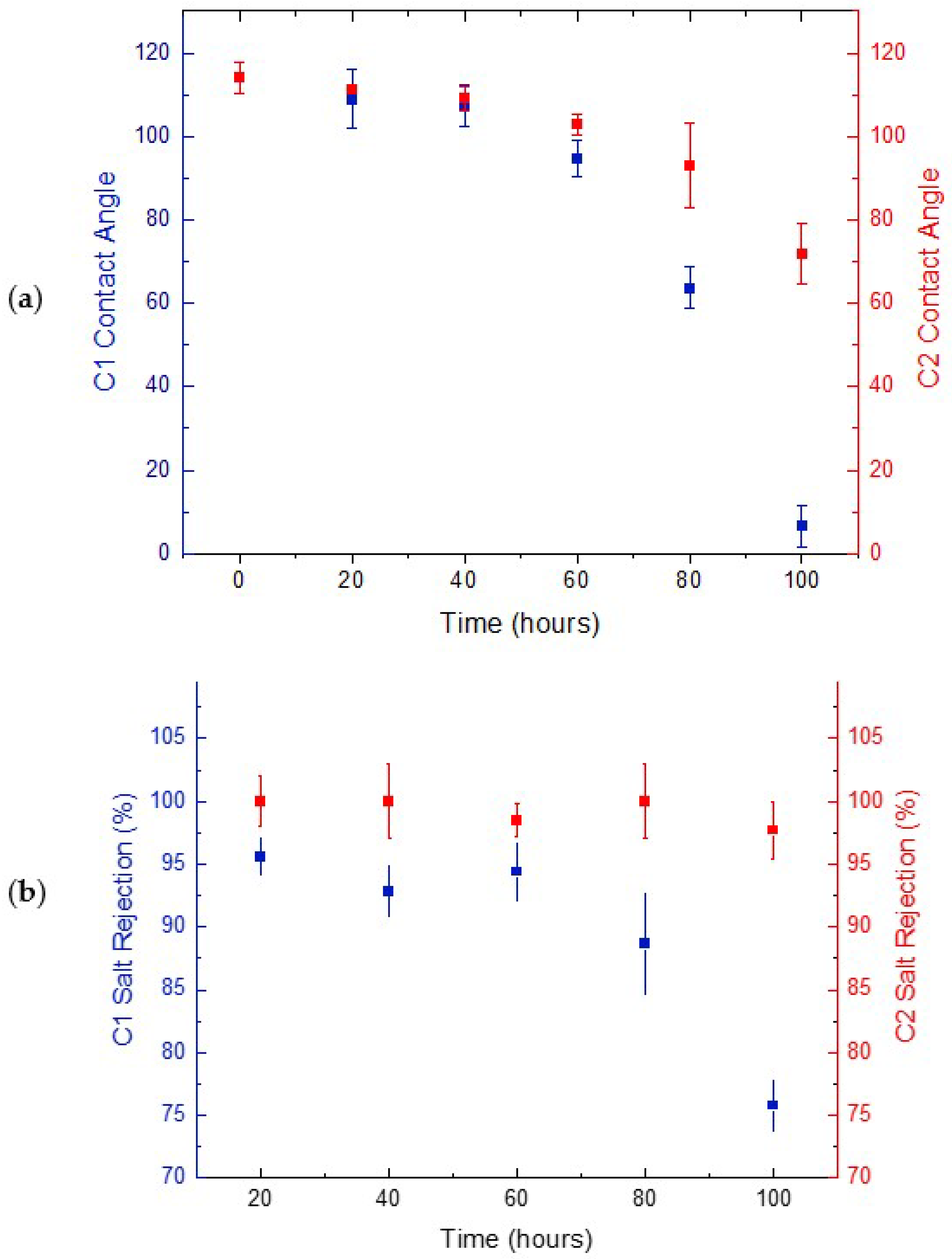
3.1. Long-Term MD Pilot Performance
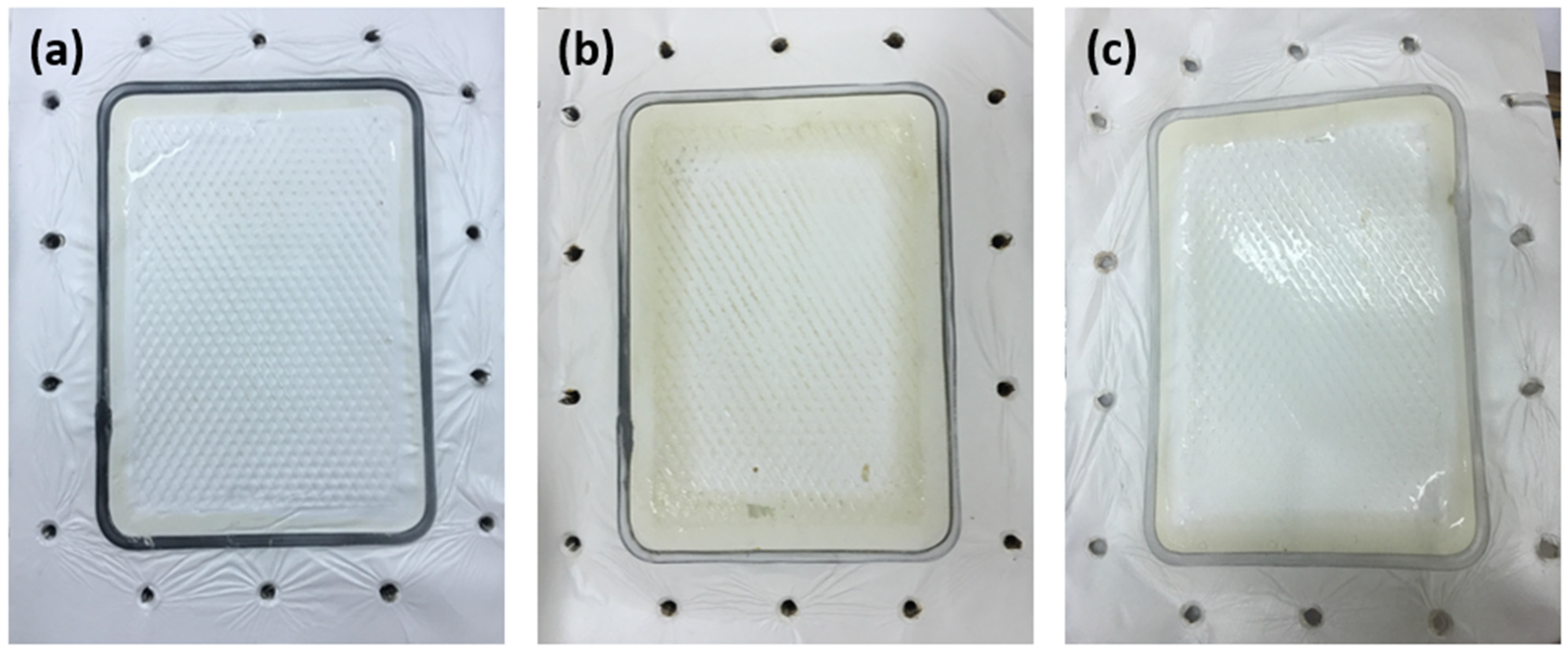
3.1.1. Membrane Surface Analysis
3.1.2. Membrane Performance at Pilot-Scale
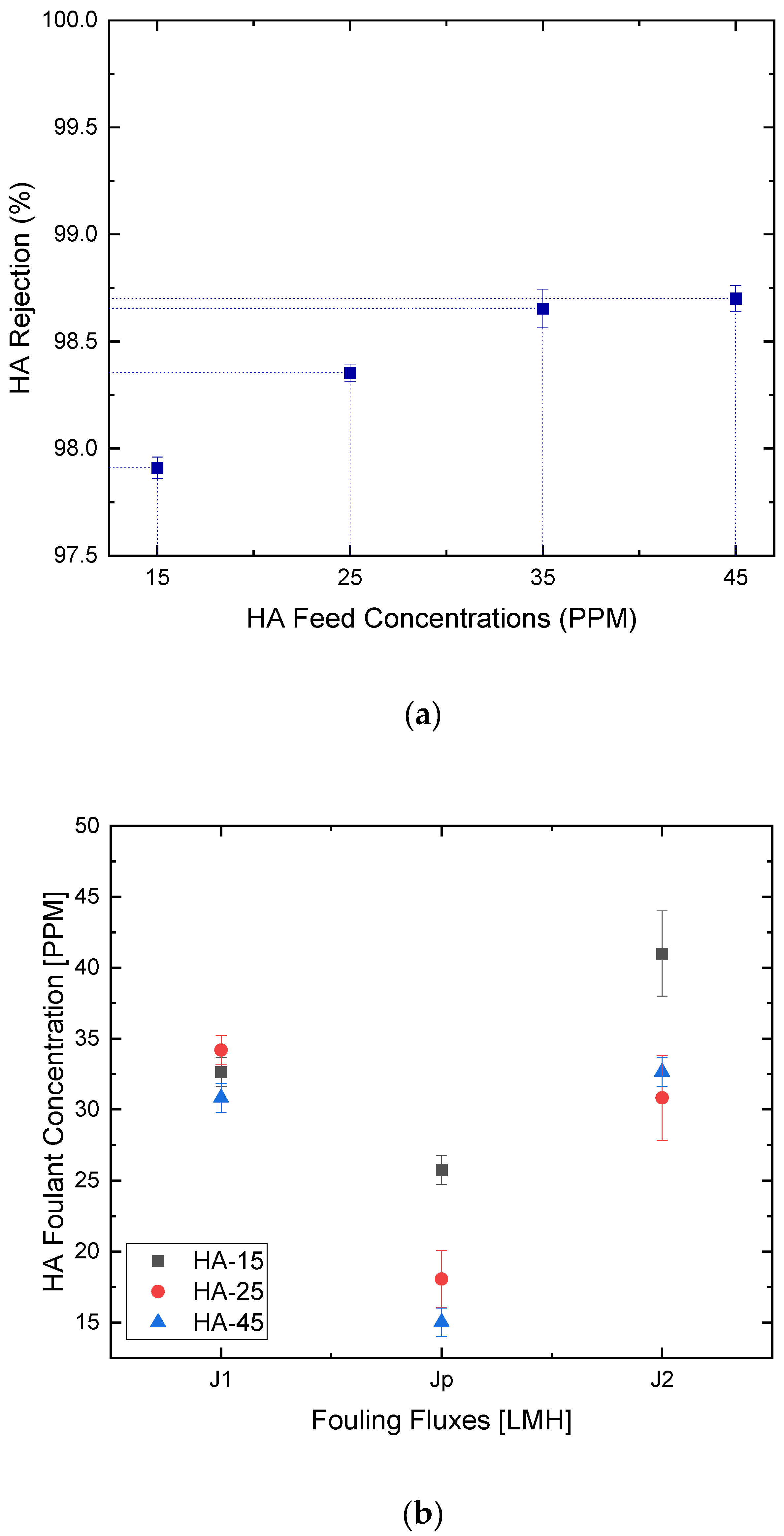
3.2. Membrane Fouling Analysis
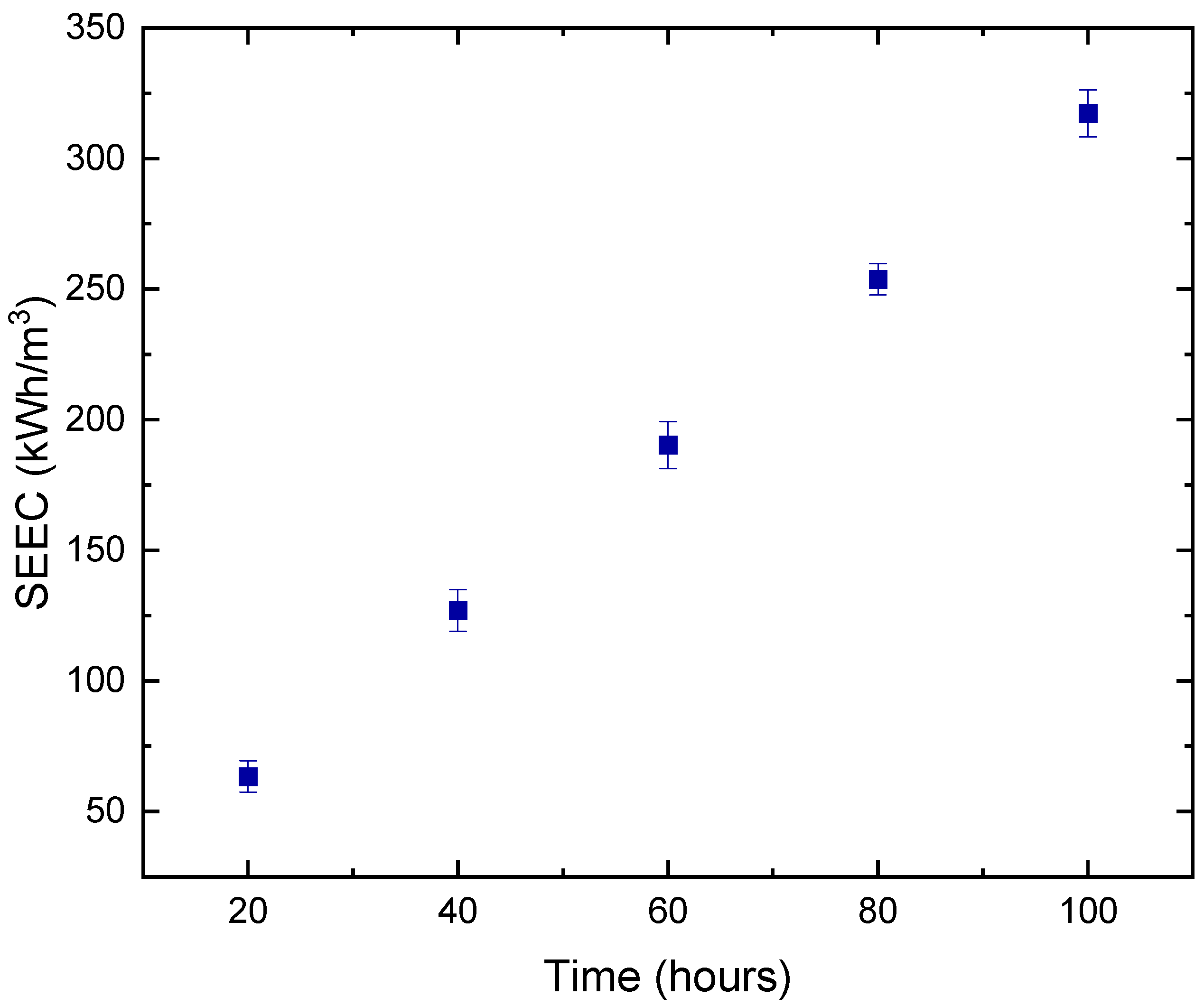
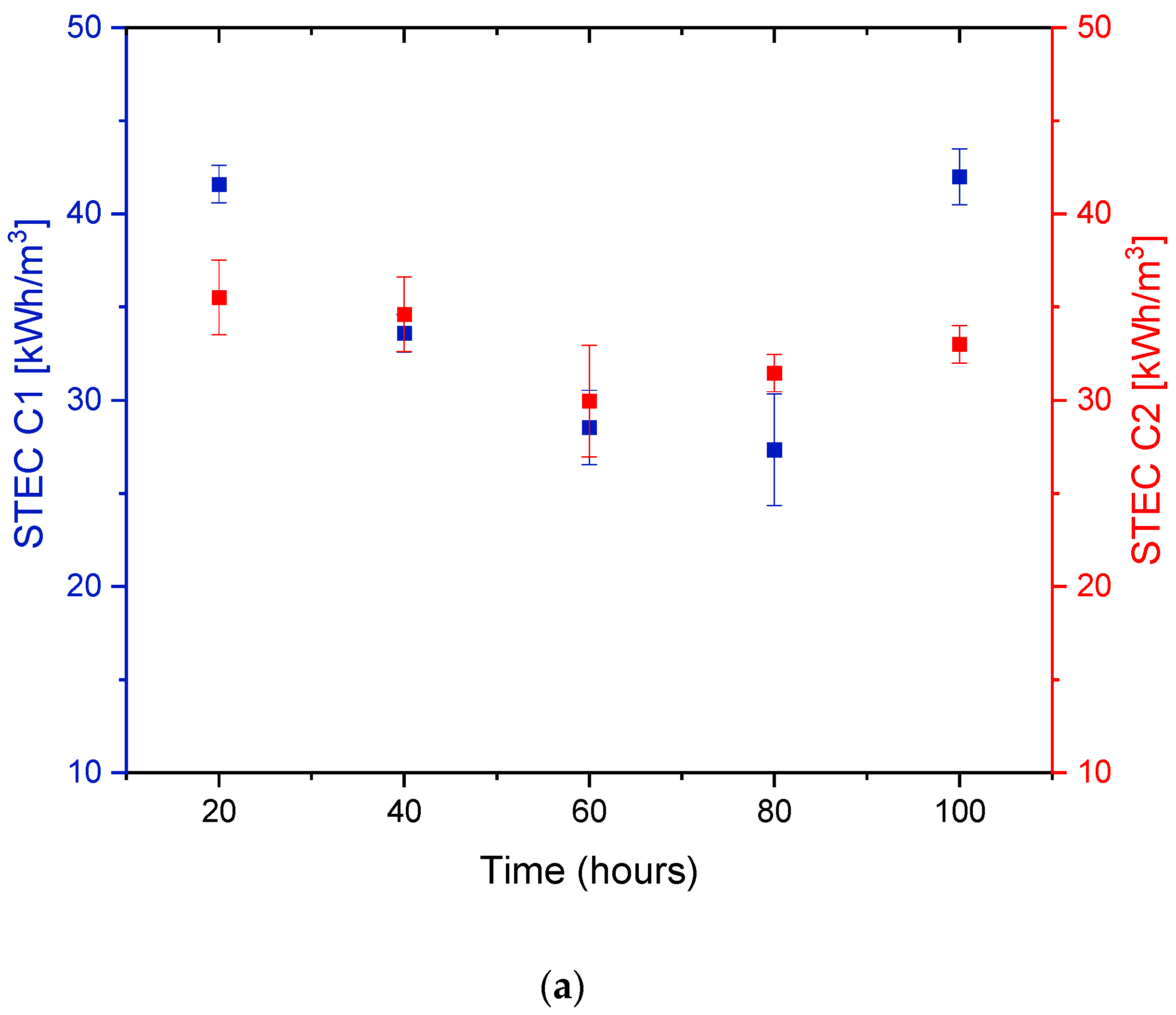
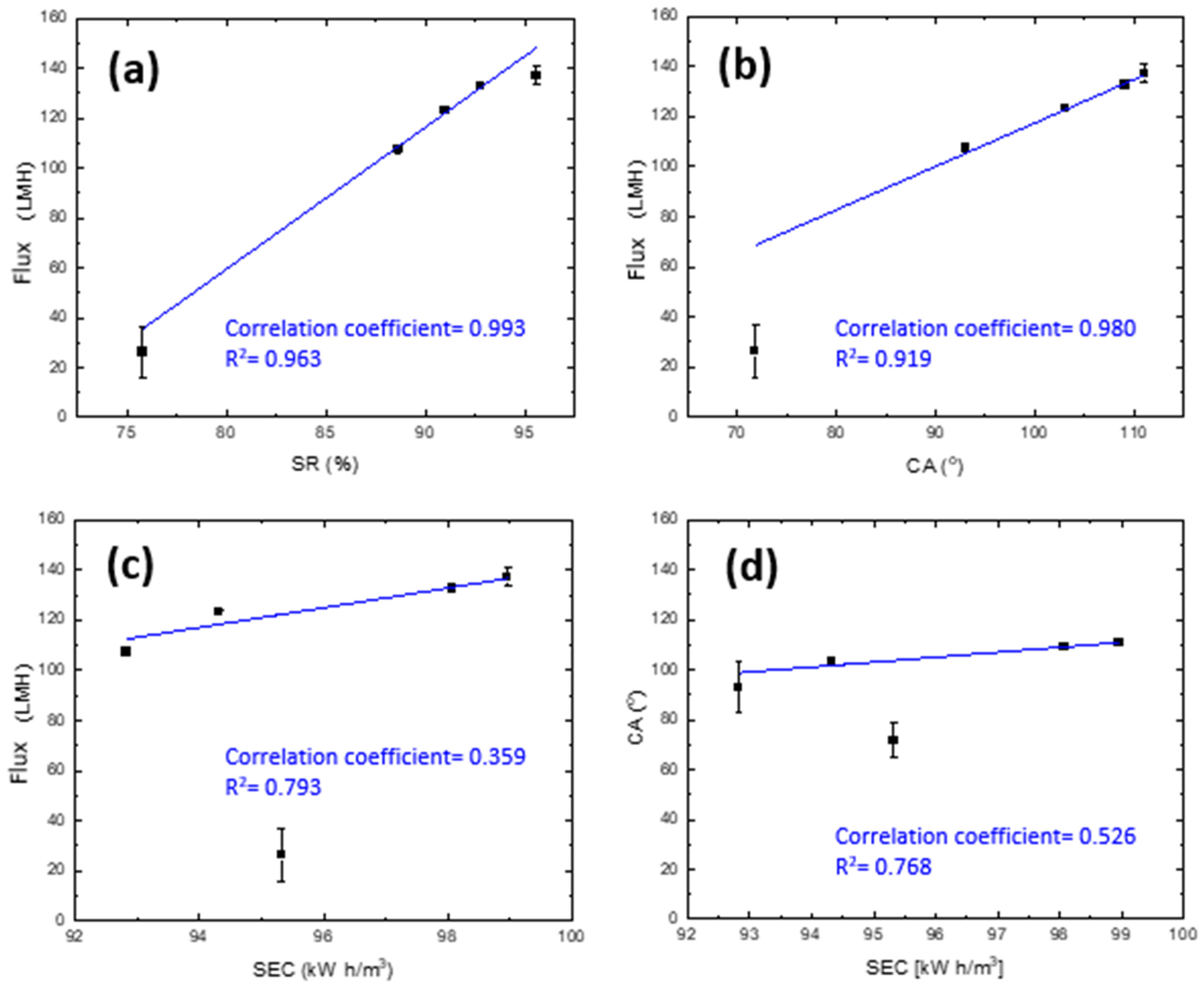
3.3. Specific Energy Consumption (SEC) of DCMD Pilot Unit
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cyganowski, J.; Lutz, H. Post-processing. In Ultrafiltration for Bioprocessing; Woodhead Publishing: Sawston, UK, 2015; Chapter 8; pp. 131–149. [Google Scholar]
- Kim, B.; Kwak, R.; Kwon, H.J.; Pham, V.S.; Kim, M.; Al-Anzi, B.; Lim, G.; Han, J. Purification of high salinity brine by multi-stage ion concentration polarization desalination. Sci. Rep. 2016, 6, 31850. [Google Scholar] [CrossRef] [Green Version]
- Abdelrazeq, H.; Khraisheh, M.; Ashraf, H.M.; Ebrahimi, P.; Kunju, A. Sustainable innovation in membrane technologies for produced water treatment: Challenges and limitations. Sustainability 2021, 13, 6759. [Google Scholar] [CrossRef]
- Criscuoli, A.; Capuano, A.; Andreucci, M.; Drioli, E. Low-temperature direct contact membrane distillation for the treatment of aqueous solutions containing urea. Membranes 2020, 10, 176. [Google Scholar] [CrossRef]
- Cohen-Tanugi, D.; McGovern, R.K.; Dave, S.H.; Lienhard, J.H.; Grossman, J.C. Quantifying the potential of ultra-permeable membranes for water desalination. Energy Environ. Sci. 2014, 7, 1134–1141. [Google Scholar] [CrossRef] [Green Version]
- Kebria, M.R.S.; Rahimpour, A. Membrane Distillation: Basics, Advances, and Applications. In Advances in Membrane Technologies; IntechOpen: London, UK, 2020; pp. 1–16. [Google Scholar]
- Miladi, R.; Frikha, N.; Kheiri, A.; Gabsi, S. Energetic performance analysis of seawater desalination with a solar membrane distillation. Energy Convers. Manag. 2019, 185, 143–154. [Google Scholar] [CrossRef]
- Kiss, A.A.; Kattan Readi, O.M. An industrial perspective on membrane distillation processes. J. Chem. Technol. Biotechnol. 2018, 93, 2047–2055. [Google Scholar] [CrossRef]
- Woo, Y.C.; Kim, Y.; Shim, W.; Tijing, L.D.; Yao, M.; Nghiem, L.D.; Choi, J.; Kim, S.; Kyong, H. Graphene/PVDF flat-sheet membrane for the treatment of RO brine from coal seam gas produced water by air gap membrane distillation. J. Memb. Sci. 2016, 16, 74–84. [Google Scholar] [CrossRef]
- Seman, M.A.; Khayet, M.; Ali, Z.B.; Hilal, N. Reduction of nanofiltration membrane fouling by UV-initiated graft polymerization technique. J. Memb. Sci. 2010, 355, 133–141. [Google Scholar] [CrossRef]
- Tao, G.; Viswanath, B.; Kekre, K.; Lee, L.Y.; Ng, H.Y.; Ong, S.L.; Seah, H. RO brine treatment and recovery by biological activated carbon and capacitive deionization process. Water Sci. Technol. 2011, 64, 77–82. [Google Scholar] [CrossRef]
- Chen, L.; Wang, C.; Liu, S.; Hu, Q.; Zhu, L.; Cao, C. Investigation of the long-term desalination performance of membrane capacitive deionization at the presence of organic foulants. Chemosphere 2017, 193, 989–997. [Google Scholar] [CrossRef]
- Yu, J.; Qin, J.; Kekre, K.A.; Viswanath, B.; Tao, G.; Seah, H. Impact of operating conditions on performance of capacitive deionisation for reverse osmosis brine recovery Jing Yu, Jianjun Qin, Kiran A. Kekre, Balakrishnan Viswanath, Guihe Tao. J. Water Reuse Desalin. 2014, 4, 59–64. [Google Scholar] [CrossRef]
- Horseman, T.; Su, C.; Christie, K.S.S.; Lin, S. Highly effective scaling mitigation in membrane distillation using a superhydrophobic membrane with gas purging. Environ. Sci. Technol. Lett. 2019, 6, 423–429. [Google Scholar] [CrossRef]
- Jiang, S.; Li, Y.; Ladewig, B.P. Science of the Total Environment A review of reverse osmosis membrane fouling and control strategies. Sci. Total Environ. 2017, 595, 567–583. [Google Scholar] [CrossRef]
- Liu, E.; Lee, L.Y.; Ong, S.L.; Ng, H.Y. Treatment of industrial brine using Capacitive Deionization (CDI) towards zero liquid discharge–Challenges and optimization. Water Res. 2020, 183, 116059. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Zheng, R.; Liu, Y.; He, H.; Yuan, X.; Ji, Y.; Li, D.; Yin, H.; Zhang, Y.; Li, X.; et al. Slippery for scaling resistance in membrane distillation: A novel porous micropillared superhydrophobic surface. Water Res. 2019, 155, 152–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Julian, H.; Julian, H.; Ye, Y.; Li, H.; Chen, V. Scaling Mitigation in Submerged Vacuum Membrane Distillation and Crystallization (VMDC) with Periodic Air-backwash Scaling mitigation in submerged vacuum membrane distillation and crystallization (VMDC) with periodic air-backwash. J. Memb. Sci. 2017, 547, 19–33. [Google Scholar] [CrossRef]
- Xiao, Z.; Li, Z.; Guo, H.; Liu, Y.; Wang, Y.; Yin, H. Scaling mitigation in membrane distillation: From superhydrophobic to slippery. Desalination 2019, 466, 36–43. [Google Scholar] [CrossRef]
- Deshmukh, A.; Boo, C.; Karanikola, V.; Lin, S.; Straub, A.P.; Tong, T.; Warsinger, D.M.; Elimelech, M. Membrane distillation at the water-energy nexus: Limits, opportunities, and challenges. Energy Environ. Sci. 2018, 11, 1177–1196. [Google Scholar] [CrossRef]
- Laurijssen, J.; Faaij, A.; Worrell, E. Energy conversion strategies in the European paper industry—A case study in three countries. Appl. Energy 2012, 98, 102–113. [Google Scholar] [CrossRef]
- Neelis, M.; Ramirez-Ramirez, A.; Patel, M.; Farla, J.; Boonekamp, P.; Blok, K. Energy efficiency developments in the Dutch energy-intensive manufacturing industry, 1980–2003. Energy Policy 2007, 35, 6112–6131. [Google Scholar] [CrossRef] [Green Version]
- González, D.; Amigo, J.; Suárez, F. Membrane distillation: Perspectives for sustainable and improved desalination. Renew. Sustain. Energy Rev. 2017, 80, 238–259. [Google Scholar] [CrossRef]
- Xu, J.; Singh, Y.B.; Amy, G.L.; Ghaffour, N. Effect of operating parameters and membrane characteristics on air gap membrane distillation performance for the treatment of highly saline water. J. Memb. Sci. 2016, 512, 73–82. [Google Scholar] [CrossRef] [Green Version]
- Minier-matar, J.; Hussain, A.; Janson, A.; Benyahia, F.; Adham, S. Field evaluation of membrane distillation technologies for desalination of highly saline brines. Desalination 2014, 351, 101–108. [Google Scholar] [CrossRef]
- Abdelrazeq, H.; Khraisheh, M.; Al Momani, F.; McLeskey, J.T.; Hassan, M.K.; Gad-el-Hak, M.; Tafreshi, H.V. Performance of electrospun polystyrene membranes in synthetic produced industrial water using direct-contact membrane distillation. Desalination 2020, 493, 114663. [Google Scholar] [CrossRef]
- Chou, S.; Torres, J.; Li, X.; Fane, A.G.; Fang, W.; Tang, C.Y.; Chaitra, G.; Hu, X.; Shi, L.; Wang, R. Nature gives the best solution for desalination: Aquaporin-based hollow fiber composite membrane with superior performance. J. Memb. Sci. 2015, 494, 68–77. [Google Scholar] [CrossRef]
- Field, R.W.; Pearce, G.K. Critical, sustainable and threshold fluxes for membrane filtration with water industry applications. Adv. Colloid Interface Sci. 2011, 164, 38–44. [Google Scholar] [CrossRef]
- Field, R.W.; Wu, D.; Howell, J.A.; Gupta, B.B. Critical flux concept for microfiltration fouling. J. Memb. Sci. 1995, 100, 259–272. [Google Scholar] [CrossRef]
- Abdel-Karim, A.; Leaper, S.; Alberto, M.; Vijayaraghavan, A.; Fan, X.; Holmes, S.M.; Souaya, E.R.; Badawy, M.I.; Gorgojo, P. High flux and fouling resistant flat sheet polyethersulfone membranes incorporated with graphene oxide for ultrafiltration applications. Chem. Eng. J. 2018, 334, 789–799. [Google Scholar] [CrossRef] [Green Version]
- Mansour, S.; Giwa, A.; Hasan, S.W. Novel graphene nanoplatelets-coated polyethylene membrane for the treatment of reject brine by pilot-scale direct contact membrane distillation: An optimization study. Desalination 2018, 441, 9–20. [Google Scholar] [CrossRef]
- Phattaranawik, J.; Jiraratananon, R.; Fane, A.G. Effect of pore size distribution and air flux on mass transport in direct contact membrane distillation. J. Memb. Sci. 2003, 215, 75–85. [Google Scholar] [CrossRef]
- Khayet, M.; Mengual, J.I.; Matsuura, T. Porous hydrophobic/hydrophilic composite membranes: Application in desalination using direct contact membrane distillation. J. Memb. Sci. 2005, 252, 101–113. [Google Scholar] [CrossRef]
- Khayet, M.; Matsuura, T.; Mengual, J.I.; Qtaishat, M. Design of novel direct contact membrane distillation membranes. Desalination 2006, 192, 105–111. [Google Scholar] [CrossRef]
- Li, G.P.; Zhang, L.Z. Investigation of a solar energy driven and hollow fiber membrane-based humidification-dehumidification desalination system. Appl. Energy 2016, 177, 393–408. [Google Scholar] [CrossRef]
- Luo, A.; Lior, N. Critical review of membrane distillation performance criteria. Desalin. Water Treat. 2016, 57, 20093–20140. [Google Scholar] [CrossRef]
- Meng, S.; Ye, Y.; Mansouri, J.; Chen, V. Fouling and crystallisation behaviour of superhydrophobic nano-composite PVDF membranes in direct contact membrane distillation. J. Memb. Sci. 2014, 463, 102–112. [Google Scholar] [CrossRef]
- Alklaibi, A.M.; Lior, N. Membrane-distillation desalination: Status and potential. Desalination 2004, 171, 111–131. [Google Scholar] [CrossRef]
- Abdelatty, R.; Khan, A.; Yusuf, M.; Alashraf, A.; Shakoor, R.A. Effect of silicon nitride and graphene nanoplatelets on the properties of aluminum metal matrix composites. Materials 2021, 14, 1898. [Google Scholar] [CrossRef]
- Alsawaftah, N.; Abuwatfa, W.; Darwish, N.; Husseini, G. A Comprehensive Review on Membrane Fouling: Mathematical Modelling, Prediction, Diagnosis, and Mitigation. Water 2021, 13, 1327. [Google Scholar] [CrossRef]
- Gryta, M.; Markowska, A.; Karakulski, K. The influence of polypropylene degradation on the membrane wettability during membrane distillation. J. Ofmembrane Sci. J. 2009, 326, 493–502. [Google Scholar] [CrossRef]
- Chen, L.; Xu, P.; Wang, H. Interplay of the factors affecting water flux and salt rejection in membrane distillation: A state-of-the-art critical review. Water 2020, 12, 2841. [Google Scholar] [CrossRef]
- Vigneswaran, S.; Kwon, D. Effect of Ionic Strength and Permeate Flux on Membrane Fouling: Analysis of Forces acting on Particle Deposit and Cake Formation. KSCE J. Civ. Eng. 2015, 19, 1604–1611. [Google Scholar] [CrossRef]
- Zuo, J.; Bonyadi, S.; Chung, T.S. Exploring the potential of commercial polyethylene membranes for desalination by membrane distillation. J. Memb. Sci. 2016, 497, 239–247. [Google Scholar] [CrossRef]
- McGaughey, A.L.; Gustafson, R.D.; Childress, A.E. Effect of long-term operation on membrane surface characteristics and performance in membrane distillation. J. Memb. Sci. 2017, 543, 143–150. [Google Scholar] [CrossRef]
- Ding, Z.; Liu, L.; Liu, Z.; Ma, R. Fouling resistance in concentrating TCM extract by direct contact membrane distillation. J. Memb. Sci. 2010, 362, 317–325. [Google Scholar] [CrossRef]
- Wang, Z.; Lin, S. Membrane fouling and wetting in membrane distillation and their mitigation by novel membranes with special wettability. Water Res. 2017, 112, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Khayet, M.; Mengual, J.I. Effect of salt concentration during the treatment of humic acid solutions by membrane distillation. Desalination 2004, 168, 373–381. [Google Scholar] [CrossRef]
- Shen, X.; Liu, J.; Feng, X.; Zhao, Y.; Chen, L. Preliminary investigation on hemocompatibility of poly (vinylidene fluoride) membrane grafted with acryloylmorpholine via ATRP. J. Biomed. Mater. Res. A 2015, 103, 683–692. [Google Scholar] [CrossRef]
- Cho, D.; Kwon, G. Influence of humic acid on the long-term performance of direct contact membrane distillation. Energy Environ. 2019, 30, 109–120. [Google Scholar] [CrossRef]
- Miao, R.; Wu, Y.; Wang, P.; Wu, G.; Wang, L.; Li, X.; Wang, J.; Lv, Y.; Liu, T. New insights into the humic acid fouling mechanism of ultrafiltration membranes for different Ca2+ dosage ranges: Results from micro- and macro-level analyses. Water Sci. Technol. 2018, 77, 2265–2273. [Google Scholar] [CrossRef]
- Srisurichan, S.; Jiraratananon, R.; Fane, A.G. Mass transfer mechanisms and transport resistances in direct contact membrane distillation process. J. Memb. Sci. 2006, 277, 186–194. [Google Scholar] [CrossRef]
- Shen, X.; Xie, T.; Wang, J.; Wang, F. Improved fouling resistance of poly (vinylidene fluoride) membrane modified with poly (acryloyl morpholine) -based amphiphilic copolymer. Colloid Polym. Sci. 2017, 295, 1211–1221. [Google Scholar] [CrossRef]
- Shen, X.; Xie, T.; Wang, J.; Liu, P.; Wang, F. An anti-fouling poly(vinylidene fluoride) hybrid membrane blended with functionalized ZrO2 nanoparticles for efficient oil/water separation. RSC Adv. 2017, 7, 5262–5271. [Google Scholar] [CrossRef] [Green Version]
- Alkhouzaam, A.; Qiblawey, H. Novel polysulfone ultrafiltration membranes incorporating polydopamine functionalized graphene oxide with enhanced flux and fouling resistance. J. Memb. Sci. 2020, 620, 118900. [Google Scholar] [CrossRef]
- Fane, A.G. (Tony). A grand challenge for membrane desalination: More water, less carbon. Desalination 2018, 426, 155–163. [Google Scholar] [CrossRef]
- Simionescu, L.N.; Gherghina, Ș.C.; Sheikha, Z.; Tawil, H. Does water, waste, and energy consumption influence firm performance? Panel data evidence from S &P 500 information technology sector. Int. J. Environ. Res. Public Health 2020, 17, 5206. [Google Scholar] [CrossRef]
- Duong, H.C.; Cooper, P.; Nelemans, B.; Cath, T.Y.; Nghiem, L.D. Evaluating energy consumption of air gap membrane distillation for seawater desalination at pilot scale level. Sep. Purif. Technol. 2016, 166, 55–62. [Google Scholar] [CrossRef] [Green Version]
- Al-Obaidani, S.; Curcio, E.; Macedonio, F.; Di Profio, G.; Al-Hinai, H.; Drioli, E. Potential of membrane distillation in seawater desalination: Thermal efficiency, sensitivity study and cost estimation. J. Memb. Sci. 2008, 323, 85–98. [Google Scholar] [CrossRef]
- Lin, S.; Yip, N.Y.; Elimelech, M. Direct contact membrane distillation with heat recovery: Thermodynamic insights from module scale modeling. J. Memb. Sci. 2014, 453, 498–515. [Google Scholar] [CrossRef]
- Duong, H.C. Membrane Distillation for Strategic Desalination Applications; University of Wollongong: Wollongong, Australia, 2017. [Google Scholar]
- Laurijssen, J.; Faaij, A.; Worrell, E. Benchmarking energy use in the paper industry: A benchmarking study on process unit level. Energy Effic. 2013, 6, 49–63. [Google Scholar] [CrossRef] [Green Version]
- Criscuoli, A.; Concetta, M.; Drioli, E. Evaluation of energy requirements in membrane distillation. Chem. Eng. Process. 2008, 47, 1098–1105. [Google Scholar] [CrossRef]
- Gordon, A. Evaluation of hollow fiber - based direct contact and vacuum membrane distillation systems using aspen process simulation. J. Memb. Sci. 2014, 464, 127–139. [Google Scholar]
- Dow, N.; Gray, S.; Li, J.; Zhang, J.; Ostarcevic, E.; Liubinas, A.; Atherton, P.; Roeszler, G.; Gibbs, A.; Duke, M. Pilot trial of membrane distillation driven by low grade waste heat: Membrane fouling and energy assessment. Desalination 2016, 391, 30–42. [Google Scholar] [CrossRef]
- Lawrence, A.; Thollander, P.; Andrei, M.; Karlsson, M. Specific energy consumption/use (SEC) in energy management for improving energy efficiency in industry: Meaning, usage and differences. Energies 2019, 12, 247. [Google Scholar] [CrossRef] [Green Version]
- Suhr, M.; Klein, G.; Kourti, I.; Rodrigo Gonzalo, M.; Giner Santonja, G.; Roudier, S.; Delgado Sancho, L. Best Available Techniques (BAT) Reference Document for the Production of Pulp, Paper and Board; Publications Office of the European Union, European Commision: Luxembourg, 2015; ISBN 9789279481673. [Google Scholar]
- Jantaporn, W.; Ali, A.; Aimar, P. Specific energy requirement of direct contact membrane distillation. Chem. Eng. Res. Des. 2017, 128, 15–26. [Google Scholar] [CrossRef] [Green Version]
- Rauf, A.; Li, H.; Ullah, S.; Meng, L.; Wang, B.; Wang, M. Statistical study about the influence of particle precipitation on mesosphere summer echoes in polar latitudes during July 2013. Earth Planets Sp. 2018, 70, 108. [Google Scholar] [CrossRef] [Green Version]







| Chemicals | Feed Composition [g/L] | |
|---|---|---|
| C1 | C2 | |
| Na | 23,876 | 11,938 |
| Mg | 2520 | 1260 |
| Ca | 765 | 382 |
| K | 793 | 396 |
| Sr | 11 | 5 |
| B | 9 | 4 |
| Cl | 42,682 | 21,341 |
| SO4 | 4229 | 2114 |
| HCO3 | 726 | 363 |
| Br | 67 | 33 |
| Membrane | Feed Type | Temperature Inlets [°C] | Duration of MD [hours] | PWP [LMH] | SEC [kWh/m3] | Plant Capacity [m3/h] | Ref | |
|---|---|---|---|---|---|---|---|---|
| Feed | Permeate | |||||||
| PP | Distilled Water | 59.2 | 14.3 | 3 | 56.2 | 3550–4580 | - | [63] |
| PVDF | Simulated RO Brine | 80 | 30 | - | 10.80–12.6 | 130–1700 | - | [64] |
| PTFE | Wastewater | 60 | 18–21 | 840–1800 | 2–5 | 1500 | 3.85 | [65] |
| PE | Synthetic Brine | 70 | 20 | 20 | 122.2 | 107.1 | 207.31 | This work |
| 100 | 12.6 | 90.8 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelrazeq, H.; Khraisheh, M.; Hassan, M.K. Long-Term Treatment of Highly Saline Brine in a Direct Contact Membrane Distillation (DCMD) Pilot Unit Using Polyethylene Membranes. Membranes 2022, 12, 424. https://doi.org/10.3390/membranes12040424
Abdelrazeq H, Khraisheh M, Hassan MK. Long-Term Treatment of Highly Saline Brine in a Direct Contact Membrane Distillation (DCMD) Pilot Unit Using Polyethylene Membranes. Membranes. 2022; 12(4):424. https://doi.org/10.3390/membranes12040424
Chicago/Turabian StyleAbdelrazeq, Haneen, Majeda Khraisheh, and Mohammad K. Hassan. 2022. "Long-Term Treatment of Highly Saline Brine in a Direct Contact Membrane Distillation (DCMD) Pilot Unit Using Polyethylene Membranes" Membranes 12, no. 4: 424. https://doi.org/10.3390/membranes12040424
APA StyleAbdelrazeq, H., Khraisheh, M., & Hassan, M. K. (2022). Long-Term Treatment of Highly Saline Brine in a Direct Contact Membrane Distillation (DCMD) Pilot Unit Using Polyethylene Membranes. Membranes, 12(4), 424. https://doi.org/10.3390/membranes12040424