Novel Sandwich-Structured Hollow Fiber Membrane for High-Efficiency Membrane Distillation and Scale-Up for Pilot Validation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Polymer Characterization
2.3. Fabrication of Hollow Fiber Membranes
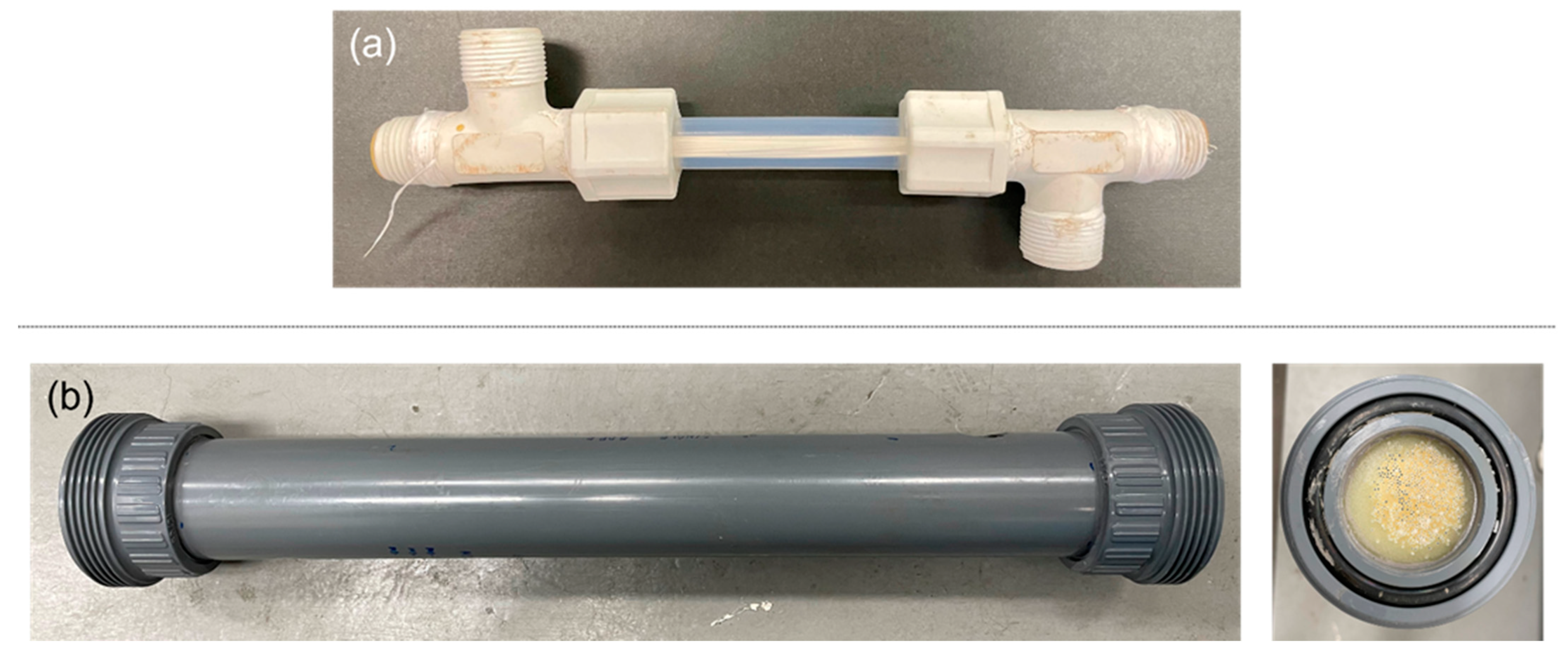
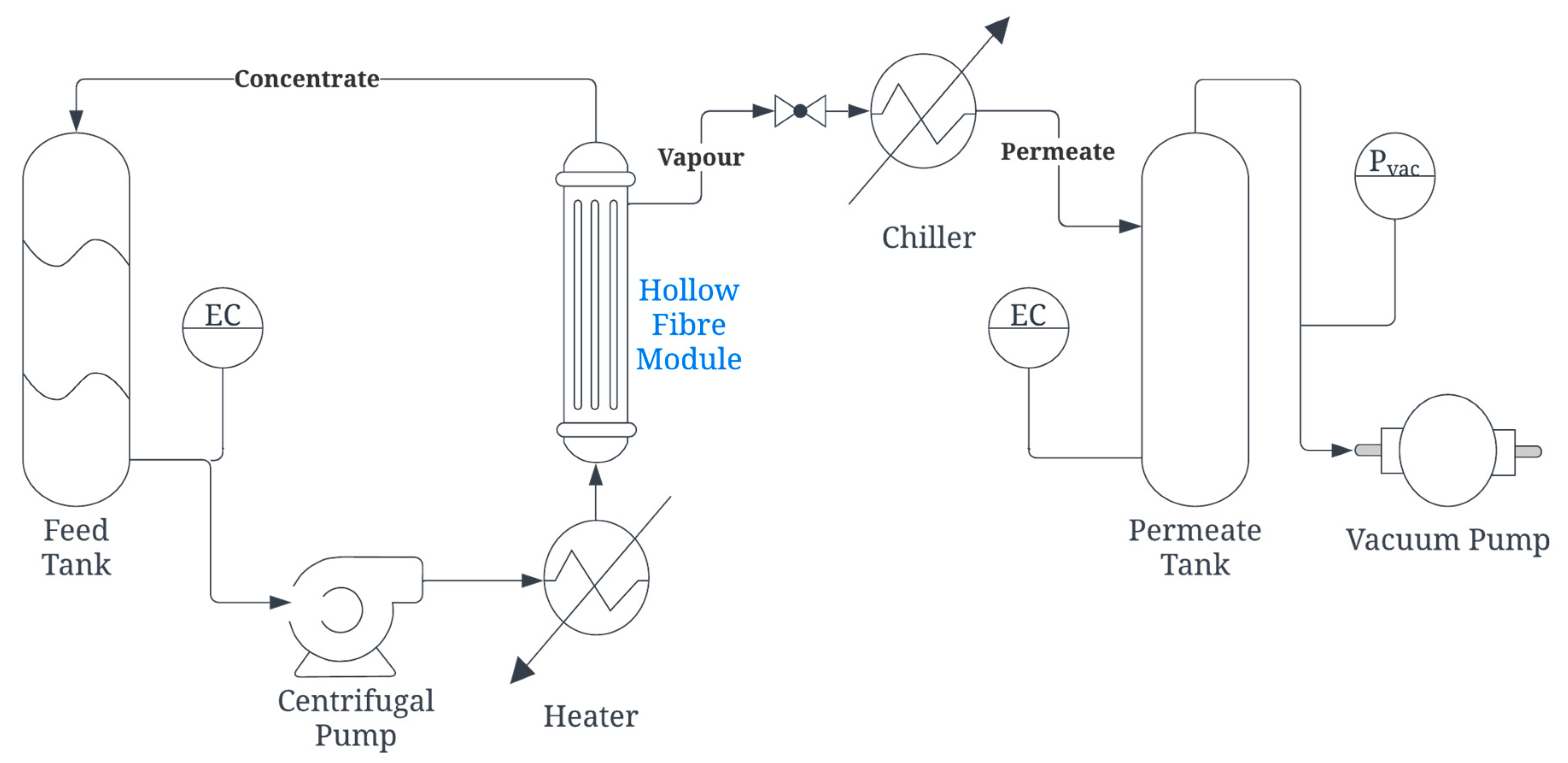
2.4. Membrane Module Testing
3. Results and Discussion
3.1. Characterization of Polymers and Dopes
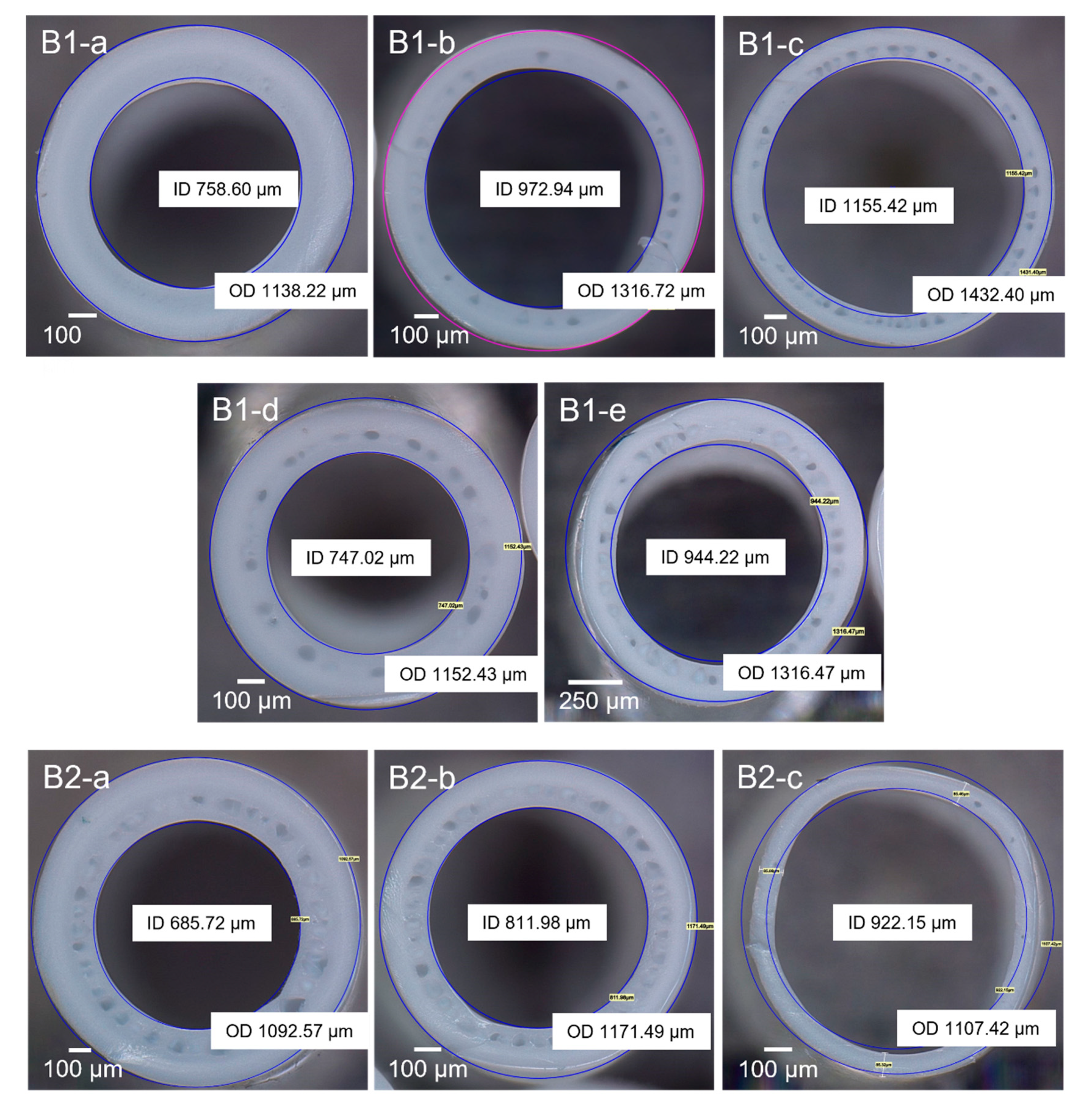
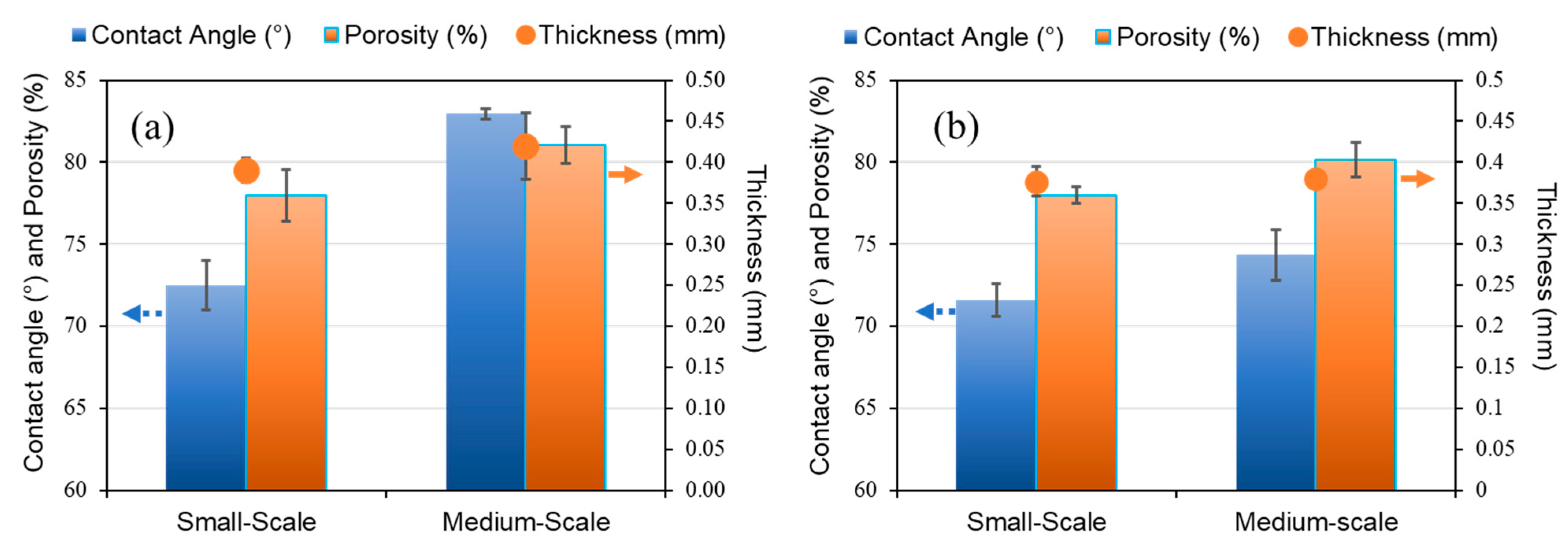
3.2. Characterization of PVDF Hollow Fiber Membranes
3.3. VMD Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Escobar, I.C. Chapter 14 Conclusion: A Summary of Challenges still Facing Desalination and Water Reuse. In Sustainable Water for the Future: Water Recycling versus Desalination; Escobar, I.C., Schafer, A.I., Eds.; Elsevier: Amsterdam, The Netherlands, 2010; Volume 2, pp. 389–397. [Google Scholar]
- Christidis, N.; Scott, P.A. The influence of anthropogenic climate change on wet and dry summers in Europe. Sci. Bull. 2021, 66, 813–823. [Google Scholar] [CrossRef]
- World Health Organization; UNICEF. Progress on Drinking Water, Sanitation and Hygiene: 2017 Update and SDG Baselines; World Health Organization: Geneva, Switzerland, 2017; ISBN 9789241512893. [Google Scholar]
- Ahmed, F.E.; Khalil, A.; Hilal, N. Emerging desalination technologies: Current status, challenges and future trends. Desalination 2021, 517, 115183. [Google Scholar] [CrossRef]
- Zapata-Sierra, A.; Cascajares, M.; Alcayde, A.; Manzano-Augliaro, F. Worldwide research trends on desalination. Desalination 2022, 519, 115305. [Google Scholar] [CrossRef]
- Li, B.-J.; Choi, S.-M.; Cho, S.-H.; Master, G.-R.L.; Park, C.-D. Design and performance analysis of vertical multi-effect difusion solar distiller: A review. Desalination 2022, 527, 115572. [Google Scholar] [CrossRef]
- Jhon Jairo Feria-Díaz, J.J.; López-Méndez, M.C.; Rodríguez-Miranda, J.P.; Sandoval-Herazo, L.C.; Correa-Mahecha, F. Commercial Thermal Technologies for Desalination of Water from Renewable Energies: A State of the Art Review. Processes 2021, 9, 262. [Google Scholar] [CrossRef]
- Curto, D.; Franzitta, V.; Guercio, A. A review of the water desalination technologies. Appl. Sci. 2021, 11, 670. [Google Scholar] [CrossRef]
- Lim, Y.J.; Goh, K.; Kurihara, M.; Wang, R. Seawater desalination by reverse osmosis: Current development and future challenges in membrane fabrication—A review. J. Membr. Sci. 2021, 629, 119292. [Google Scholar] [CrossRef]
- Edwie, F.; Chung, T.S. Development of simultaneous membrane distillation-crystallization (SMDC) technology for treatment of saturated brine. Chem. Eng. Sci. 2013, 98, 160–172. [Google Scholar] [CrossRef]
- Choudhury, M.R.; Anwar, N.; Jassby, D.; Rahaman, M.S. Fouling and wetting in the membrane distillation driven wastewater reclamation process—A review. Adv. Colloid Interface Sci. 2019, 269, 370–399. [Google Scholar] [CrossRef]
- Yan, Z.; Jiang, Y.; Liu, L.; Li, Z.; Chen, X.; Xia, M.; Fan, G.; Ding, A. Membrane distillation for wastewater treatment: A mini Review. Water 2021, 13, 3480. [Google Scholar] [CrossRef]
- Deshmukh, A.; Boo, C.; Karanikola, V.; Lin, S.; Straub, A.P.; Tong, T.; Warsinger, D.M.; Elimelech, M. Membrane distillation at the water-energy nexus: Limits, opportunities, and challenges. Energy Environ. Sci. 2018, 11, 1177–1196. [Google Scholar] [CrossRef]
- Ibrar, I.; Yadav, S.; Naji, O.; Alanezi, A.A.; Ghaffour, N.; Deon, S.; Subbiah, S.; Altaee, A. Development in forward Osmosis-Membrane distillation hybrid system for wastewater treatment. Sep. Purif. Technol. 2022, 286, 120498. [Google Scholar] [CrossRef]
- Meng, L.; Mansouri, J.; Li, X.; Liang, J.; Huang, M.; Lv, Y.; Wang, Z.; Chen, V. Omniphobic membrane via bioinspired silicification for the treatment of RO concentrate by membrane distillation. J. Membr. Sci. 2022, 647, 120267. [Google Scholar] [CrossRef]
- Kharraz, J.A.; Khanzada, N.K.; Farid, M.U.; Kim, J.; Jeong, S.; An, A.K. Membrane distillation bioreactor (MDBR) for wastewater treatment, water reuse, and resource recovery: A review. J. Water Proc. Eng. 2002, 47, 102687. [Google Scholar] [CrossRef]
- Parani, S.; Oluwafemi, O.S. Membrane Distillation: Recent Configurations, Membrane Surface Engineering, and Applications. Membranes 2021, 11, 934. [Google Scholar] [CrossRef]
- Abdelkareem, M.A.; El Haj Assad, M.; Sayed, E.T.; Soudan, B. Recent progress in the use of renewable energy sources to power water desalination plants. Desalination 2018, 435, 97–113. [Google Scholar] [CrossRef]
- Zuo, J.; Chung, T.S. PVDF hollow fibers with novel sandwich structure and superior wetting resistance for vacuum membrane distillation. Desalination 2017, 417, 94–101. [Google Scholar] [CrossRef]
- Zhong, W.; Guo, L.; Ji, C.; Dong, G.; Li, S. Membrane distillation for zero liquid discharge during treatment of wastewater from the industry of traditional Chinese medicine: A review. Environ. Chem. Lett. 2021, 19, 2317–2330. [Google Scholar] [CrossRef]
- Julian, H.; Nurgirisia, N.; Sutrisna, P.D.; Wenten, I.G. Advances in seawater membrane distillation (SWMD) towards stand-alone zero liquid discharge (ZLD) desalination. Rev. Chem. Eng. 2021, 000010151520200073. [Google Scholar] [CrossRef]
- Yadav, A.; Labhasetwar, P.K.; Shahi, V.K. Membrane distillation crystallization technology for zero liquid discharge and resource recovery: Opportunities, challenges and futuristic perspectives. Sci. Total Environ. 2022, 806, 150692. [Google Scholar] [CrossRef]
- Zuo, J.; Bonyadi, S.; Chung, T.S. Exploring the potential of commercial polyethylene membranes for desalination by membrane distillation. J. Membr. Sci. 2016, 497, 239–247. [Google Scholar] [CrossRef]
- Francis, L.; Ahmed, F.E.; Hilal, N. Advances in Membrane Distillation Module Configurations. Membranes 2022, 12, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Pagliero, M.; Khayet, M.; García-Payo, C.; García-Fernández, L. Hollow fibre polymeric membranes for desalination by membrane distillation technology: A review of different morphological structures and key strategic improvements. Desalination 2021, 516, 115235–115263. [Google Scholar] [CrossRef]
- Camacho, L.; Dumée, L.; Zhang, J.; Li, J.; Duke, M.; Gomez, J.; Gray, S. Advances in Membrane Distillation for Water Desalination and Purification Applications. Water 2013, 5, 94–196. [Google Scholar] [CrossRef] [Green Version]
- Chiam, C.K.; Sarbatly, R. Vacuum membrane distillation processes for aqueous solution treatment-A review. Chem. Eng. Process. Process Intensif. 2013, 74, 27–54. [Google Scholar] [CrossRef]
- Zuo, J.; Chung, T.S.; O’Brien, G.S.; Kosar, W. Hydrophobic/hydrophilic PVDF/Ultem® dual-layer hollow fiber membranes with enhanced mechanical properties for vacuum membrane distillation. J. Membr. Sci. 2017, 523, 103–110. [Google Scholar] [CrossRef]
- Abu-Zeid, M.A.E.R.; Zhang, Y.; Dong, H.; Zhang, L.; Chen, H.L.; Hou, L. A comprehensive review of vacuum membrane distillation technique. Desalination 2015, 356, 1–14. [Google Scholar] [CrossRef]
- Sorour, M.H.; Hani, H.A.; Shaalan, H.F.; El-Toukhy, M. Fabricatio nand characterization of hydrophobic PVDF-based hollow fiber membranes for vacuum membrane distillation of seawater and desalination brine. Egypt. J. Chem. 2021, 64, 4889–4899. [Google Scholar] [CrossRef]
- Albloushi, A.; Giwa, A.; Mukherjee, D.; Calabro, V.; Cassano, A.; Chakraborty, S.; Hasan, S.W. Chapter 7—Renewable Energy-Powered Membrane Systems for Water Desalination. In Current Trends and Future Developments on (Bio-) Membranes; Basile, A., Cassano, A., Figoli, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 153–177. ISBN 978-0-12-813545-7. [Google Scholar]
- Baghel, R.; Upadhyaya, S.; Singh, K.; Chaurasia, S.P.; Gupta, A.B.; Dohare, R.K. A review on membrane applications and transport mechanisms in vacuum membrane distillation. Rev. Chem. Eng. 2017, 34, 73–106. [Google Scholar] [CrossRef]
- Wang, P.; Teoh, M.M.; Chung, T.S. Morphological architecture of dual-layer hollow fiber for membrane distillation with higher desalination performance. Water Res. 2011, 45, 5489–5500. [Google Scholar] [CrossRef]
- Peng, N.; Chung, T.S.; Wang, K.Y. Macrovoid evolution and critical factors to form macrovoid-free hollow fiber membranes. J. Membr. Sci. 2008, 318, 363–372. [Google Scholar] [CrossRef]
- Sukitpaneenit, P.; Chung, T.S. Molecular elucidation of morphology and mechanical properties of PVDF hollow fiber membranes from aspects of phase inversion, crystallization and rheology. J. Membr. Sci. 2009, 340, 192–205. [Google Scholar] [CrossRef]
- Fontananova, E.; Jansen, J.C.; Cristiano, A.; Curcio, E.; Drioli, E. Effect of additives in the casting solution on the formation of PVDF membranes. Desalination 2006, 192, 190–197. [Google Scholar] [CrossRef]
- Zuo, J.; Chung, T.S.N. A Hollow Fiber Membrane. WO2018080398A1, 3 May 2018. [Google Scholar]
- Chen, Z.; Rana, D.; Matsuura, T.; Meng, D.; Lan, C.Q. Study on structure and vacuum membrane distillation performance of PVDF membranes: II. Influence of molecular weight. Chem. Eng. J. 2015, 276, 174–184. [Google Scholar] [CrossRef]
- Liu, Z.; Maréchal, P.; Jérôme, R. Melting and crystallization of poly(vinylidene fluoride) blended with polyamide 6. Polymer 1997, 38, 5149–5153. [Google Scholar] [CrossRef] [Green Version]
- Marega, C.; Marigo, A. Influence of annealing and chain defects on the melting behaviour of poly(vinylidene fluoride). Eur. Polym. J. 2003, 39, 1713–1720. [Google Scholar] [CrossRef]
- Ji, D.; Xiao, C.; Chen, K.; Zhou, F.; Gao, Y.; Zhang, T.; Ling, H. Solvent-free fabrication of PVDF hollow fiber membranes with controlled pore structure via melt spinning and stretching. J. Membr. Sci. 2021, 621, 118593. [Google Scholar] [CrossRef]
- Dikshit, A.K. Effect of solvent on thermal transitions and conductivity of poly(vinylidene fluoride) gel electrolytes. Polym. Plast. Technol. Mater. 2020, 59, 822–834. [Google Scholar] [CrossRef]
- Rosenberg, Y.; Siegmann, A.; Narkis, M.; Shkolnik, S. The sol/gel contribution to the behavior of γ-irradiated poly(vinylidene fluoride). J. Appl. Polym. Sci. 1991, 43, 535–541. [Google Scholar] [CrossRef]
- Silva, A.J.D.J.; Contreras, M.M.; Nascimento, C.R.; da Costa, M.F. Kinetics of thermal degradation and lifetime study of poly(vinylidene fluoride) (PVDF) subjected to bioethanol fuel accelerated aging. Heliyon 2020, 6, e04573. [Google Scholar] [CrossRef]
- Xu, B.; Hou, S.; Chu, M.; Cao, G.; Yang, Y. An activation-free method for preparing microporous carbon by the pyrolysis of poly(vinylidene fluoride). Carbon 2010, 48, 2812–2814. [Google Scholar] [CrossRef]
- Liu, S.-H.; Liu, M.; Xu, Z.-L.; Wei, Y.-M. A polyethersulfone–bisphenol sulfuric acid hollow fiber ultrafiltration membrane fabricated by a reverse thermally induced phase separation process. RSC Adv. 2018, 8, 7800–7809. [Google Scholar] [CrossRef] [Green Version]
- Sathiya, S.; Norlisa, H.; Syed, M.S. Effect of coagulation bath temperature during preparation of PES hollow fiber supported liquid membrane for acetic acid removal. Chem. Eng. Res. Bull. 2017, 19, 118–122. [Google Scholar]
- Xu, J.; Tang, Y.; Wang, Y.; Shan, B.; Yu, L.; Gao, C. Effect of Coagulation Bath Conditions on the Morphology and Performance of PSf Membrane Blended with a Capsaicin-Mimic Copolymer. J. Membr. Sci. 2014, 455, 121–130. [Google Scholar] [CrossRef]
- Blanco, F.J.; Sublet, J.; Nguyen, Q.T.; Schaetzel, P. Formation and Morphology Studies of Different Polysulfones-Based Membranes Made by Wet Phase Inversion Process. J. Membr. Sci. 2006, 283, 27–37. [Google Scholar] [CrossRef]
- Bildyukevich, A.V.; Plisko, T.V.; Usosky, V.V. The formation of polysulfone hollow fiber membranes by the free fall spinning method. Pet. Chem. 2016, 56, 379–400. [Google Scholar] [CrossRef]
- Bonyadi, S.; Chung, T.S.; Krantz, W.B. Investigation of corrugation phenomenon in the inner contour of hollow fibers during the non-solvent induced phase-separation process. J. Membr. Sci. 2007, 299, 200–210. [Google Scholar] [CrossRef]
- Li, G.; Kujawski, W.; Knozowska, K.; Kujawa, J. The Effects of PEI Hollow Fiber Substrate Characteristics on PDMS/PEI Hollow Fiber Membranes for CO2/N2 Separation. Membranes 2021, 11, 56. [Google Scholar] [CrossRef]
- Yang, X.; Wang, R.; Fane, A.G. Novel designs for improving the performance of hollow fiber membrane distillation modules. J. Membr. Sci. 2011, 384, 52–62. [Google Scholar] [CrossRef] [Green Version]
- Shin, Y.; Choi, J.; Park, Y.; Choi, Y.; Lee, S. Influence of operation conditions on the performance of pilot-scale vacuum membrane distillation (VMD). Desalin. Water Treat. 2017, 97, 1–7. [Google Scholar] [CrossRef]
- Han, F.; Bian, Y.; Zhang, G. Conductive heating vacuum membrane distillation for brine desalination: Study on operational conditions, temperature polarization and energy consumption. Desalination 2022, 531, 115726. [Google Scholar] [CrossRef]
- Anvari, A.; Yancheshme, A.A.; Kekre, K.M.; Ronen, A. State-of-the-art methods for overcoming temperature polarization in membrane distillation process: A review. J. Membr. Sci. 2020, 616, 118413. [Google Scholar] [CrossRef]

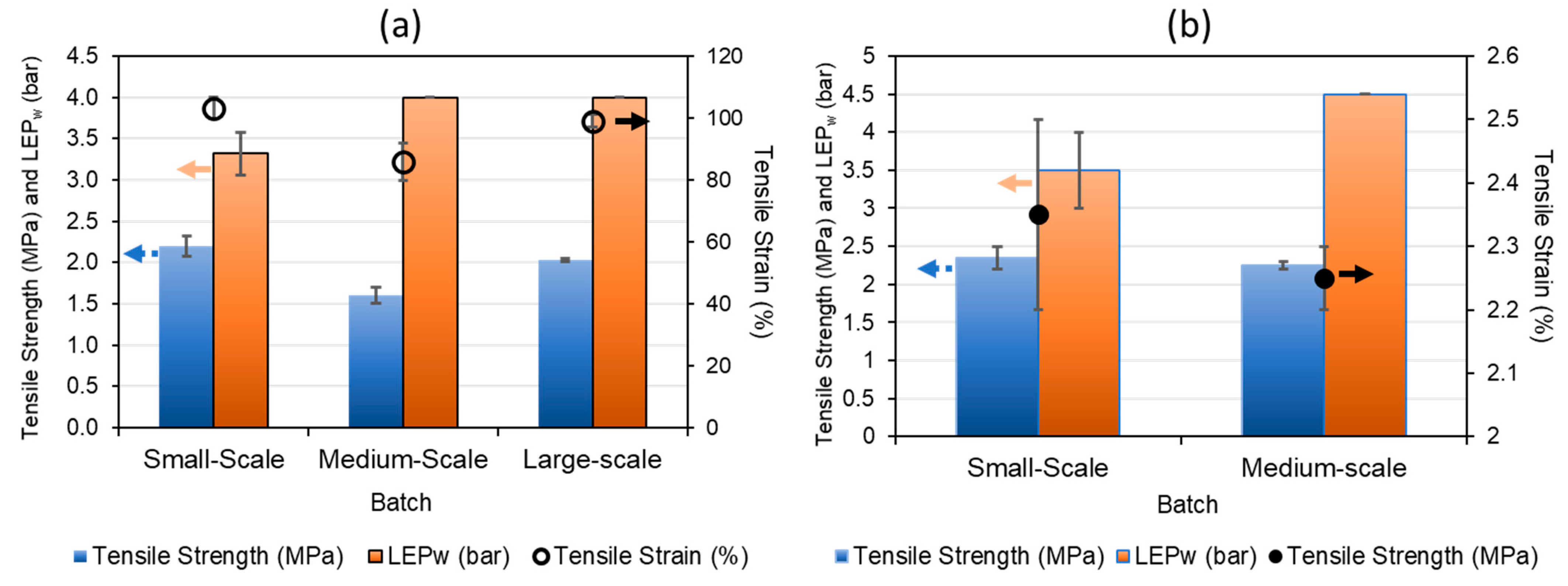
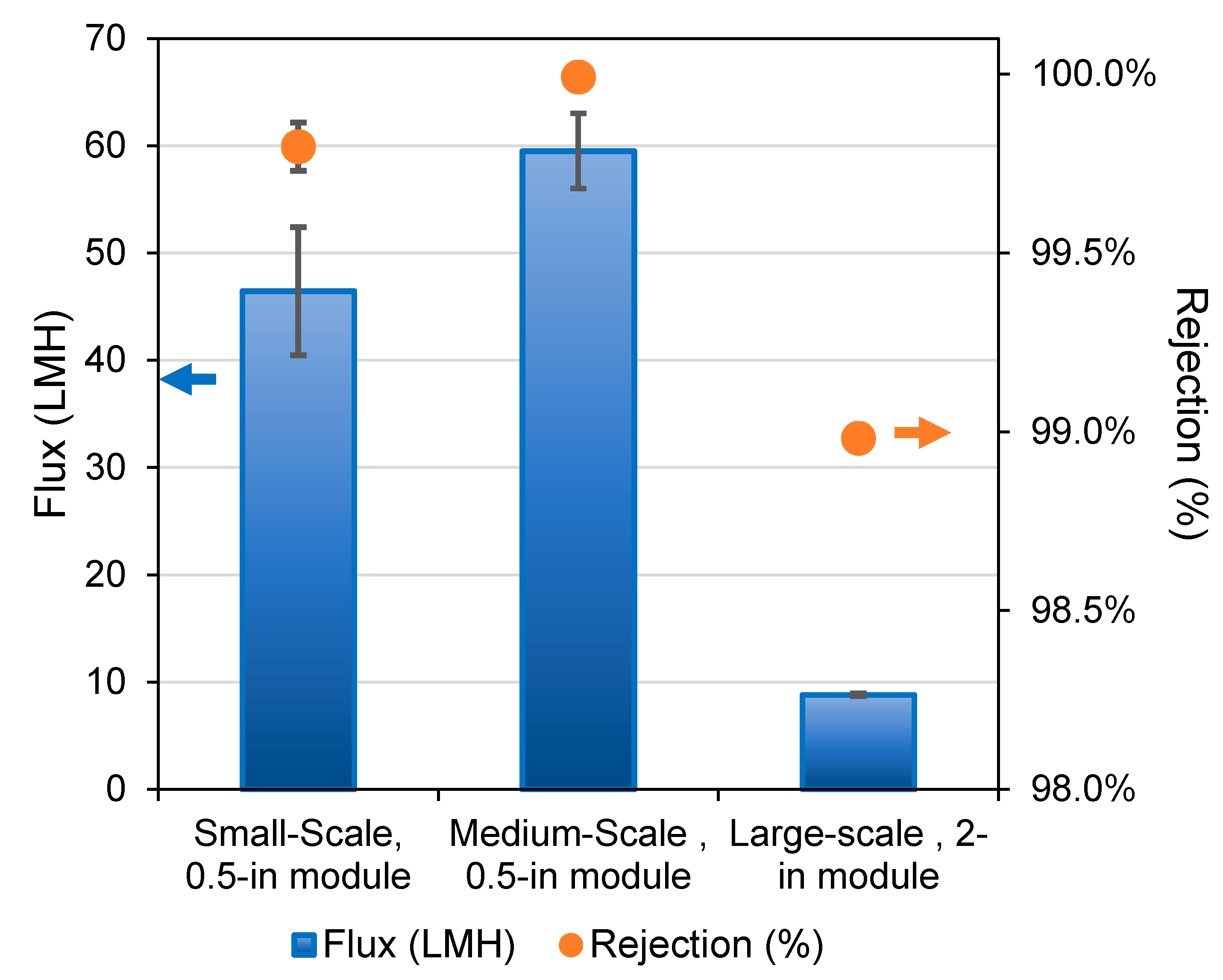
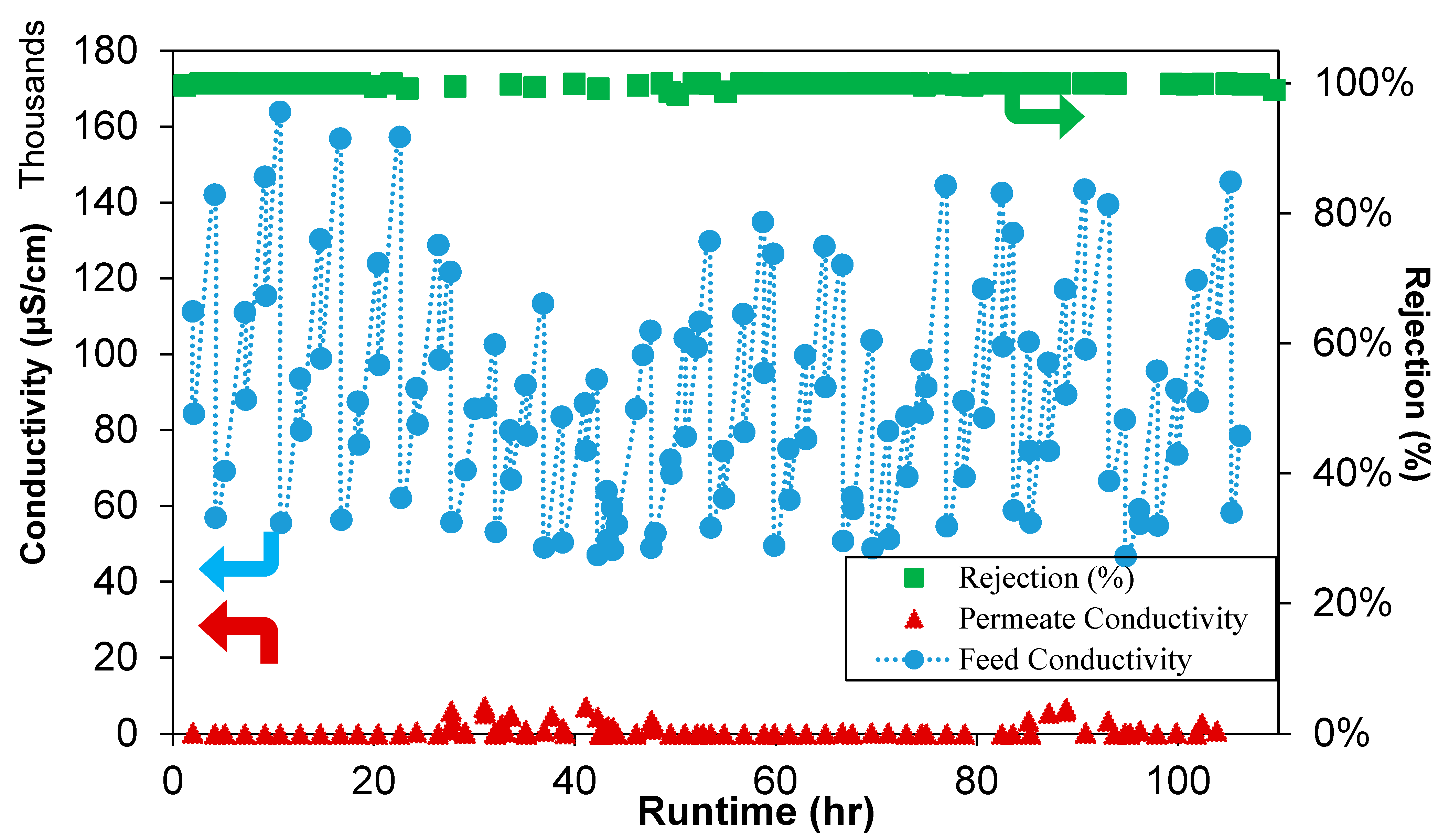
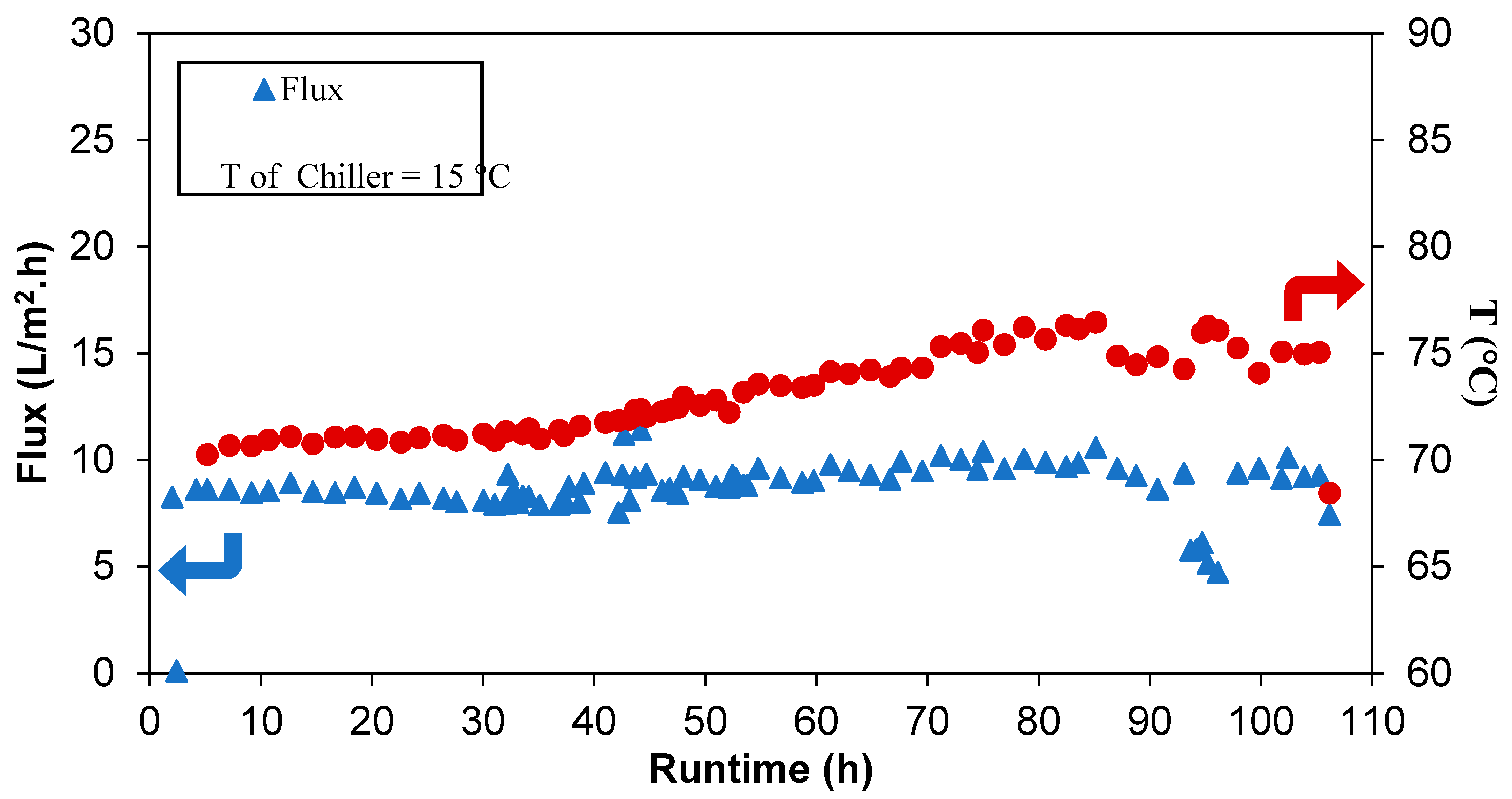
| Polymer | PVDF 1 | PVDF 2 |
|---|---|---|
| Melting point (°C) | 162.65 | 162.64 |
| Crystallization point (°C) | 127.68 | 126.81 |
| Max. thermal degradation (°C) | 472.49 | 472.66 |
| Melting enthalpy (J/g) | 36.08 | 33.92 |
| Crystallization enthalpy (J/g) | 41.41 | 36.64 |
| Dope viscosity (Pa·s) | 101.93 (@50.1 °C) | 167.27(@ 51.7 °C) |
| Batch Number | B1 | B2 | B3 | B4 | B5 | B6 | B7 | B8 Onwards |
|---|---|---|---|---|---|---|---|---|
| Dope (wt. %) | PVDF/LiCl/EG/NMP: 13/5/5/77 | |||||||
| Bore solution (wt. %) | NMP/Water: 50/50 | |||||||
| Scale-up (kg Dope) | 1.5 | 1.5 | 1.5 | 1.5 | 20 | 20 | 50 | 50 |
| Air gap (mm) | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 |
| PVDF source | 1 | 2 | 1 | 1 | 2 | 1 | 2 | 1 |
| Coagulation bath, tap water (°C) | RT and 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 |
| Line speed (m/min) | 3 | 9 | 3 | 9 | 9 | 9 | 9 | 9 |
| Dope flowrate (mL/min) | 4.5 | 13.5 | 4.5 | 13.5 | 13.5 | 13.5 | 13.5 | 13.5 |
| Bore flowrate (mL/min) | 1.5–4.5 | 4.5–9.0 | 1.5–3.0 | 4.5–6.8 | 4.5–6.8 | 4.5–6.8 | 4.5–6.8 | 4.5 |
| PVDF | Batch No | Bore Fluid Flowrate (mL/min) | Coagulation Bath Temperature (°C) | Outer Diameter (mm) | Inner Diameter (mm) | Contact Angle (°) |
|---|---|---|---|---|---|---|
| 1 | B1-a | 1.5 | ≈24 | 1.13 ± 0.01 | 0.73 ± 0.01 | 69.3 |
| 1 | B1-b | 3 | ≈24 | 1.29 ± 0.02 | 0.95 ± 0.01 | 77.2 |
| 1 | B1-c | 4.5 | ≈24 | 1.38 ± 0.01 | 1.09 ± 0.01 | 72.3 |
| 1 | B1-d | 1.5 | 38.3 | 1.12 ± 0.01 | 0.70 ± 0.00 | 66.6 |
| 1 | B1-e | 3 | 38.3 | 1.25 ± 0.03 | 0.89 ± 0.01 | 64.1 |
| 2 | B2-a | 4.5 | 38.6 | 1.07 ± 0.02 | 0.66 ± 0.03 | 70.6 |
| 2 | B2-b | 6.8 | 38.6 | 1.16 ± 0.01 | 0.79 ± 0.01 | 72.6 |
| 2 | B2-c | 9 | 38.6 | 1.25 ± 0.01 | 0.90 ± 0.01 | n/a |
| Testing Site | Lab-Scale Module | Pilot-Scale Module |
|---|---|---|
| Module (nominal inches) | 0.5 | 2 |
| VMD configuration | in-to-out | in-to-out |
| Number of fibers | 15 | 560 |
| Effective length (mm) | 120 | 370 |
| Effective membrane area (m2) | 0.0035–0.0051 | 0.456 |
| Packing density (%) | ≈13 † | 35 |
| Feed flowrate (L/min) | 0.5 | 8.5–9.5 |
| Feed temperature (°C) | 88 | ≥70 |
| Vacuum (bar) | −0.80 | −0.85 |
| Test duration (hr) | ≥1 | >100 |
| Feed concentration (g/L NaCl) | 35 | ≈35.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qua, M.S.; Zhao, Y.; Zhang, J.; Hernandez, S.; Paing, A.T.; Mottaiyan, K.; Zuo, J.; Dhalla, A.; Chung, T.-S.; Gudipati, C. Novel Sandwich-Structured Hollow Fiber Membrane for High-Efficiency Membrane Distillation and Scale-Up for Pilot Validation. Membranes 2022, 12, 423. https://doi.org/10.3390/membranes12040423
Qua MS, Zhao Y, Zhang J, Hernandez S, Paing AT, Mottaiyan K, Zuo J, Dhalla A, Chung T-S, Gudipati C. Novel Sandwich-Structured Hollow Fiber Membrane for High-Efficiency Membrane Distillation and Scale-Up for Pilot Validation. Membranes. 2022; 12(4):423. https://doi.org/10.3390/membranes12040423
Chicago/Turabian StyleQua, Marn Soon, Yan Zhao, Junyou Zhang, Sebastian Hernandez, Aung Thet Paing, Karikalan Mottaiyan, Jian Zuo, Adil Dhalla, Tai-Shung Chung, and Chakravarthy Gudipati. 2022. "Novel Sandwich-Structured Hollow Fiber Membrane for High-Efficiency Membrane Distillation and Scale-Up for Pilot Validation" Membranes 12, no. 4: 423. https://doi.org/10.3390/membranes12040423
APA StyleQua, M. S., Zhao, Y., Zhang, J., Hernandez, S., Paing, A. T., Mottaiyan, K., Zuo, J., Dhalla, A., Chung, T.-S., & Gudipati, C. (2022). Novel Sandwich-Structured Hollow Fiber Membrane for High-Efficiency Membrane Distillation and Scale-Up for Pilot Validation. Membranes, 12(4), 423. https://doi.org/10.3390/membranes12040423









