Experimental and Computational Approaches to Sulfonated Poly(arylene ether sulfone) Synthesis Using Different Halogen Atoms at the Reactive Site
Abstract
Highlights
- The effect of differences in the halogen atoms at the ends of the biphenyl sulfone monomers on polymerization spontaneity was investigated through computer simulations and experiments.
- The polymerization process of SPAES was predicted using the first-principle calculation method.
- The simulation feasibility of polymer synthesis was verified experimentally.
- This approach based on quantum mechanics can even be effective in underlying polymer synthesis.
Abstract
1. Introduction
2. Materials and Methods
2.1. Computational Details
2.2. Materials
2.3. Synthesis of the SPAES Copolymer
2.4. Membrane Preparation
2.5. Characterization of the SPAES Copolymer
3. Results and Discussion
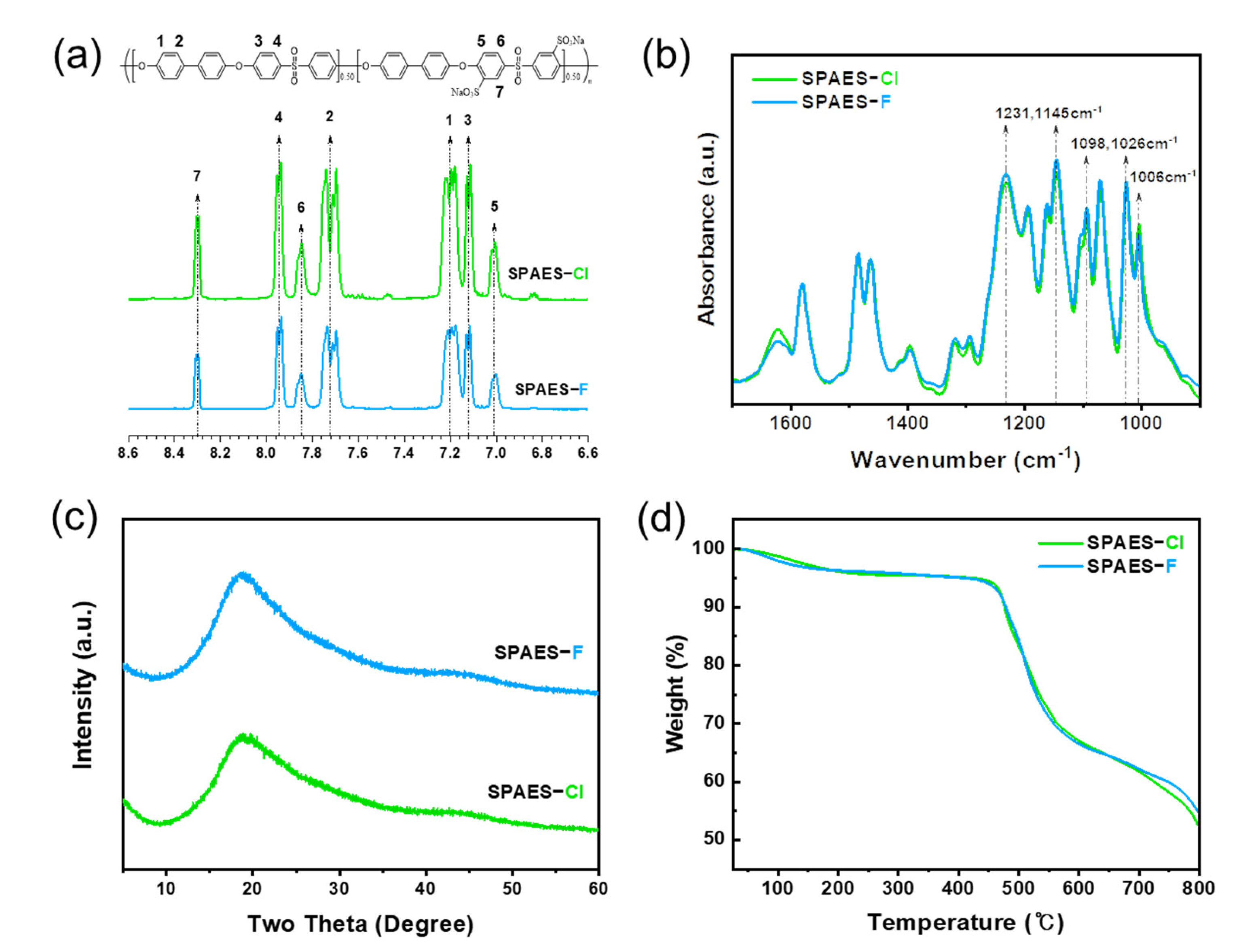
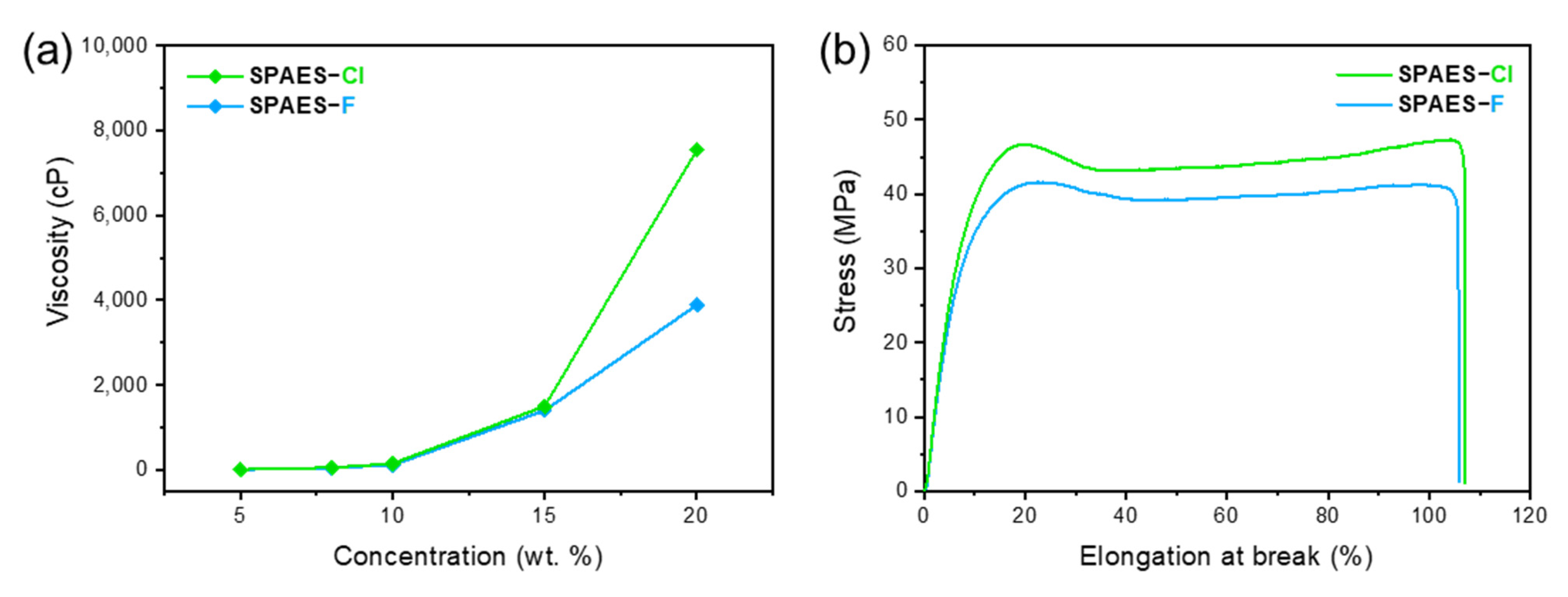
3.1. Synthesis and Characterization of the SPAES Copolymer
3.2. DFT Calculations
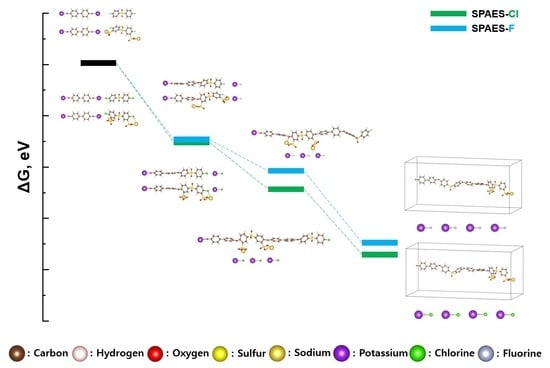
3.3. Gibbs Free Energy Diagram
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| DFT | density functional theory |
| BP | 4,4′-dihydroxy biphenyl |
| DCDPS | bis(4-chlorophenyl) sulfone |
| DFDPS | bis(4-fluorophenyl) sulfone |
| SDCDPS | bis(4-chlorophenyl-3-sulfophenyl) sulfone disodium salt |
| SDFDPS | bis(4-fluorophenyl-3-sulfophenyl) sulfone disodium salt |
| SPAES | sulfonated poly(arylene ether sulfone) |
| VASP | Vienna ab initio simulation package |
| RPBE | revised Perdew–Burke–Ernzerhof |
| GGA | generalized gradient approximation |
References
- Huang, Z.; Lv, B.; Zhou, L.; Tao, W.; Qin, X.; Shao, Z. Ultra-thin h-BN doped high sulfonation sulfonated poly (ether-ether-ketone) of PTFE-reinforced proton exchange membrane. J. Membr. Sci. 2022, 644, 120099. [Google Scholar] [CrossRef]
- Lv, B.; Yin, H.; Huang, Z.; Geng, K.; Qin, X.; Song, W.; Shao, Z. Polyethersulfone/polyvinylpyrrolidone/boron nitride composite membranes for high proton conductivity and long-term stability high-temperature proton exchange membrane fuel cells. J. Membr. Sci. 2022, 653, 120512. [Google Scholar] [CrossRef]
- Waribam, P.; Jaiyen, K.; Samart, C.; Ogawa, M.; Guan, G.; Kongparakul, S. MXene-copper oxide/sulfonated polyether ether ketone as a hybrid composite proton exchange membrane in electrochemical water electrolysis. Catal. Today 2022, 407, 96–106. [Google Scholar] [CrossRef]
- Ahmad, S.; Nawaz, T.; Ali, A.; Orhan, M.F.; Samreen, A.; Kannan, A.M. An overview of proton exchange membranes for fuel cells: Materials and manufacturing. Int. J. Hydrog. Energy 2022, 47, 19086–19131. [Google Scholar] [CrossRef]
- He, D.; Liu, G.; Wang, A.; Ji, W.; Wu, J.; Tang, H.; Lin, W.; Zhang, T.; Zhang, H. Alkali-free quaternized polybenzimidazole membranes with high phosphoric acid retention ability for high temperature proton exchange membrane fuel cells. J. Membr. Sci. 2022, 650, 120442. [Google Scholar] [CrossRef]
- Chen, Q.; Luo, J.; Liao, J.; Zhu, C.; Li, J.; Xu, J.; Xu, Y.; Ruan, H.; Shen, J. Tuning the length of aliphatic chain segments in aromatic poly(arylene ether sulfone) to tailor the micro-structure of anion-exchange membrane for improved proton blocking performance. J. Membr. Sci. 2022, 641, 119860. [Google Scholar] [CrossRef]
- Liu, D.; Xie, Y.; Zhong, J.; Yang, F.; Pang, J.; Jiang, Z. High methanol resistance semi-crystalline sulfonated poly(ether ketone) proton exchange membrane for direct methanol fuel cell. J. Membr. Sci. 2022, 650, 120413. [Google Scholar] [CrossRef]
- Wei, P.; Sui, Y.; Li, X.; Liu, Q.; Zhu, B.; Cong, C.; Meng, X.; Zhou, Q. Sandwich-structure PI/SPEEK/PI proton exchange membrane developed for achieving the high durability on excellent proton conductivity and stability. J. Membr. Sci. 2022, 644, 120116. [Google Scholar] [CrossRef]
- Namsheer, K.; Rout, C.S. Conducting polymers: A comprehensive review on recent advances in synthesis, properties and applications. RSC Adv. 2021, 11, 5659–5697. [Google Scholar]
- Alam, M.W.; Bhat, S.I.; Al Qahtani, H.S.; Aamir, M.; Amin, M.N.; Farhan, M.; Aldabal, S.; Khan, M.S.; Jeelani, I.; Nawaz, A. Recent Progress, Challenges, and Trends in Polymer-Based Sensors: A Review. Polymers 2022, 14, 2164. [Google Scholar] [CrossRef]
- Chepurnaya, I.; Smirnova, E.; Karushev, M. Electrochemically Active Polymer Components in Next-Generation LiFePO4 Cathodes: Can Small Things Make a Big Difference? Batteries 2022, 8, 185. [Google Scholar] [CrossRef]
- Fujiki, S.; Amaike, K.; Yagi, A.; Itami, K. Synthesis, properties, and material hybridization of bare aromatic polymers enabled by dendrimer support. Nat. Commun. 2022, 13, 5358. [Google Scholar] [CrossRef]
- Kushwaha, C.S.; Singh, P.; Shukla, S.K.; Chehimi, M.M. Advances in conducting polymer nanocomposite based chemical sensors: An overview. Mater. Sci. Eng. B 2022, 284, 115856. [Google Scholar] [CrossRef]
- Lee, D.H.; Park, T.; Yoo, H. Biodegradable polymer composites for electrophysiological signal sensing. Polymers 2022, 14, 2875. [Google Scholar] [CrossRef] [PubMed]
- Kang, P.-L.; Liu, Z.-P. Reaction prediction via atomistic simulation: From quantum mechanics to machine learning. iScience 2021, 24, 102013. [Google Scholar] [CrossRef]
- Bennett, S.; Szczypiński, F.T.; Turcani, L.; Briggs, M.E.; Greenaway, R.L.; Jelfs, K.E. Materials Precursor Score: Modeling Chemists’ Intuition for the Synthetic Accessibility of Porous Organic Cage Precursors. J. Chem. Inf. Model. 2021, 61, 4342–4356. [Google Scholar] [CrossRef]
- Schmidt, J.A.; Weatherby, J.A.; Sugden, I.J.; Santana-Bonilla, A.; Salerno, F.; Fuchter, M.J.; Johnson, E.R.; Nelson, J.; Jelfs, E.K. Computational Screening of Chiral Organic Semiconductors: Exploring Side-Group Functionalization and Assembly to Optimize Charge Transport. Cryst. Growth Des. 2021, 21, 5036–5049. [Google Scholar] [CrossRef]
- Shen, Y.; Borowski, J.E.; Hardy, M.A.; Sarpong, R.; Doyle, A.G.; Cernak, T. Automation and computer-assisted planning for chemical synthesis. Nat. Rev. Methods Primers 2021, 1, 23. [Google Scholar] [CrossRef]
- Schwaller, P.; Vaucher, A.C.; Laino, T.; Reymond, J.-L. Prediction of chemical reaction yields using deep learning. Mach. Learn. Sci. Technol. 2021, 2, 015016. [Google Scholar] [CrossRef]
- Tao, H.; Wu, T.; Aldeghi, M.; Wu, T.C.; Aspuru-Guzik, A.; Kumacheva, E. Nanoparticle synthesis assisted by machine learning. Nat. Rev. Mater. 2021, 6, 701–716. [Google Scholar] [CrossRef]
- Schön, C.-F.; van Bergerem, S.; Mattes, C.; Yadav, A.; Grohe, M.; Kobbelt, L.; Wuttig, M. Classification of properties and their relation to chemical bonding: Essential steps toward the inverse design of functional materials. Sci. Adv. 2022, 8, eade0828. [Google Scholar] [CrossRef] [PubMed]
- Bartel, C.J. Review of computational approaches to predict the thermodynamic stability of inorganic solids. J. Mater. Sci. 2022, 57, 10475–10498. [Google Scholar] [CrossRef]
- Golov, A.; Carrasco, J. Enhancing first-principles simulations of complex solid-state ion conductors using topological analysis of procrystal electron density. npj Comput. Mater. 2022, 8, 187. [Google Scholar] [CrossRef]
- Kalantarian, M.M.; Haghipour, A. Estimation of electrochemical cell potentials and reaction energies using Fermi energies. Phys. Chem. Chem. Phys. 2022, 24, 25–29. [Google Scholar] [CrossRef]
- Shinde, R.A.; Adole, V.A.; Shinde, R.S.; Desale, B.S.; Jagdale, B.S. Synthesis, antibacterial, antifungal and computational study of (E)-4-(3-(2,3-dihydrobenzo[b][1,4]dioxin-6-yl)-3-oxoprop-1-en-1-yl)benzonitrile. Results Chem. 2022, 4, 100553. [Google Scholar] [CrossRef]
- Yang, R.X.; McCandler, C.A.; Andriuc, O.; Siron, M.; Woods-Robinson, R.; Horton, M.K.; Persson, K.A. Big Data in a Nano World: A Review on Computational, Data-Driven Design of Nanomaterials Structures, Properties, and Synthesis. ACS Nano 2022. [Google Scholar] [CrossRef]
- Le, T.-T. Prediction of tensile strength of polymer carbon nanotube composites using practical machine learning method. J. Compos. Mater. 2020, 55, 787–811. [Google Scholar] [CrossRef]
- Lin, T.; Hu, L.; Wisely, W.; Gu, X.; Cai, J.; Litster, S.; Kara, L.B. Prediction of high frequency resistance in polymer electrolyte membrane fuel cells using long short term memory based model. Energy AI 2021, 3, 100045. [Google Scholar] [CrossRef]
- Hosseini, S.-A.; Nematzadeh, M.; Chastre, C. Prediction of shear behavior of steel fiber-reinforced rubberized concrete beams reinforced with glass fiber-reinforced polymer (GFRP) bars. Compos. Struct. 2021, 256, 113010. [Google Scholar] [CrossRef]
- Chen, L.; Pilania, G.; Batra, R.; Huan, T.D.; Kim, C.; Kuenneth, C.; Ramprasad, R. Polymer informatics: Current status and critical next steps. Mater. Sci. Eng. R Rep. 2021, 144, 100595. [Google Scholar] [CrossRef]
- Gormley, A.J.; Webb, M.A. Machine learning in combinatorial polymer chemistry. Nat. Rev. Mater. 2021, 6, 642–644. [Google Scholar] [CrossRef] [PubMed]
- Caddeo, C.; Ackermann, J.; Mattoni, A. A Theoretical Perspective on the Thermodynamic Stability of Polymer Blends for Solar Cells: From Experiments to Predictive Modeling. Sol. RRL 2022, 6, 2200172. [Google Scholar] [CrossRef]
- Orasugh, J.T.; Ray, S.S. Prospect of DFT Utilization in Polymer-Graphene Composites for Electromagnetic Interference Shielding Application: A Review. Polymers 2022, 14, 704. [Google Scholar] [CrossRef] [PubMed]
- Alesadi, A.; Xia, W.; Kilin, D. Photo-Induced Charge Transfer of Fullerene and Non-Fullerene Conjugated Polymer Blends via Ab Initio Excited-State Dynamics. J. Phys. Chem. C 2022, 126, 12015–12024. [Google Scholar] [CrossRef]
- Tran, H.; Toland, A.; Stellmach, K.; Paul, M.K.; Gutekunst, W.; Ramprasad, R. Toward Recyclable Polymers: Ring-Opening Polymerization Enthalpy from First-Principles. J. Phys. Chem. Lett. 2022, 13, 4778–4785. [Google Scholar] [CrossRef]
- Thompson, R.B. An interpretation of quantum foundations based on density functional theory and polymer self-consistent field theory. Quantum Stud. Math. Found. 2022, 9, 405–416. [Google Scholar] [CrossRef]
- Noudem, P.; Fouejio, D.; Mveme, C.D.D.; Zekeng, S.S.; Nya, F.T.; Ejuh, G.W. Hartree-Fock and DFT studies of the optoelectronic, thermodynamic, structural and nonlinear optical properties of photochromic polymers containing styrylquinoline fragments. Mater. Chem. Phys. 2022, 281, 125883. [Google Scholar] [CrossRef]
- Mombrú, D.; Romero, M.; Faccio, R.; Mombrú, Á.W. Roles of amorphous and crystalline regions in determining the optical and electronic properties of donor:acceptor systems comprising poly(3-hexylthiophene) embedded with nitrogen/sulfur-doped graphene quantum dots. Polym. J. 2022, 54, 1465–1476. [Google Scholar] [CrossRef]
- Lenzi, V.; Crema, A.; Pyrlin, S.; Marques, L. Current State and Perspectives of Simulation and Modeling of Aliphatic Isocyanates and Polyisocyanates. Polymers 2022, 14, 1642. [Google Scholar] [CrossRef]
- Keya, K.N.; Xia, W.; Kilin, D. DFT simulation of conductivity of the p-type doped and charge-injected cis-polyacetylene. Mol. Phys. 2022, 120, e2110167. [Google Scholar] [CrossRef]
- Bedoura, S.; Xi, H.-W.; Goh, H.W.; Lim, K.H. DFT/TDDFT Investigation on donor-acceptor triazole-based copolymers for organic photovoltaics. J. Mol. Struct. 2022, 1248, 131406. [Google Scholar] [CrossRef]
- Takagi, T.; Toda, T.; Miya, M.; Takenaka, K. DFT study on the anionic polymerization of phenyl-substituted [3]dendralene derivatives: Reactivities of monomer and chain end carbanion. Polym. J. 2022, 54, 643–652. [Google Scholar] [CrossRef]
- Hohenberg, P.; Kohn, W. Inhomogeneous Electron Gas. Phys. Rev. 1964, 136, B864–B871. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Self-Consistent Equations Including Exchange and Correlation Effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef]
- Han, J.; Kim, K.; Kim, J.; Kim, S.; Choi, S.-W.; Lee, H.; Kim, J.-J.; Kim, T.-H.; Sung, Y.-E.; Lee, J.-C. Cross-linked highly sulfonated poly(arylene ether sulfone) membranes prepared by in-situ casting and thiol-ene click reaction for fuel cell application. J. Membr. Sci. 2019, 579, 70–78. [Google Scholar] [CrossRef]
- Haragirimana, A.; Li, N.; Hu, Z.; Chen, S. A facile, effective thermal crosslinking to balance stability and proton conduction for proton exchange membranes based on blend sulfonated poly(ether ether ketone)/sulfonated poly(arylene ether sulfone). Int. J. Hydrog. Energy 2021, 46, 15866–15877. [Google Scholar] [CrossRef]
- Liu, L.; Pu, Y.; Lu, Y.; Li, N.; Hu, Z.; Chen, S. Superacid sulfated SnO2 doped with CeO2: A novel inorganic filler to simultaneously enhance conductivity and stabilities of proton exchange membrane. J. Membr. Sci. 2021, 621, 118972. [Google Scholar] [CrossRef]
- Sharma, P.P.; Tinh, V.D.C.; Kim, D. Enhanced Ion Cluster Size of Sulfonated Poly (Arylene Ether Sulfone) for Proton Exchange Membrane Fuel Cell Application. Polymers 2021, 13, 1111. [Google Scholar] [CrossRef]
- Tinh, V.D.C.; Thuc, V.D.; Kim, D. Chemically sustainable fuel cells via layer-by-layer fabrication of sulfonated poly(arylene ether sulfone) membranes containing cerium oxide nanoparticles. J. Membr. Sci. 2021, 634, 119430. [Google Scholar] [CrossRef]
- Yuan, C.; Wang, Y. Synthesis and characterization of a crosslinked membrane based on sulfonated poly (aryl ether sulfone) and sulfonated polyvinyl alcohol applied in direct methanol fuel cells. J. Polym. Res. 2020, 27, 1–13. [Google Scholar] [CrossRef]
- Cavallo, G.; Metrangolo, P.; Milani, R.; Pilati, T.; Priimagi, A.; Resnati, G.; Terraneo, G. The Halogen Bond. Chem. Rev. 2016, 116, 2478–2601. [Google Scholar] [CrossRef] [PubMed]
- Metrangolo, P.; Resnati, G. Halogen bonding: A paradigm in supramolecular chemistry. Chem. A Eur. J. 2001, 7, 2511–2519. [Google Scholar] [CrossRef]
- Que, C.; Qi, Q.; Zemlyanov, D.Y.; Mo, H.; Deac, A.; Zeller, M.; Indulkar, A.S.; Gao, Y.; Zhang, G.G.Z.; Taylor, L.S. Evidence for Halogen Bonding in Amorphous Solid Dispersions. Cryst. Growth Des. 2020, 20, 3224–3235. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Franchini, C.; Podloucky, R.; Paier, J.; Marsman, M.; Kresse, G. Ground-state properties of multivalent manganese oxides: Density functional and hybrid density functional calculations. Phys. Rev. B 2007, 75, 195128. [Google Scholar] [CrossRef]
- Wang, L.; Maxisch, T.; Ceder, G. Oxidation energies of transition metal oxides within the GGA+ U framework. Phys. Rev. B 2006, 73, 195107. [Google Scholar] [CrossRef]
- Harrison, W.L.; Wang, F.; Mecham, J.B.; Bhanu, V.A.; Hill, M.; Kim, Y.S.; McGrath, J.E. Influence of the bisphenol structure on the direct synthesis of sulfonated poly(arylene ether) copolymers. I. J. Polym. Sci. Part A Polym. Chem. 2003, 41, 2264–2276. [Google Scholar] [CrossRef]
- Harrison, W.L.; Hickner, M.A.; Kim, Y.S.; McGrath, J.E. Poly(Arylene Ether Sulfone) Copolymers and Related Systems from Disulfonated Monomer Building Blocks: Synthesis, Characterization, and Performance—A Topical Review. Fuel Cells 2005, 5, 201–212. [Google Scholar] [CrossRef]
- Wang, F.; Hickner, M.; Kim, Y.S.; Zawodzinski, T.A.; McGrath, J.E. Direct polymerization of sulfonated poly (arylene ether sulfone) random (statistical) copolymers: Candidates for new proton exchange membranes. J. Membr. Sci. 2002, 197, 231–242. [Google Scholar] [CrossRef]
- Kang, M.-S.; Choi, Y.-J.; Choi, I.-J.; Yoon, T.-H.; Moon, S.-H. Electrochemical characterization of sulfonated poly(arylene ether sulfone) (S-PES) cation-exchange membranes. J. Membr. Sci. 2003, 216, 39–53. [Google Scholar] [CrossRef]
- Ryu, S.K.; Kim, A.R.; Vinothkannan, M.; Lee, K.H.; Chu, J.Y.; Yoo, D.J. Enhancing physicochemical properties and single cell performance of sulfonated poly (arylene ether)(SPAE) membrane by incorporation of phosphotungstic acid and graphene oxide: A potential electrolyte for proton exchange membrane fuel cells. Polymers 2021, 13, 2364. [Google Scholar] [CrossRef] [PubMed]
- Bowen, C.H.; Reed, T.J.; Sargent, C.J.; Mpamo, B.; Galazka, J.M.; Zhang, F. Seeded chain-growth polymerization of proteins in living bacterial cells. ACS Synth. Biol. 2019, 8, 2651–2658. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Kim, W.; Kim, D.; Kim, S.; Chae, J.; Choi, S.Q.; Kim, F.S.; Kim, T.-S.; Kim, B.J. Importance of critical molecular weight of semicrystalline n-type polymers for mechanically robust, efficient electroactive thin films. Chem. Mater. 2019, 31, 3163–3173. [Google Scholar] [CrossRef]
- Nunes, R.W.; Martin, J.R.; Johnson, J.F. Influence of molecular weight and molecular weight distribution on mechanical properties of polymers. Polym. Eng. Sci. 1982, 22, 205–228. [Google Scholar] [CrossRef]
- Jiang, K.; Siahrostami, S.; Akey, A.J.; Li, Y.; Lu, Z.; Lattimer, J.; Hu, Y.; Stokes, C.; Gangishetty, M.; Chen, G.; et al. Transition-Metal Single Atoms in a Graphene Shell as Active Centers for Highly Efficient Artificial Photosynthesis. Chem 2017, 3, 950–960. [Google Scholar] [CrossRef]


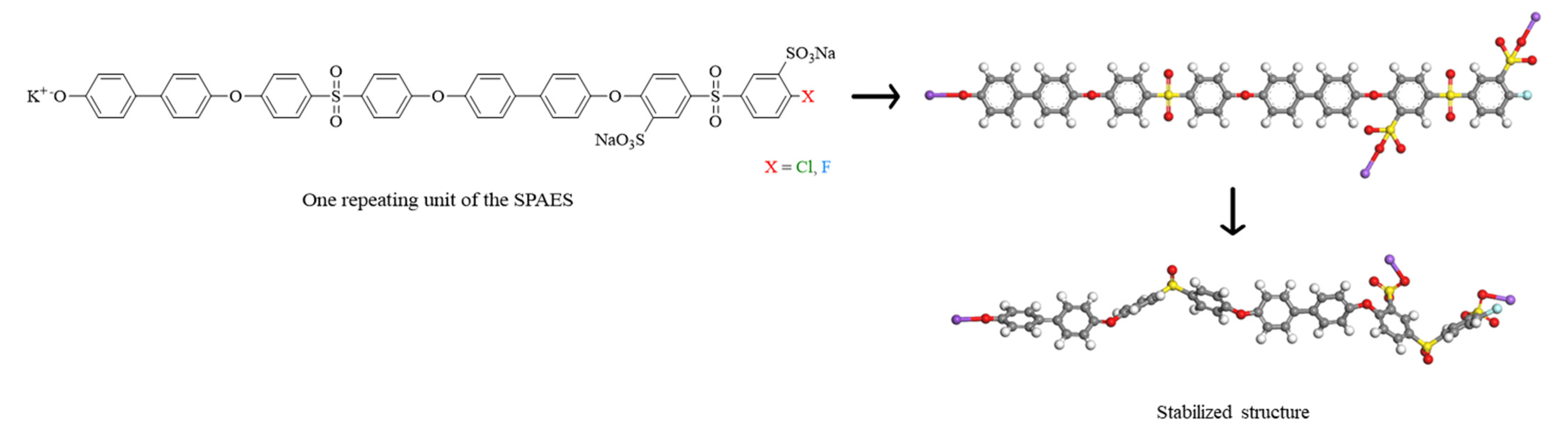
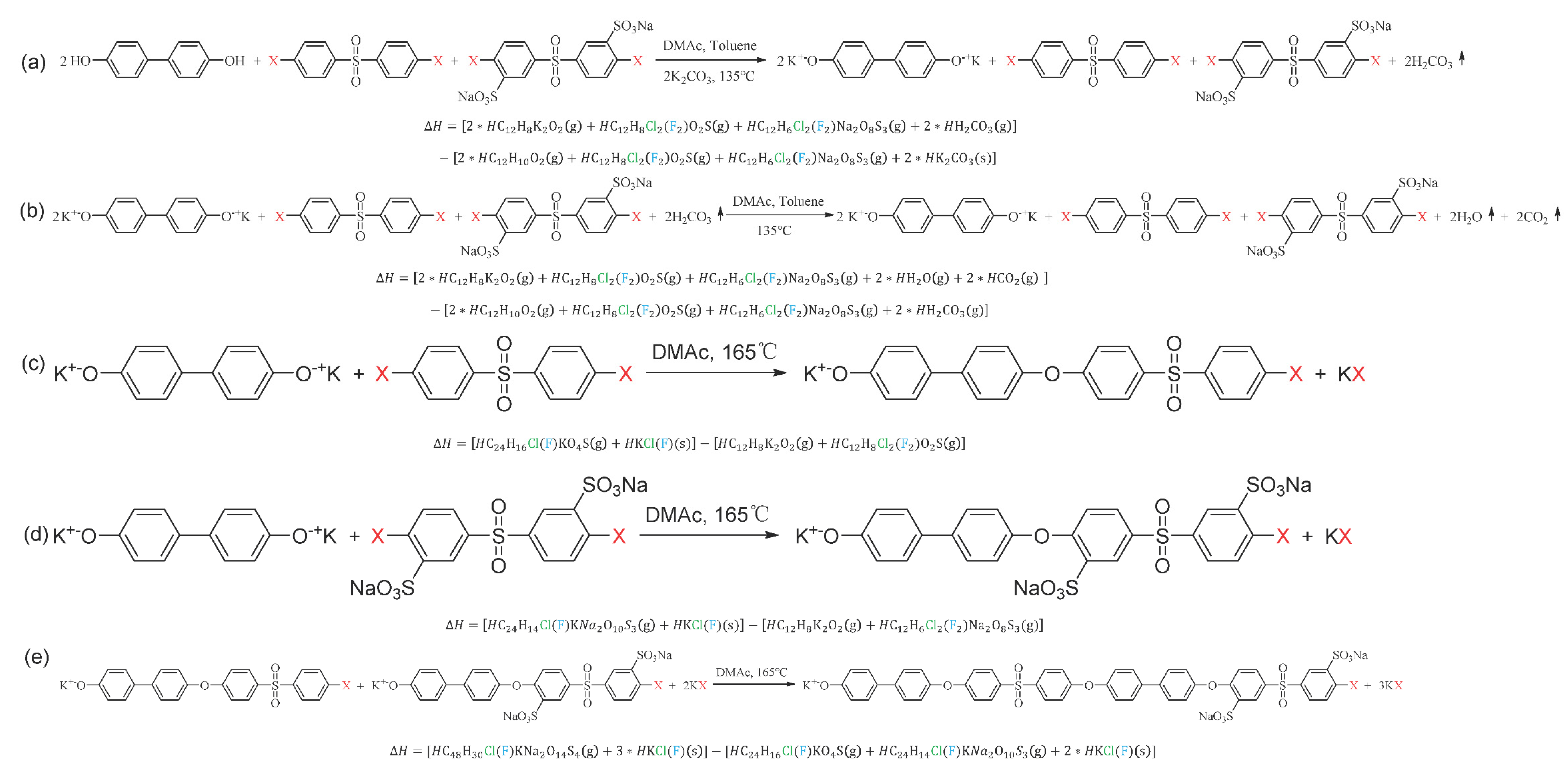

| Polymer | IEC (mmol∙g−1) 1 | Proton Conductivity (S∙cm−1) 2 | |
|---|---|---|---|
| Theoretical | Experimental | ||
| SPAES-Cl | 2.08 | 1.82 | 0.119 |
| SPAES-F | 1.81 | 0.124 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, S.; Cha, J.-E.; Moon, S.J.; Albers, J.G.; Seo, M.H.; Choi, Y.-W.; Kim, J.H. Experimental and Computational Approaches to Sulfonated Poly(arylene ether sulfone) Synthesis Using Different Halogen Atoms at the Reactive Site. Membranes 2022, 12, 1286. https://doi.org/10.3390/membranes12121286
Jang S, Cha J-E, Moon SJ, Albers JG, Seo MH, Choi Y-W, Kim JH. Experimental and Computational Approaches to Sulfonated Poly(arylene ether sulfone) Synthesis Using Different Halogen Atoms at the Reactive Site. Membranes. 2022; 12(12):1286. https://doi.org/10.3390/membranes12121286
Chicago/Turabian StyleJang, Seol, Jung-Eun Cha, Seung Jae Moon, Justin Georg Albers, Min Ho Seo, Young-Woo Choi, and Jong Hak Kim. 2022. "Experimental and Computational Approaches to Sulfonated Poly(arylene ether sulfone) Synthesis Using Different Halogen Atoms at the Reactive Site" Membranes 12, no. 12: 1286. https://doi.org/10.3390/membranes12121286
APA StyleJang, S., Cha, J.-E., Moon, S. J., Albers, J. G., Seo, M. H., Choi, Y.-W., & Kim, J. H. (2022). Experimental and Computational Approaches to Sulfonated Poly(arylene ether sulfone) Synthesis Using Different Halogen Atoms at the Reactive Site. Membranes, 12(12), 1286. https://doi.org/10.3390/membranes12121286