Development of Lignin-Containing Cellulose Nanofibrils Coated Paper-Based Filters for Effective Oil-Water Separation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
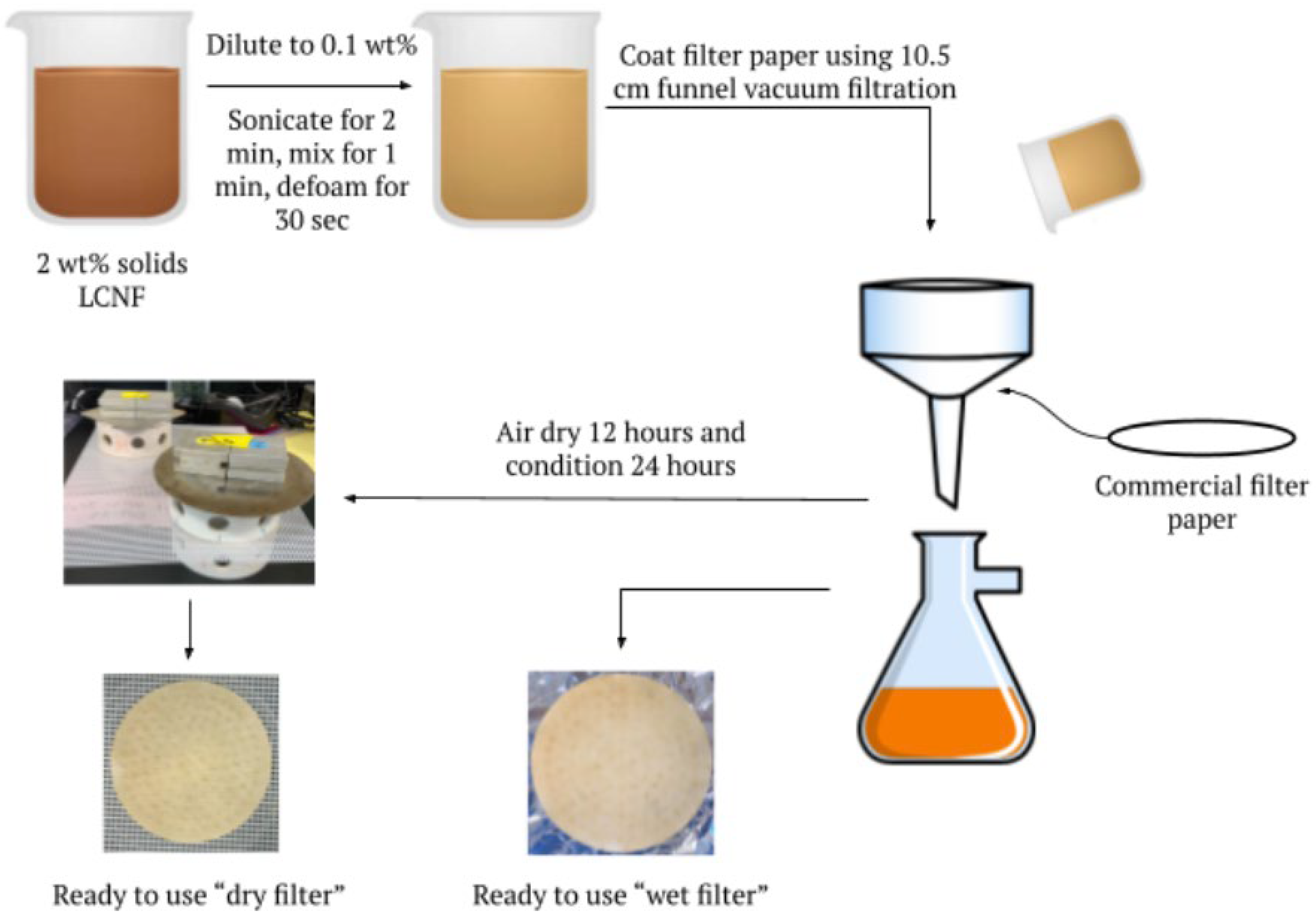
2.2. Preparation of LCNF-Modified Filter Papers
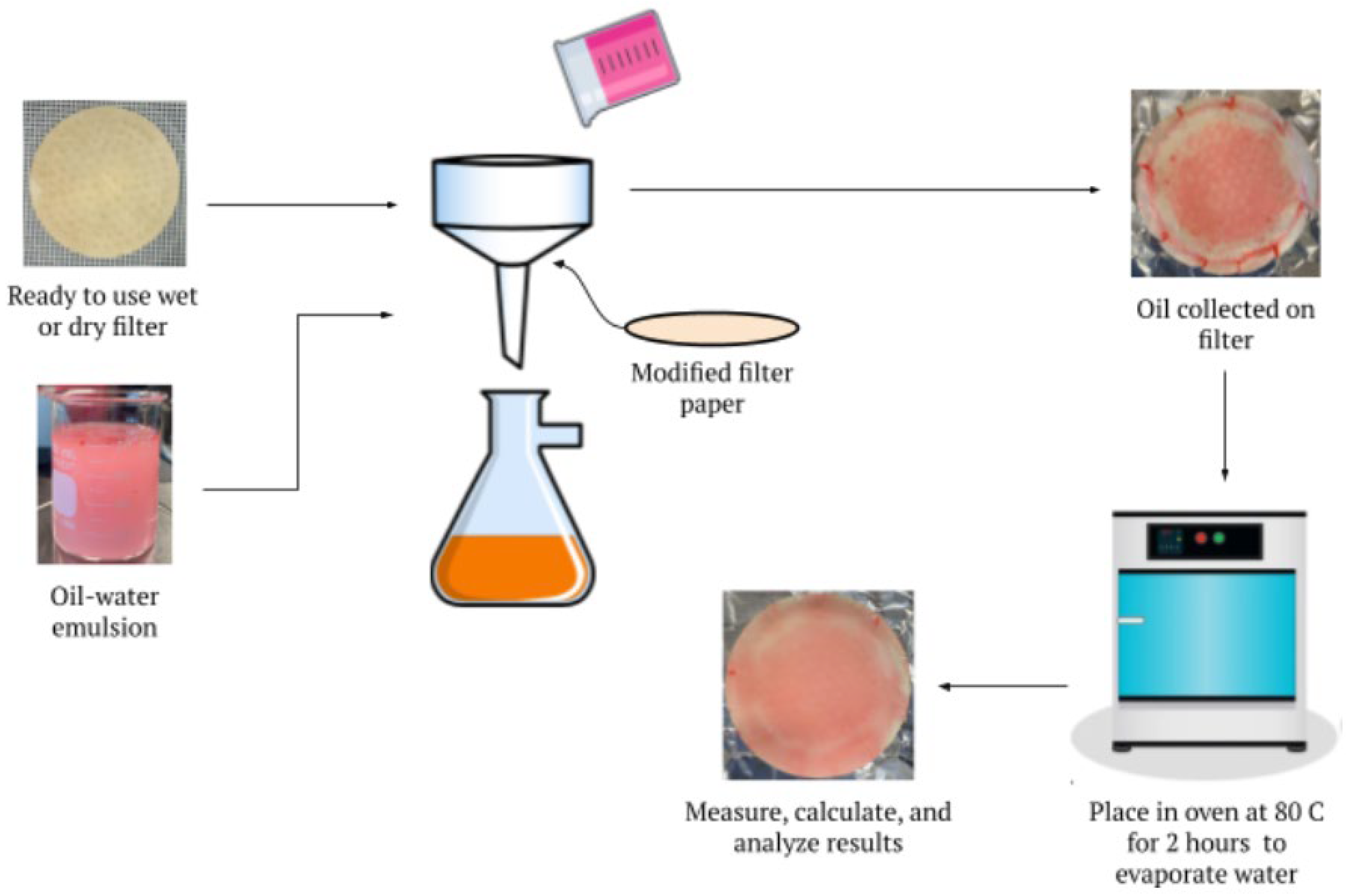
2.3. Filter Characterization
2.4. Preparation of Oil-Water Emulsions
2.5. Oil-Water Separation Process
3. Results and Discussion
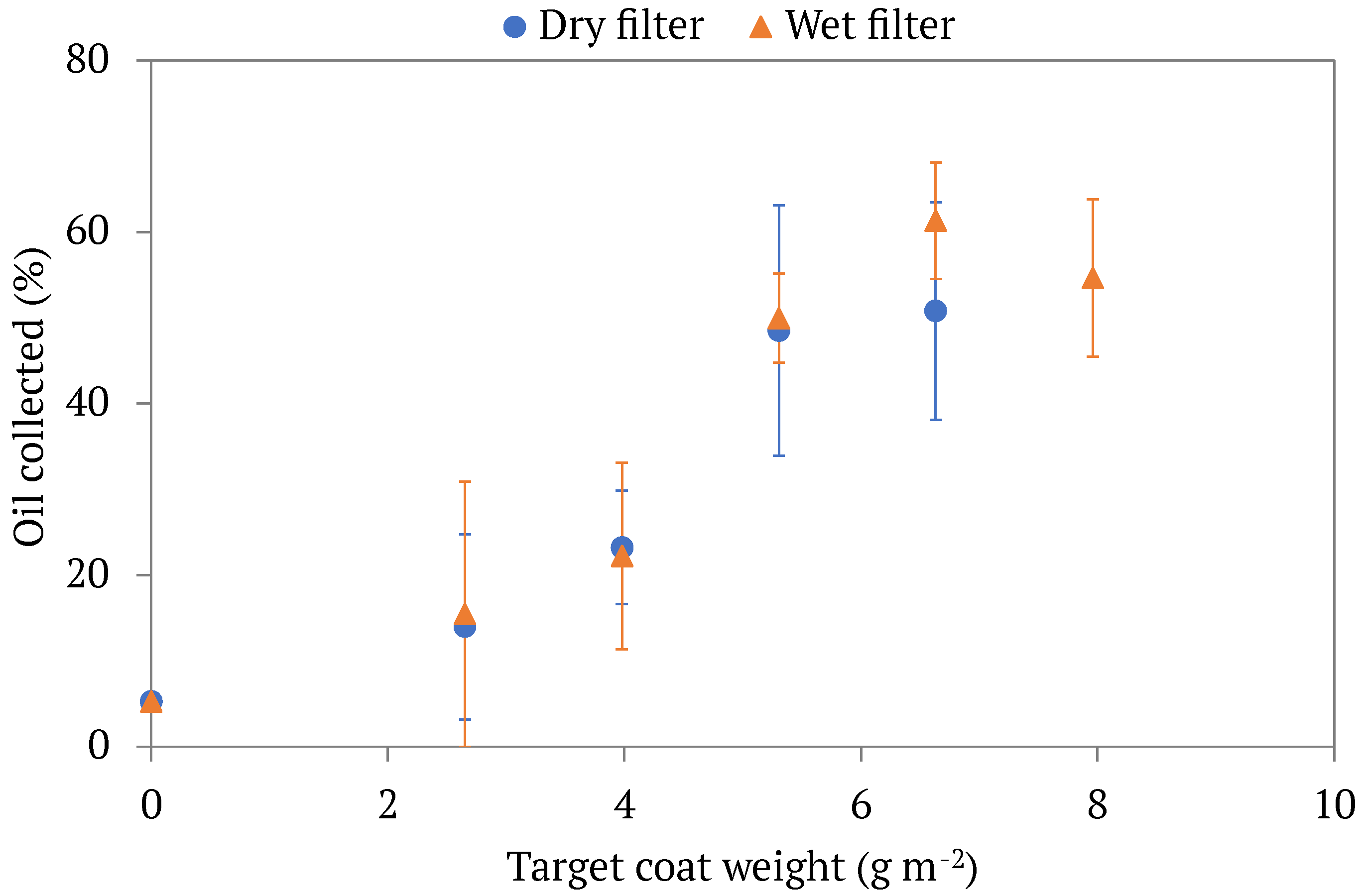
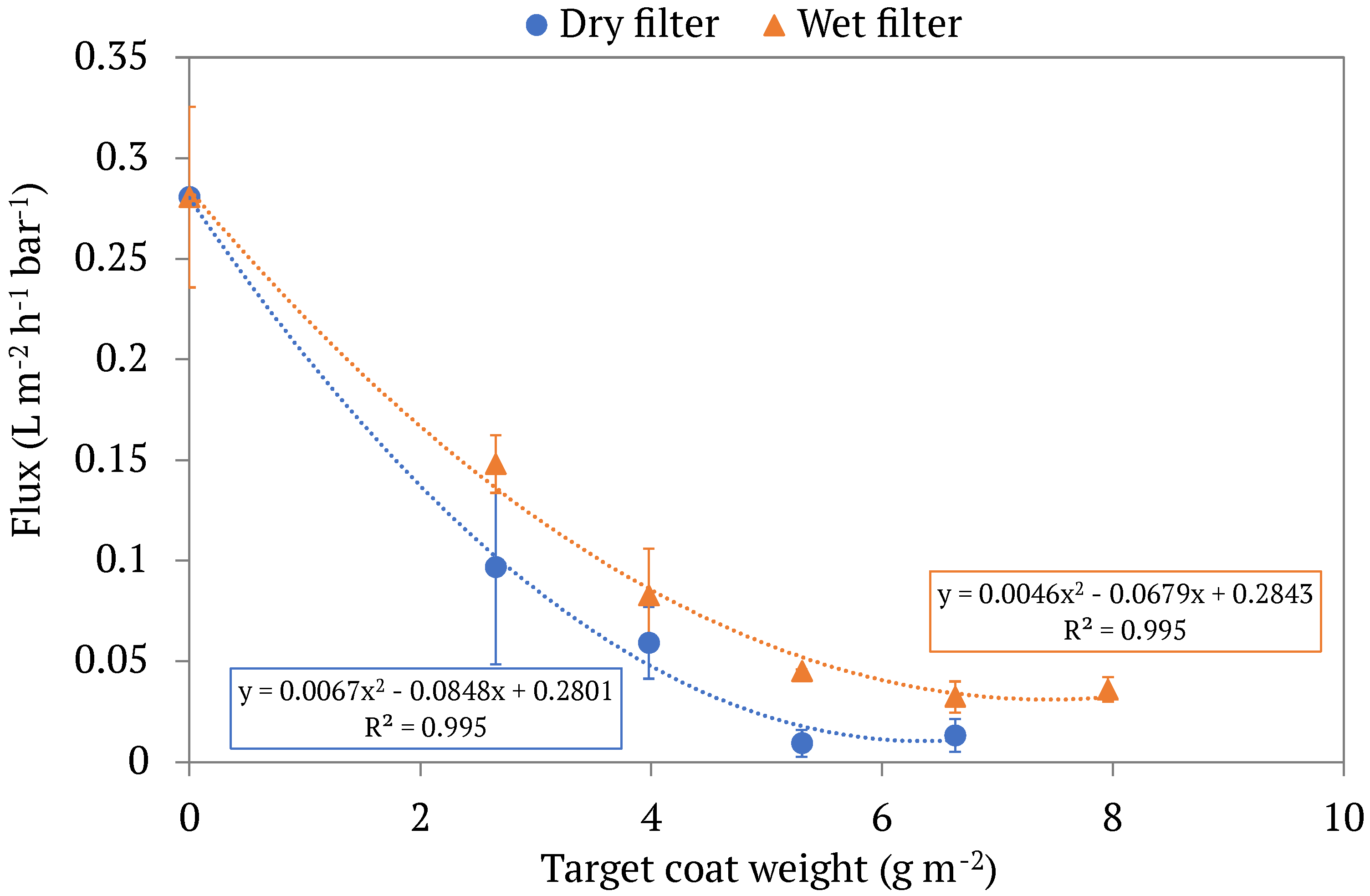
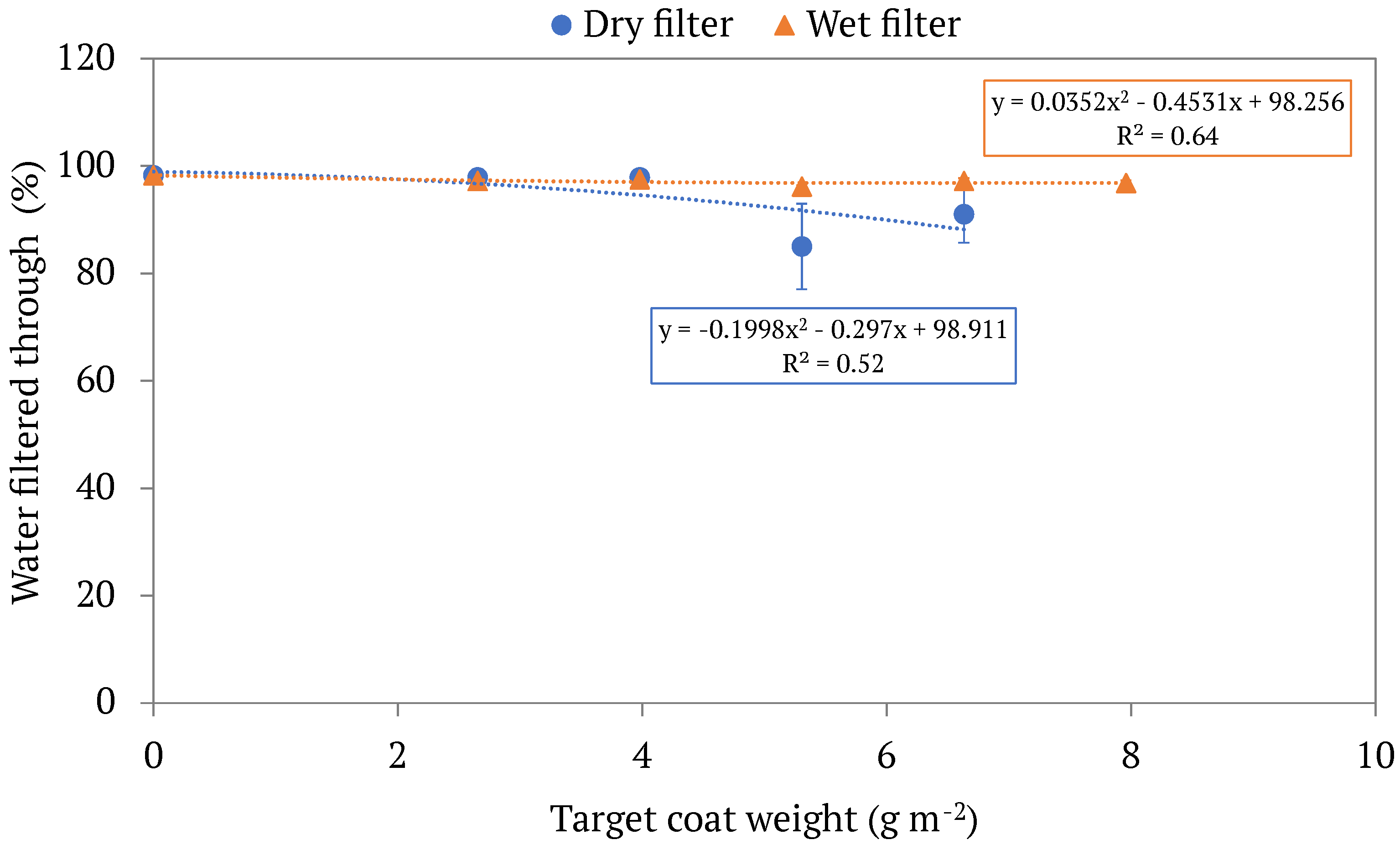
3.1. Filtration Outcomes
3.2. Filter Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, W.; Huang, W.; Zhou, W.; Qiu, Z.; Wang, Z.; Li, H.; Wang, Y.; Li, J.; Xie, Y. Sustainable and antibacterial sandwich-like Ag-Pulp/CNF composite paper for oil/water separation. Carbohydr. Polym. 2020, 245, 116587. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Han, M.; He, F. A review of treating oily wastewater. Arab. J. Chem. 2017, 10, S1913–S1922. [Google Scholar] [CrossRef]
- Kummu, M.; Guillaume, J.H.; de Moel, H.; Eisner, S.; Flörke, M.; Porkka, M.; Siebert, S.; Veldkamp, T.I.; Ward, P.J. The world’s road to water scarcity: Shortage and stress in the 20th century and pathways towards sustainability. Sci. Rep. 2016, 6, 38495. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhan, H.; Li, D.; Tian, H.; Chang, C. Tunicate cellulose nanocrystals modified commercial filter paper for efficient oil/water separation. J. Membr. Sci. 2019, 591, 117362. [Google Scholar] [CrossRef]
- Tayeb, A.H.; Tajvidi, M.; Bousfield, D. Paper-based oil barrier packaging using lignin-containing cellulose nanofibrils. Molecules 2020, 25, 1344. [Google Scholar] [CrossRef]
- Chen, C.; Weng, D.; Mahmood, A.; Chen, S.; Wang, J. Separation mechanism and construction of surfaces with special wettability for oil/water separation. ACS Appl. Mater. Interfaces 2019, 11, 11006–11027. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhong, L.; Zhang, T.; Qiu, F.; Yue, X.; Yang, D. Sustainable, flexible, and superhydrophobic functionalized cellulose aerogel for selective and versatile oil/water separation. ACS Sustain. Chem. Eng. 2019, 7, 9984–9994. [Google Scholar] [CrossRef]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose nanocrystals: Chemistry, self-assembly, and applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef]
- Hubbe, M.A.; Pruszynski, P. Greaseproof paper products: A review emphasizing ecofriendly approaches. BioResources 2020, 15, 1978–2005. [Google Scholar] [CrossRef]
- Tayeb, A.H.; Amini, E.; Ghasemi, S.; Tajvidi, M. Cellulose nanomaterials—Binding properties and applications: A review. Molecules 2018, 23, 2684. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Aguilar, M.; González, I.; Tarrés, Q.; Pèlach, M.À.; Alcalà, M.; Mutjé, P. The key role of lignin in the production of low-cost lignocellulosic nanofibres for papermaking applications. Ind. Crops Prod. 2016, 86, 295–300. [Google Scholar] [CrossRef]
- Rojo, E.; Peresin, M.S.; Sampson, W.W.; Hoeger, I.C.; Vartiainen, J.; Laine, J.; Rojas, O.J. Comprehensive elucidation of the effect of residual lignin on the physical, barrier, mechanical and surface properties of nanocellulose films. Green Chem. 2015, 17, 1853–1866. [Google Scholar] [CrossRef]
- Horseman, T.; Tajvidi, M.; Diop, C.I.; Gardner, D.J. Preparation and property assessment of neat lignocellulose nanofibrils (LCNF) and their composite films. Cellulose 2017, 24, 2455–2468. [Google Scholar] [CrossRef]
- Mazhari Mousavi, S.M.; Afra, E.; Tajvidi, M.; Bousfield, D.W.; Dehghani-Firouzabadi, M. Cellulose nanofiber/carboxymethyl cellulose blends as an efficient coating to improve the structure and barrier properties of paperboard. Cellulose 2017, 24, 3001–3014. [Google Scholar] [CrossRef]
- Kumar, V.; Elfving, A.; Koivula, H.; Bousfield, D.; Toivakka, M. Roll-to-Roll Processed Cellulose Nanofiber Coatings. Ind. Eng. Chem. Resour. 2016, 55, 3603–3613. [Google Scholar] [CrossRef]
- Wang, L.; Chen, C.; Wang, J.; Gardner, D.J.; Tajvidi, M. Cellulose nanofibrils versus cellulose nanocrystals: Comparison of performance in flexible multilayer films for packaging applications. Food Packag. Shelf Life 2020, 23, 100464. [Google Scholar] [CrossRef]
- Yang, C.; Topuz, F.; Sang-Hee, P.; Szekely, G. Biobased thin-film composite membranes comprising priamine–genipin selective layer on nanofibrous biodegradable polylactic acid support for oil and solvent-resistant nanofiltration. Green Chem. 2022, 13, 5291–5303. [Google Scholar] [CrossRef]
- Wang, Y.; He, Y.; Li, H.; Yu, J.; Zhang, L.; Chen, L.; Bai, Y. Layer-by-layer construction of CS-CNCs multilayer modified mesh with robust anti-crude-oil-fouling performance for efficient oil/water separation. J. Membr. Sci. 2021, 639, 119776. [Google Scholar] [CrossRef]
- Alammar, A.; Hardian, R.; Szekely, G. Upcycling agricultural waste into membranes: From date seed biomass to oil and solvent-resistant nanofiltration. Green Chem. 2021, 1, 365–374. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, Y.; Gao, C.; An, Q.; Xiao, Z.; Zhai, S. Superhydrophobic aerogel membrane with integrated functions of biopolymers for efficient oil/water separation. Sep. Purif. Technol. 2022, 282, 120138. [Google Scholar] [CrossRef]
- Liu, Z.; Yu, J.; Lin, W.; Yang, W.; Li, R.; Chen, H.; Zhang, X. Facile method for the hydrophobic modification of filter paper for applications in water-oil separation. Surf. Coat. Technol. 2018, 352, 313–319. [Google Scholar] [CrossRef]
- Wu, H.; Wu, L.; Lu, S.; Lin, X.; Xiao, H.; Ouyang, X.; Cao, S.; Chen, L.; Huang, L. Robust superhydrophobic and superoleophilic filter paper via atom transfer radical polymerization for oil/water separation. Carbohydr. Polym. 2018, 181, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Amini, E.; Hafez, I.; Tajvidi, M.; Bousfield, D. Cellulose and lignocellulose nanofibril suspensions and films: A comparison. Carbohydr. Polym. 2020, 250, 117011. [Google Scholar] [CrossRef] [PubMed]
- Kaelble, D.H. Dispersion-Polar Surface Tension Properties of Organic Solids. J. Adhes. 1970, 2, 66–81. [Google Scholar] [CrossRef]
- Griffin, W.C. Classification of surface-active agents by HLB. Off. J. Soc. Cosmet. Chem. 1949, 1, 311–326. [Google Scholar]
- Ding, Q.; Zeng, J.; Wang, B.; Tang, D.; Chen, K.; Gao, W. Effect of nanocellulose fiber hornification on water fraction characteristics and hydroxyl accessibility during dehydration. Carbohydr. Polym. 2019, 207, 44–51. [Google Scholar] [CrossRef]
- Yang, S.; Wang, T.; Tang, R.; Yan, Q.; Tian, W.; Zhang, L. Enhanced permeability, mechanical and antibacterial properties of cellulose acetate ultrafiltration membranes incorporated with lignocellulose nanofibrils. Int. J. Biol. Macromol. 2020, 151, 159–167. [Google Scholar] [CrossRef]
- Yook, S.; Park, H.; Park, H.; Lee, S.Y.; Kwon, J.; Youn, H.J. Barrier coatings with various types of cellulose nanofibrils and their barrier properties. Cellulose 2020, 27, 4509–4523. [Google Scholar] [CrossRef]
- Hossain, R.; Tajvidi, M.; Bousfield, D.; Gardner, D.J. Multi-layer oil-resistant food serving containers made using cellulose nanofiber coated wood flour composites. Carbohydr. Polym. 2021, 267, 118221. [Google Scholar] [CrossRef]
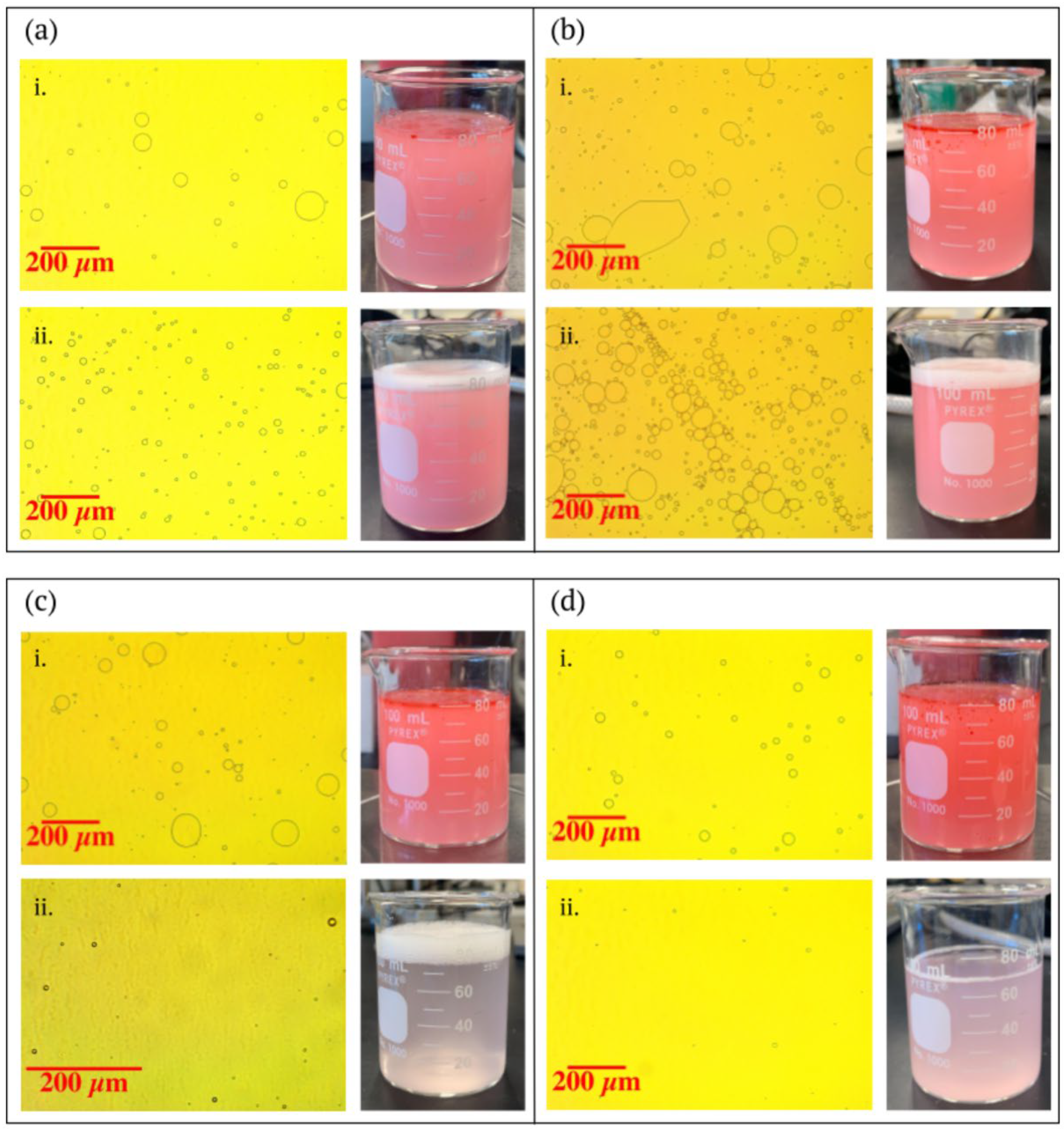


| Wet Filters | Dry Filters | ||||
|---|---|---|---|---|---|
| Coat Weight (gm−2) | Average Oil Particle Size (µm) | Coefficient of Variation (%) | Coat Weight (gm−2) | Average Oil Particle Size (µm) | Coefficient of Variation (%) |
| 0 | 8.4 | 73.1 | 0 | 8.4 | 73.1 |
| 2.44 | 7.0 | 158.0 | 7.16 | 8.1 | 94.9 |
| 3.79 | 2.3 | 660.9 | 8.22 | 4.7 | 74.8 |
| 7.49 | 5.5 | 181.6 | 8.76 | 2.8 | 60.0 |
| 8.34 | 1.0 | 170.3 | 10.62 | 2.7 | 12.9 |
| Coat Weight (gm−2) | 2.87 | 5.49 | 6.02 | 9.23 |
|---|---|---|---|---|
| Water contact angle (°) | 49.9 (±11.4) | 78.5 (±6.1) | 81.1 (±5.7) | 84.9 (±4.4) |
| Diiodomethane contact angle (°) | 19.2 (±5.4) | 46.1 (±3.1) | 43.0 (±4.5) | 45.0 (±6.2) |
| Surface free energy (mN m−1) | 62.8 (±7.5) | 41.2 (±4.0) | 41.5 (±4.3) | 39.5 (±4.7) |
| Dispersive surface energy (mN m−1) | 48.0 (±1.5) | 36.4 (±1.7) | 38.1 (±2.4) | 37.0 (±3.3) |
| Polar surface energy (mN m−1) | 14.8 (±6.0) | 4.7 (±2.4) | 3.4 (±1.9) | 2.5 (±1.4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mittag, A.; Rahman, M.M.; Hafez, I.; Tajvidi, M. Development of Lignin-Containing Cellulose Nanofibrils Coated Paper-Based Filters for Effective Oil-Water Separation. Membranes 2023, 13, 1. https://doi.org/10.3390/membranes13010001
Mittag A, Rahman MM, Hafez I, Tajvidi M. Development of Lignin-Containing Cellulose Nanofibrils Coated Paper-Based Filters for Effective Oil-Water Separation. Membranes. 2023; 13(1):1. https://doi.org/10.3390/membranes13010001
Chicago/Turabian StyleMittag, Anna, Md Musfiqur Rahman, Islam Hafez, and Mehdi Tajvidi. 2023. "Development of Lignin-Containing Cellulose Nanofibrils Coated Paper-Based Filters for Effective Oil-Water Separation" Membranes 13, no. 1: 1. https://doi.org/10.3390/membranes13010001
APA StyleMittag, A., Rahman, M. M., Hafez, I., & Tajvidi, M. (2023). Development of Lignin-Containing Cellulose Nanofibrils Coated Paper-Based Filters for Effective Oil-Water Separation. Membranes, 13(1), 1. https://doi.org/10.3390/membranes13010001








