Palladium Membrane with High Density of Large-Angle Grain Boundaries to Promote Hydrogen Diffusivity
Abstract
1. Introduction
2. Materials and Methods
2.1. Membrane Supports
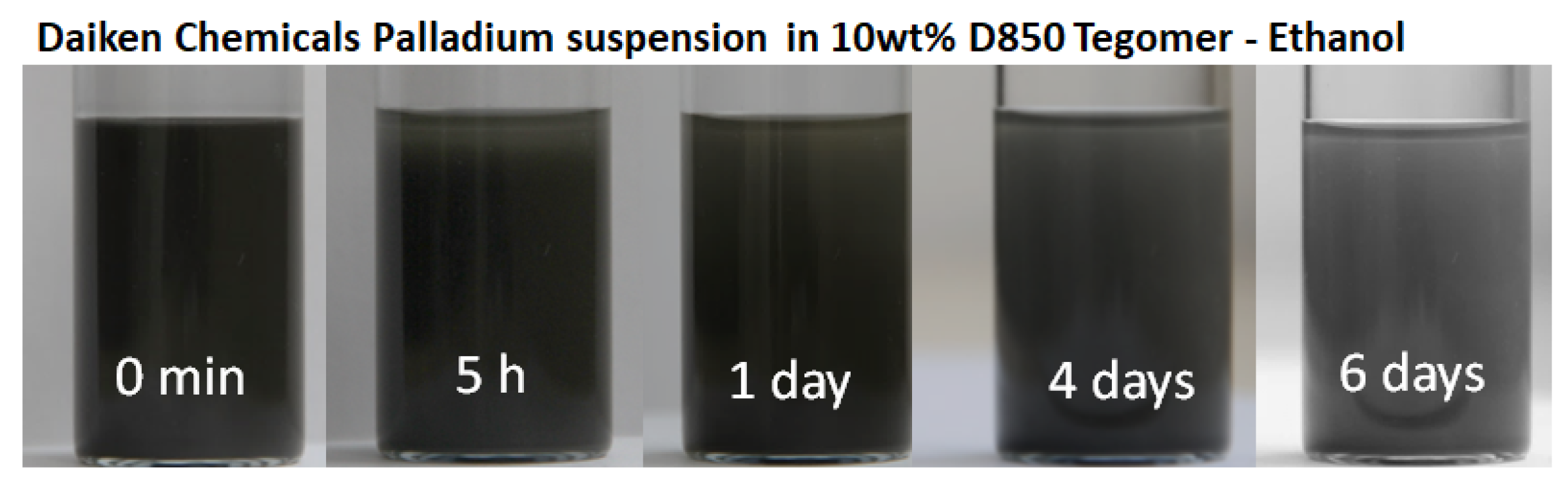
2.2. Suspension Plasma Spraying
2.3. Palladium Powders
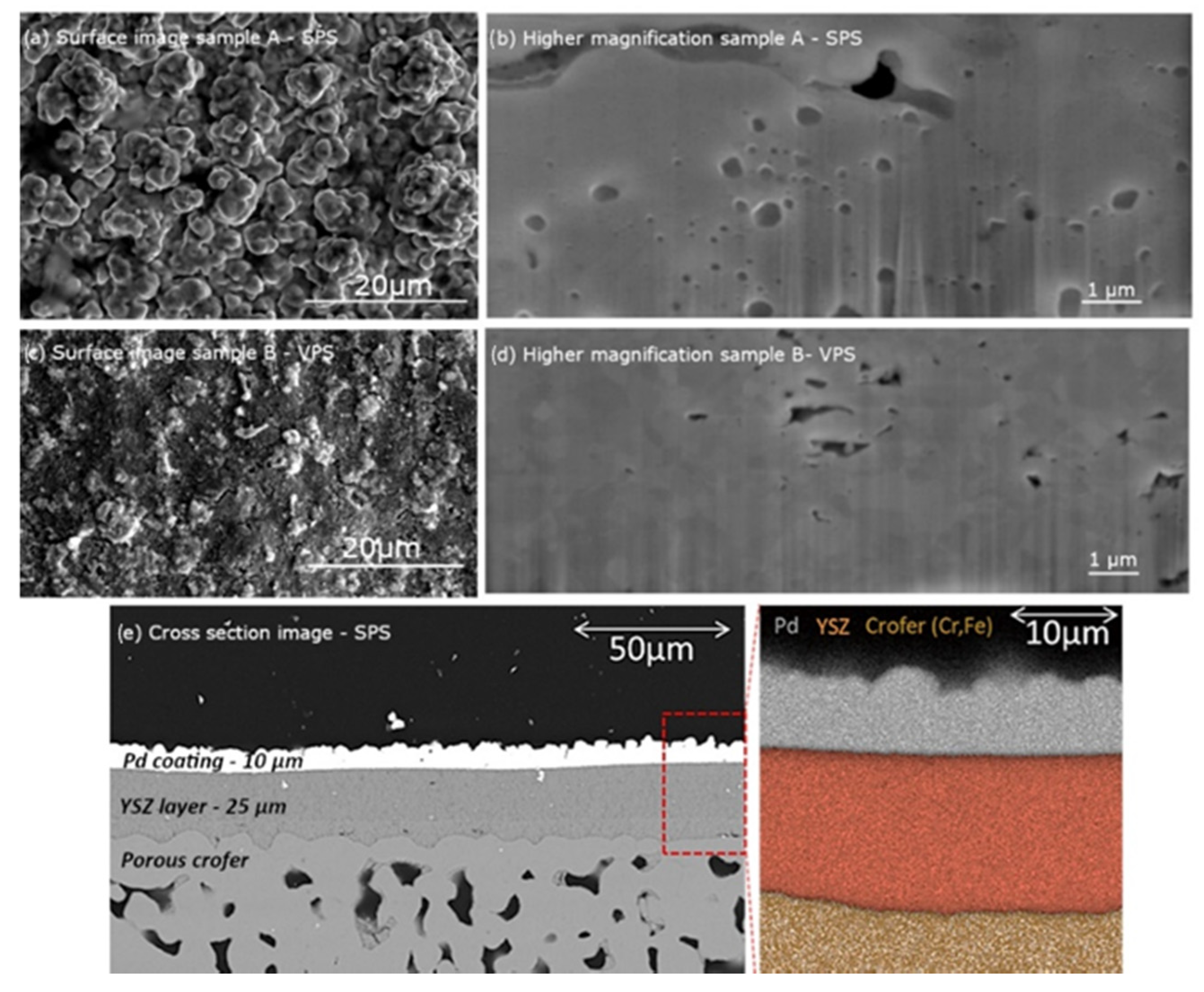
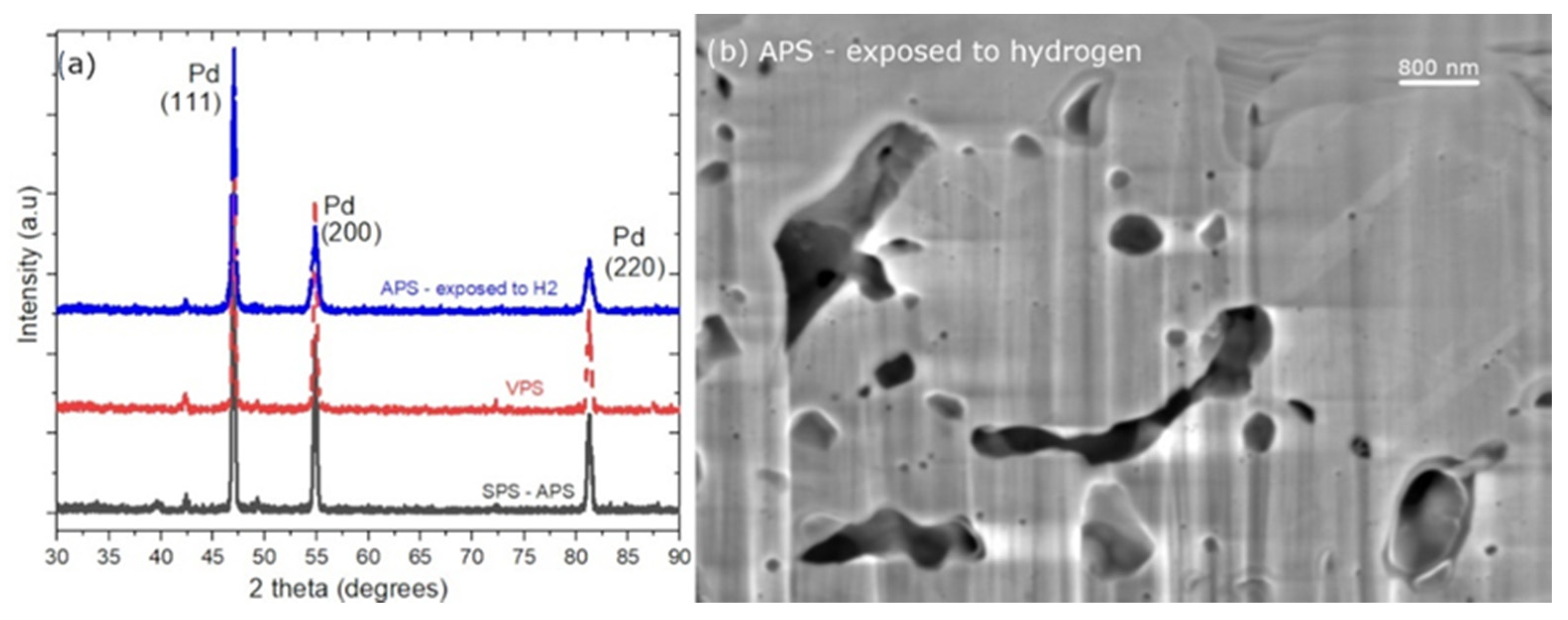
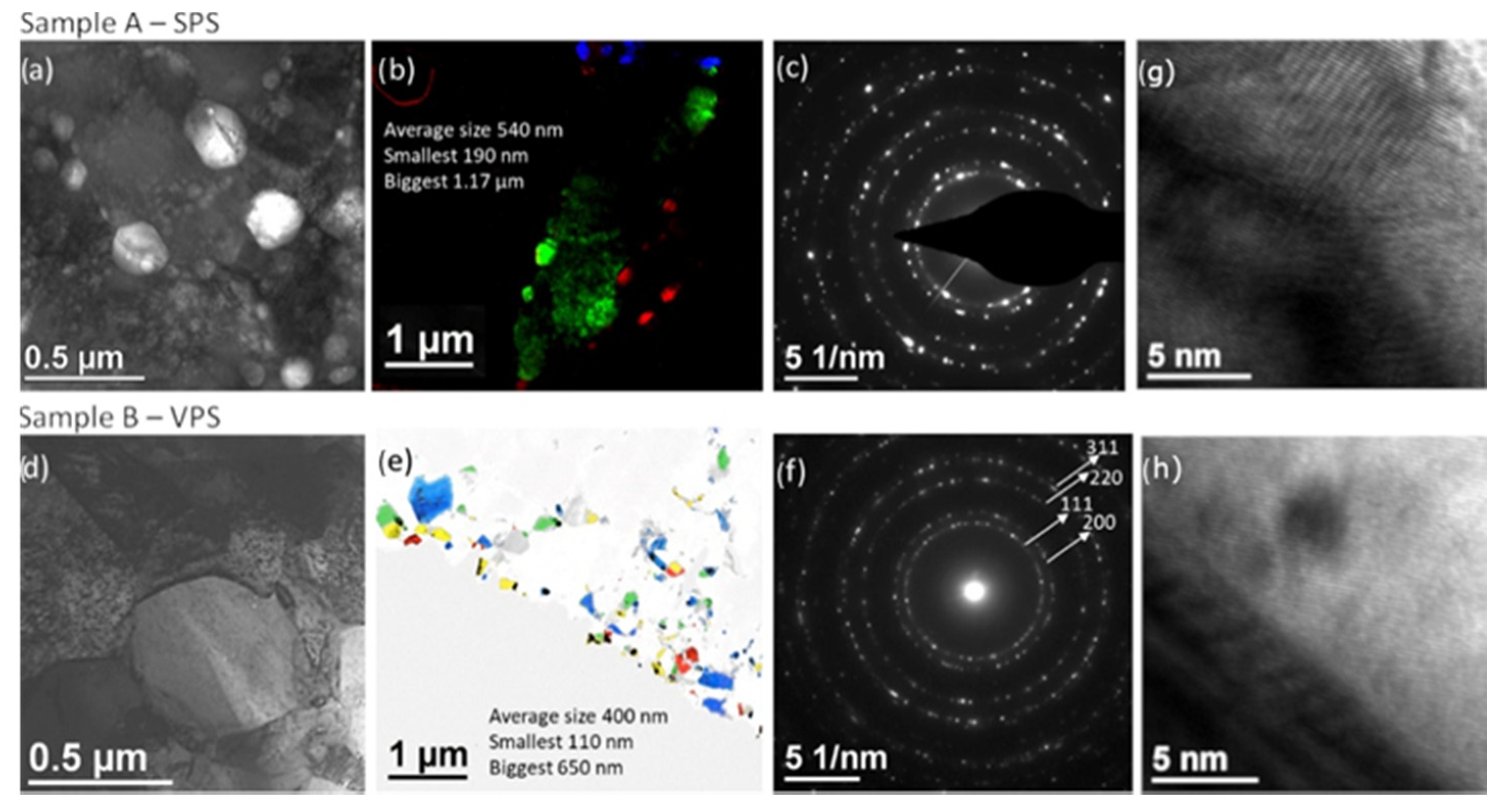
2.4. Coatings Characterization
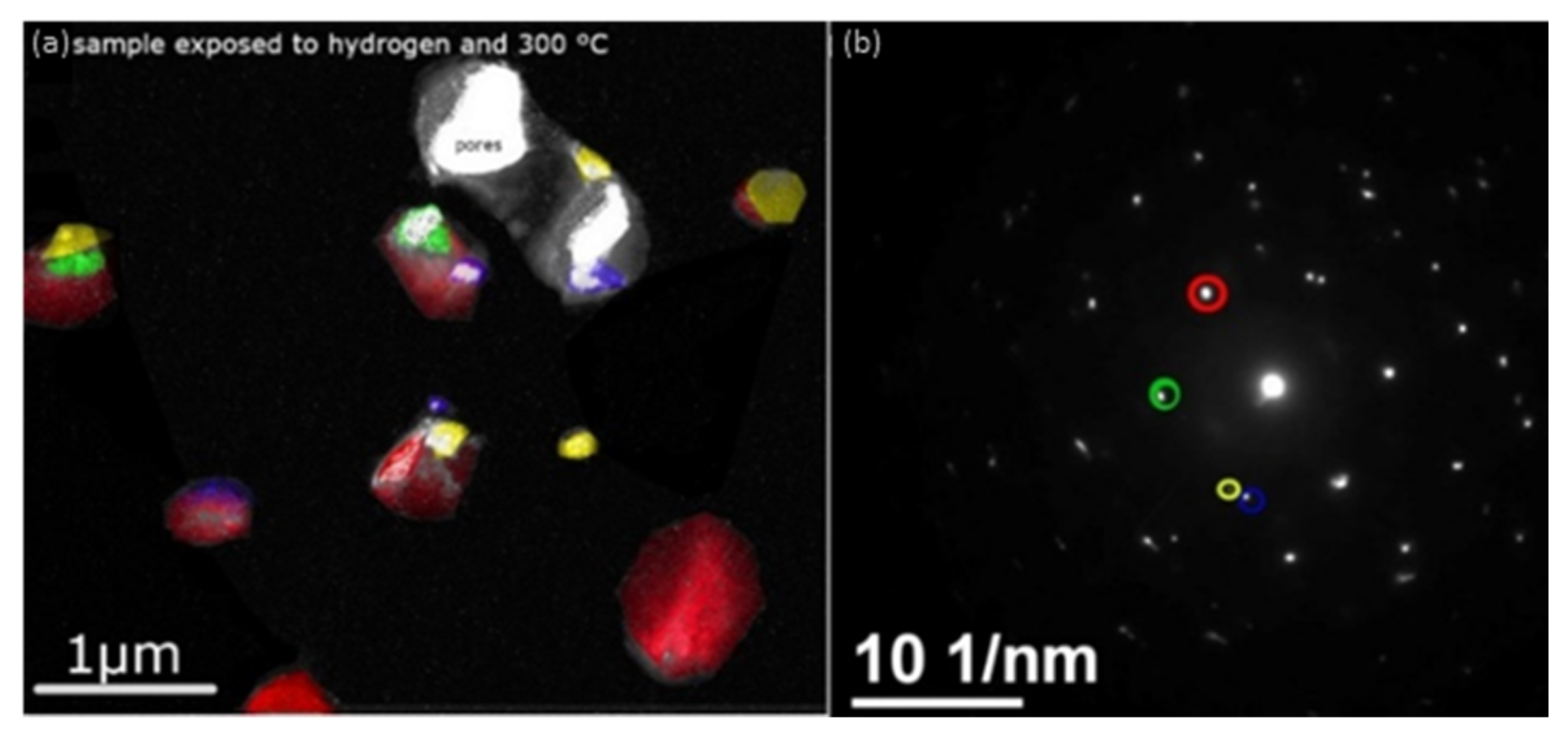
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dolan, M.D. Non-Pd BCC alloy membranes for industrial hydrogen separation. J. Membr. Sci. 2010, 362, 12–28. [Google Scholar] [CrossRef]
- Staffell, I.; Scamman, D.; Velazquez Abad, A.; Balcombe, P.; Dodds, P.E.; Ekins, P.; Shah, N.; Ward, K.R. The role of hydrogen and fuel cells in the global energy system. Energy Environ. Sci. 2019, 12, 463–491. [Google Scholar] [CrossRef]
- Peters, T.A.; Carvalho, P.A.; Stange, M.; Bredesen, R. Formation of hydrogen bubbles in Pd-Ag membranes during H2 permeation. Int. J. Hydrogen Energy 2019, 45, 7488–7496. [Google Scholar] [CrossRef]
- Peters, T.; Caravella, A. Pd-Based Membranes: Overview and Perspectives. Membranes 2019, 9, 25. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, G.; Araújo, T.; da Silva Lopes, T.; Sousa, J.; Mendes, A. Recent advances in membrane technologies for hydrogen purification. Int. J. Hydrog. Energy 2020, 45, 7313–7338. [Google Scholar] [CrossRef]
- Hafeez, S.; Al-Salem, S.; Manos, G.; Constantinou, A. Fuel production using membrane reactors: A review. Environ. Chem. Lett. 2020, 18, 1477–1490. [Google Scholar] [CrossRef]
- Freeman, B.D. Basis of Permeability/Selectivity Tradeoff Relations in Polymeric Gas Separation Membranes. Macromolecules 1999, 32, 375–380. [Google Scholar] [CrossRef]
- Ockwig, N.W.; Nenoff, T.M. Membranes for hydrogen separation. Chem. Rev. 2007, 107, 4078–4110. [Google Scholar] [CrossRef]
- Gallucci, F.; Fernandez, E.; Corengia, P.; van Sint Annaland, M. Recent advances on membranes and membrane reactors for hydrogen production. Chem. Eng. Sci. 2013, 92, 40–66. [Google Scholar] [CrossRef]
- Alique, D.; Martinez-Diaz, D.; Sanz, R.; Calles, J.A. Review of Supported Pd-Based Membranes Preparation by Electroless Plating for Ultra-Pure Hydrogen Production. Membranes 2018, 8, 5. [Google Scholar] [CrossRef]
- Wunsch, A.; Kant, P.; Mohr, M.; Haas-Santo, K.; Pfeifer, P.; Dittmeyer, R. Recent Developments in Compact Membrane Reactors with Hydrogen Separation. Membranes 2018, 8, 107. [Google Scholar] [CrossRef]
- Kiadehi, A.D.; Taghizadeh, M. Fabrication, characterization, and application of palladium composite membrane on porous stainless steel substrate with NaY zeolite as an intermediate layer for hydrogen purification. Int. J. Hydrogen Energy 2019, 44, 2889–2904. [Google Scholar] [CrossRef]
- Bryden, K. Nanostructured palladium–iron membranes for hydrogen separation and membrane hydrogenation reactions. J. Membr. Sci. 2002, 203, 29–42. [Google Scholar] [CrossRef]
- Natter, H.; Wettmann, B.; Heisel, B.; Hempelmann, R. Hydrogen in nanocrystalline palladium. J. Alloys Compd. 1997, 253–254, 84–86. [Google Scholar] [CrossRef]
- Stuhr, U.; Striffler, T.; Wipf, H.; Natter, H.; Wettmann, B.; Janssen, S.; Hempelmann, R.; Hahn, H. An investigation of hydrogen diffusion in nanocrystalline Pd by neutron spectroscopy. J. Alloys Compd. 1997, 253–254, 393–396. [Google Scholar] [CrossRef]
- McCool, B.A.; Lin, Y.S. Nanostructured thin palladium-silver membranes: Effects of grain size on gas permeation properties. J. Mater. Sci. 2001, 36, 3221–3227. [Google Scholar] [CrossRef]
- Okazaki, J.; Ikeda, T.; Pacheco Tanaka, D.A.; Suzuki, T.M.; Mizukami, F. In situ high-temperature X-ray diffraction study of thin palladium/α-alumina composite membranes and their hydrogen permeation properties. J. Membr. Sci. 2009, 335, 126–132. [Google Scholar] [CrossRef]
- Vicinanza, N.; Svenum, I.-H.; Næss, L.N.; Peters, T.A.; Bredesen, R.; Borg, A.; Venvik, H.J. Thickness dependent effects of solubility and surface phenomena on the hydrogen transport properties of sputtered Pd77%Ag23% thin film membranes. J. Membr. Sci. 2015, 476, 602–608. [Google Scholar] [CrossRef]
- Makrides, A.C.W.; Wright, M.A.; Jewett, D.A. Separation of Hydrogen by Permeation. U.S. Patent 3, 350,846, 7 November 1967. [Google Scholar]
- Zhang, K.; Gade, S.K.; Hatlevik, Ø.; Way, J.D. A sorption rate hypothesis for the increase in H2 permeability of palladium-silver (Pd–Ag) membranes caused by air oxidation. Int. J. Hydrogen Energy 2012, 37, 583–593. [Google Scholar] [CrossRef]
- Kirchheim, R.; Kownacka, I.; Filipek, S.M. Hydrogen segratation at grain boundaries in nanocrystalline nickel. Scr. Metall. Mater. 1993, 28, 1229–1234. [Google Scholar] [CrossRef]
- Lemier, C.; Weissmüller, J. Grain boundary segregation, stress and stretch: Effects on hydrogen absorption in nanocrystalline palladium. Acta Mater. 2007, 55, 1241–1254. [Google Scholar] [CrossRef]
- Lovvik, O.M.; Zhao, D.; Li, Y.; Bredesen, R.; Peters, T. Grain Boundary Segregation in Pd-Cu-Ag Alloys for High Permeability Hydrogen Separation Membranes. Membranes 2018, 8, 81. [Google Scholar] [CrossRef] [PubMed]
- Oudriss, A.; Creus, J.; Bouhattate, J.; Conforto, E.; Berziou, C.; Savall, C.; Feaugas, X. Grain size and grain-boundary effects on diffusion and trapping of hydrogen in pure nickel. Acta Mater. 2012, 60, 6814–6828. [Google Scholar] [CrossRef]
- Ievlev, V.M.; Prizhimov, A.S.; Boldyreva, A.V. Interaction of Hydrogen Atoms with Grain Boundaries in Palladium Bicrystals. Inorg. Mater. 2018, 54, 421–425. [Google Scholar] [CrossRef]
- Oudriss, A.; Creus, J.; Bouhattate, J.; Savall, C.; Peraudeau, B.; Feaugas, X. The diffusion and trapping of hydrogen along the grain boundaries in polycrystalline nickel. Scr. Mater. 2012, 66, 37–40. [Google Scholar] [CrossRef]
- Mine, Y.; Tachibana, K.; Horita, Z. Grain-boundary diffusion and precipitate trapping of hydrogen in ultrafine-grained austenitic stainless steels processed by high-pressure torsion. Mater. Sci. Eng. A 2011, 528, 8100–8105. [Google Scholar] [CrossRef]
- Mine, Y.; Horita, Z.; Murakami, Y. Effect of high-pressure torsion on hydrogen trapping in Fe–0.01mass% C and type 310S austenitic stainless steel. Acta Mater. 2010, 58, 649–657. [Google Scholar] [CrossRef]
- Lee, S.M.; Lee, J.Y. The Trapping and Transport Phenomena of Hydrogen in Nickel. Metall. Trans. A 1986, 17, 181–187. [Google Scholar] [CrossRef]
- Choo, W.Y.; Lee, J.Y. Thermal Analysis of Trapped Hydrogen in Pure Iron. Metall. Trans. A 1982, 13, 135–140. [Google Scholar] [CrossRef]
- Iwaoka, H.; Arita, M.; Horita, Z. Hydrogen diffusion in ultrafine-grained palladium: Roles of dislocations and grain boundaries. Acta Mater. 2016, 107, 168–177. [Google Scholar] [CrossRef]
- Nam, S. A study on the palladium/nickel composite membrane by vacuum electrodeposition. J. Membr. Sci. 2000, 170, 91–99. [Google Scholar] [CrossRef]
- Arratibel Plazaola, A.; Pacheco Tanaka, D.A.; Van Sint Annaland, M.; Gallucci, F. Recent Advances in Pd-Based Membranes for Membrane Reactors. Molecules 2017, 22, 51. [Google Scholar] [CrossRef] [PubMed]
- Gora, A.; Tanak, D.A.P.; Mizukami, F.; Suzuk, T.M. Lower Temperature Dehydrogenation of Methylcyclohexane by Membrane-assisted Equilibrium Shift. Chem. Lett. 2006, 35, 1372–1373. [Google Scholar] [CrossRef]
- Zhao, T.; Okazaki, J.; Tanaka, D.A.P.; Suzuki, T.M.; Wakui, Y. Hydrogen Separation with “Pore-Fill” Type Palladium Membrane. In Proceedings of the 2011 Spring Meeting & 7th Global Congress on Process Safety, Chicago, IL, USA, 13–17 March 2011. [Google Scholar]
- Lin, Y.S. Microporous and dense inorganic membranes: Current status and prospective. Sep. Purif. Technol. 2001, 25, 39–55. [Google Scholar] [CrossRef]
- Yeung, K.; Christiansen, S.C.; Varma, A. Palladium composite membranes by electroless plating technique relationships between plating kinetics, ®lm microstructure and membrane performance. J. Membr. Sci. 1999, 159, 107–122. [Google Scholar] [CrossRef]
- Guazzone, F.; Ma, Y.H. Leak growth mechanism in composite Pd membranes prepared by the electroless deposition method. AIChE J. 2008, 54, 487–494. [Google Scholar] [CrossRef]
- Rahimpour, M.R.; Samimi, F.; Babapoor, A.; Tohidian, T.; Mohebi, S. Palladium membranes applications in reaction systems for hydrogen separation and purification: A review. Chem. Eng. Processing Process Intensif. 2017, 121, 24–49. [Google Scholar] [CrossRef]
- Fernandez, E.; Helmi, A.; Medrano, J.A.; Coenen, K.; Arratibel, A.; Melendez, J.; de Nooijer, N.C.A.; Spallina, V.; Viviente, J.L.; Zuñiga, J.; et al. Palladium based membranes and membrane reactors for hydrogen production and purification: An overview of research activities at Tecnalia and TU/e. Int. J. Hydrogen Energy 2017, 42, 13763–13776. [Google Scholar] [CrossRef]
- Zheng, L.; Li, H.; Xu, H. “Defect-free” interlayer with a smooth surface and controlled pore-mouth size for thin and thermally stable Pd composite membranes. Int. J. Hydrogen Energy 2016, 41, 1002–1009. [Google Scholar] [CrossRef]
- Melendez, J.; Fernandez, E.; Gallucci, F.; van Sint Annaland, M.; Arias, P.L.; Pacheco Tanaka, D.A. Preparation and characterization of ceramic supported ultra-thin (~1 µm) Pd-Ag membranes. J. Membr. Sci. 2017, 528, 12–23. [Google Scholar] [CrossRef]
- Altinisik, O.; Dogan, M.; Dogu, G. Preparation and characterization of palladium-plated porous glass for hydrogen enrichment. Catal. Today 2005, 105, 641–646. [Google Scholar] [CrossRef]
- Ye, J.; Dan, G.; Yuan, Q. The preparation of ultrathin palladium membrane. In Key Engineering Materials; Trans Tech Publications, Ltd.: Bäch, Switzerland, 1992; pp. 437–442. [Google Scholar]
- Itoh, N.; Akiha, T.; Sato, T. Preparation of thin palladium composite membrane tube by a CVD technique and its hydrogen permselectivity. Catal. Today 2005, 104, 231–237. [Google Scholar] [CrossRef]
- Xomeritakis, G.; Lin, Y. Fabrication of a thin palladium membrane supported in a porous ceramic substrate by chemical vapor deposition. J. Membr. Sci. 1996, 120, 261–272. [Google Scholar] [CrossRef]
- Zhao, H.; Xiong, G.; Stroh, N.; Brunner, H. Preparation and characterization of Pd-Ag alloy composite membrane with magnetron sputtering. Sci. China Ser. B Chem. 1999, 42, 581–588. [Google Scholar] [CrossRef][Green Version]
- Yun, S.; Oyama, S.T. Correlations in palladium membranes for hydrogen separation: A review. J. Membr. Sci. 2011, 375, 28–45. [Google Scholar] [CrossRef]
- Okazaki, J.; Ikeda, T.; Tanaka, D.A.P.; Sato, K.; Suzuki, T.M.; Mizukami, F. An investigation of thermal stability of thin palladium–silver alloy membranes for high temperature hydrogen separation. J. Membr. Sci. 2011, 366, 212–219. [Google Scholar] [CrossRef]
- Asif Ansar, G.S.; Patz, O.; Gregoire, J.B.; Ilhan, Z. Plasma Sprayed Oxygen Electrode for Solid Oxide Fuel Cells and High Temperature Electrolyzers. In Proceedings of the 8th European SOFC Forum, Lucerne, Switzerland, 30 June–3 July 2008. [Google Scholar]
- Fauchais, P.; Vardelle, M.; Vardelle, A.; Goutier, S. What Do We Know, What are the Current Limitations of Suspension Plasma Spraying? J. Therm. Spray Technol. 2015, 24, 1120–1129. [Google Scholar] [CrossRef]
- Quicker, P.; Höllein, V.; Dittmeyer, R. Dittmeyer Catalytic dehydrogenation of hydrocarbons in palladium composite membrane reactors. Catal. Today 2000, 56, 21–34. [Google Scholar] [CrossRef]
- Höllein, V.; Thornton, M.; Quicker, P.; Dittmeyer, R. Preparation and characterization of palladium composite membranes for hydrogen removal in hydrocarbon dehydrogenation membrane reactors. Catal. Today 2001, 67, 33–42. [Google Scholar] [CrossRef]
- Fauchais, P. Current status and future directions of thermal spray coatings and techniques. In Future Development of Thermal Spray Coatings; Elsevier: Amsterdam, The Netherlands, 2015; pp. 17–49. [Google Scholar]
- Lee, T.H.; Park, C.Y.; Lee, G.; Dorris, S.E.; Balachandran, U. Hydrogen transport properties of palladium film prepared by colloidal spray deposition. J. Membr. Sci. 2012, 415–416, 199–204. [Google Scholar] [CrossRef]
- Boeltken, T.; Soysal, D.; Lee, S.; Straczewski, G.; Gerhards, U.; Peifer, P.; Arnold, J.; Dittmeyer, R. Perspectives of suspension plasma spraying of palladium nanoparticles for preparation of thin palladium composite membranes. J. Membr. Sci. 2014, 468, 233–241. [Google Scholar] [CrossRef]
- Fauchais, P.; Vardelle, A.; Dussoubs, B. Quo VadisThermal Spraying? J. Therm. Spray Technol. 2001, 10, 44. [Google Scholar] [CrossRef]
- Crawmer, D.E. Thermal Spray Processes. In Handbook of Thermal Spray Technology; ASM International: Materials Park, OH, USA, 2013; pp. 54–76. [Google Scholar]
- Fauchais, P. Understanding plasma spraying. J. Phys. D Appl. Phys. 2004, 37, R86–R108. [Google Scholar] [CrossRef]
- Tejero-Martin, D.; Rezvani Rad, M.; McDonald, A.; Hussain, T. Beyond Traditional Coatings: A Review on Thermal-Sprayed Functional and Smart Coatings. J. Therm. Spray Technol. 2019, 28, 598–644. [Google Scholar] [CrossRef]
- Vaßen, R.; Kaßner, H.; Mauer, G.; Stöver, D. Suspension Plasma Spraying: Process Characteristics and Applications. J. Therm. Spray Technol. 2009, 19, 219–225. [Google Scholar] [CrossRef]
- Pawlowski, L. Suspension and solution thermal spray coatings. Surf. Coat. Technol. 2009, 203, 2807–2829. [Google Scholar] [CrossRef]
- Killinger, A.; Gadow, R.; Mauer, G.; Guignard, A.; Vaßen, R.; Stöver, D. Review of New Developments in Suspension and Solution Precursor Thermal Spray Processes. J. Therm. Spray Technol. 2011, 20, 677–695. [Google Scholar] [CrossRef]
- Kassner, H.; Siegert, R.; Hathiramani, D.; Vassen, R.; Stoever, D. Application of Suspension Plasma Spraying (SPS) for Manufacture of Ceramic Coatings. J. Therm. Spray Technol. 2007, 17, 115–123. [Google Scholar] [CrossRef]
- Fauchais, P.; Joulia, A.; Goutier, S.; Chazelas, C.; Vardelle, M.; Vardelle, A.; Rossignol, S. Suspension and solution plasma spraying. J. Phys. D Appl. Phys. 2013, 46, 224015. [Google Scholar] [CrossRef]
- Killinger, A. Status and future trends in suspension spray techniques. In Future Development of Thermal Spray Coatings; Elsevier: Amsterdam, The Netherlands, 2015; pp. 81–122. [Google Scholar]
- Fauchais, P.; Montavon, G.; Vardelle, M.; Cedelle, J. Developments in direct current plasma spraying. Surf. Coat. Technol. 2006, 201, 1908–1921. [Google Scholar] [CrossRef]
- Felipe Miranda, F.C. Alexei Essiptchouk and Gilberto Pertraconi. In Atmospheric Plasma Spray Processes: From Micro to Nanostructures; IntechOpen: London, UK, 2018. [Google Scholar]
- Mahade, S.; Narayan, K.; Govindarajan, S.; Bjorklund, S.; Curry, N.; Joshi, S. Exploiting Suspension Plasma Spraying to Deposit Wear-Resistant Carbide Coatings. Materials 2019, 12, 2344. [Google Scholar] [CrossRef] [PubMed]
- Hadjixenophontos, E.; Roussel, M.; Sato, T.; Weigel, A.; Stender, P.; Orimo, S.-I.; Schmitz, G. Imaging the hydrogenation of Mg thin films. Int. J. Hydrogen Energy 2017, 42, 22411–22416. [Google Scholar] [CrossRef]
- Peters, T.A.; Carvalho, P.A.; van Wees, J.F.; Overbeek, J.P.; Sagvolden, E.; van Berkel, F.P.F.; Løvvik, O.M.; Bredesen, R. Leakage evolution and atomic-scale changes in Pd-based membranes induced by long-term hydrogen permeation. J. Membr. Sci. 2018, 563, 398–404. [Google Scholar] [CrossRef]
- Bryden, K.J.; Ying, J.Y. Nanostructured palladium membrane synthesis by magnetron sputtering. Mater. Sci. Eng. A 1995, 204, 140–145. [Google Scholar] [CrossRef]
- Huang, Y.; Dittmeyer, R. Preparation and characterization of composite palladium membranes on sinter-metal supports with a ceramic barrier against intermetallic diffusion. J. Membr. Sci. 2006, 282, 296–310. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hadjixenophontos, E.; Mahmoudizadeh, M.; Rubin, M.; Ullmer, D.; Razmjooei, F.; Hanf, A.C.; Brien, J.; Dittmeyer, R.; Ansar, A. Palladium Membrane with High Density of Large-Angle Grain Boundaries to Promote Hydrogen Diffusivity. Membranes 2022, 12, 617. https://doi.org/10.3390/membranes12060617
Hadjixenophontos E, Mahmoudizadeh M, Rubin M, Ullmer D, Razmjooei F, Hanf AC, Brien J, Dittmeyer R, Ansar A. Palladium Membrane with High Density of Large-Angle Grain Boundaries to Promote Hydrogen Diffusivity. Membranes. 2022; 12(6):617. https://doi.org/10.3390/membranes12060617
Chicago/Turabian StyleHadjixenophontos, Efi, Masoud Mahmoudizadeh, Michael Rubin, Dirk Ullmer, Fatemeh Razmjooei, Alexander C. Hanf, Jan Brien, Roland Dittmeyer, and Asif Ansar. 2022. "Palladium Membrane with High Density of Large-Angle Grain Boundaries to Promote Hydrogen Diffusivity" Membranes 12, no. 6: 617. https://doi.org/10.3390/membranes12060617
APA StyleHadjixenophontos, E., Mahmoudizadeh, M., Rubin, M., Ullmer, D., Razmjooei, F., Hanf, A. C., Brien, J., Dittmeyer, R., & Ansar, A. (2022). Palladium Membrane with High Density of Large-Angle Grain Boundaries to Promote Hydrogen Diffusivity. Membranes, 12(6), 617. https://doi.org/10.3390/membranes12060617







