All-in-One Process for Mass Production of Membrane-Type Carbon Aerogel Electrodes for Solid-State Rechargeable Zinc-Air Batteries
Abstract
:1. Introduction
2. Materials and Methods
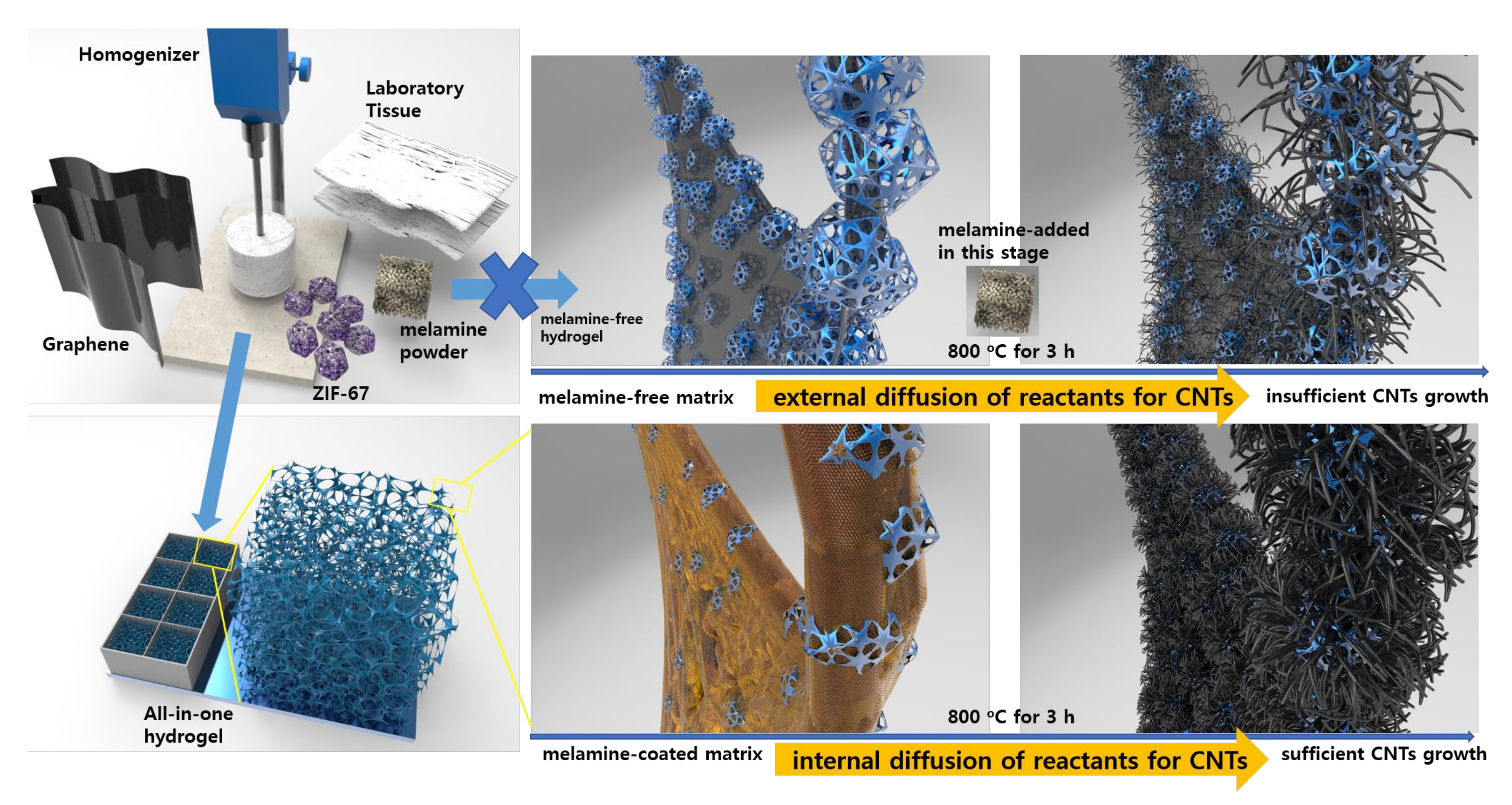
2.1. Preparation of Membrane-Type Carbon Sponge Aerogel Electrodes
2.2. Preparation of Conventional Air Electrodes with Commercial Noble Metal Catalysts
2.3. Electrochemical Analysis
2.4. Preparation and Evaluation of Rechargeable Zinc-Air Batteries
2.5. Characteristics
3. Results & Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zheng, Y.; Yang, D.-S.; Kweun, J.M.; Li, C.; Tan, K.; Kong, F.; Liang, C.; Chabal, Y.J.; Kim, Y.Y.; Cho, M.; et al. Rational design of common transition metal-nitrogen-carbon catalysts for oxygen reduction reaction in fuel cells. Nano Energy 2016, 30, 443–449. [Google Scholar] [CrossRef]
- Dunn, B.; Kamath, H.; Tarascon, J.M. Electrical Energy Storage for the Grid: A Battery of Choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Ji, Y.J.; Wang, P.T.; Shao, Q.; Li, Y.Y.; Huang, X. Adsorbing and Activating N2 on Heterogeneous Au-Fe3O4 Nanoparticles for N-2 Fixation. Adv. Funct. Mater. 2020, 30, 1906579. [Google Scholar] [CrossRef]
- Nielsen, D.U.; Hu, X.M.; Daasbjerg, K.; Skrydstrup, T. Chemically and electrochemically catalysed conversion of CO2 to CO with follow-up utilization to value-added chemicals. Nature Catal. 2018, 1, 244–254. [Google Scholar] [CrossRef]
- Zhu, D.D.; Liu, J.L.; Qiao, S.Z. Recent Advances in Inorganic Heterogeneous Electrocatalysts for Reduction of Carbon Dioxide. Adv. Mater. 2016, 28, 3423–3452. [Google Scholar] [CrossRef]
- Ganesh, I. Conversion of carbon dioxide into methanol—A potential liquid fuel: Fundamental challenges and opportunities (a review). Renew. Sustain. Energy Rev. 2014, 31, 221–257. [Google Scholar] [CrossRef]
- Burdyny, T.; Smith, W.A. CO2 reduction on gas-diffusion electrodes and why catalytic performance must be assessed at commercially-relevant conditions. Energy Environ. Sci. 2019, 12, 1442–1453. [Google Scholar] [CrossRef] [Green Version]
- Bidault, F.; Brett, D.J.L.; Middleton, P.H.; Brandon, N.P. Review of gas diffusion cathodes for alkaline fuel cells. J. Power Sources 2009, 187, 39–48. [Google Scholar] [CrossRef]
- Lee, J.-S.; Tai Kim, S.; Cao, R.; Choi, N.-S.; Liu, M.; Lee, K.T.; Cho, J. Metal–Air Batteries with High Energy Density: Li–Air versus Zn–Air. Adv. Energy Mater. 2011, 1, 34–50. [Google Scholar] [CrossRef]
- Mechili, M.; Vaitsis, C.; Argirusis, N.; Pandis, P.K.; Sourkouni, G.; Argirusis, C. Research progress in transition metal oxide based bifunctional electrocatalysts for aqueous electrically rechargeable zinc-air batteries. Renew. Sustain. Energy Rev. 2022, 156, 111970. [Google Scholar] [CrossRef]
- Ji, D.X.; Fan, L.; Li, L.L.; Peng, S.J.; Yu, D.S.; Song, J.N.; Ramakrishna, S.; Guo, S.J. Atomically Transition Metals on Self-Supported Porous Carbon Flake Arrays as Binder-Free Air Cathode for Wearable Zinc-Air Batteries. Adv. Mater. 2019, 31, 1808267. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.C.; Jia, T.; Wang, B.G. Review-Recent Advance in Self-Supported Electrocatalysts for Rechargeable Zinc-Air Batteries. J. Electrochem. Soc. 2020, 167, 110564. [Google Scholar] [CrossRef]
- Ge, X.M.; Sumboja, A.; Wuu, D.; An, T.; Li, B.; Goh, F.W.T.; Hor, T.S.A.; Zong, Y.; Liu, Z.L. Oxygen Reduction in Alkaline Media: From Mechanisms to Recent Advances of Catalysts. ACS Catal. 2015, 5, 4643–4667. [Google Scholar] [CrossRef]
- Agarwal, S.; Yu, X.W.; Manthiram, A. A pair of metal organic framework (MOF)-derived oxygen reduction reaction (ORR) and oxygen evolution reaction (OER) catalysts for zinc-air batteries. Mater. Today Energy 2020, 16, 100405. [Google Scholar] [CrossRef]
- Zhu, L.; Zheng, D.Z.; Wang, Z.F.; Zheng, X.S.; Fang, P.P.; Zhu, J.F.; Yu, M.H.; Tong, Y.X.; Lu, X.H. A Confinement Strategy for Stabilizing ZIF-Derived Bifunctional Catalysts as a Benchmark Cathode of Flexible All-Solid-State Zinc-Air Batteries. Adv. Mater. 2018, 30, 1805268. [Google Scholar] [CrossRef]
- Wang, T.T.; Kou, Z.K.; Mu, S.C.; Liu, J.P.; He, D.P.; Amiinu, I.S.; Meng, W.; Zhou, K.; Luo, Z.X.; Chaemchuen, S.; et al. 2D Dual-Metal Zeolitic-Imidazolate-Framework-(ZIF)-Derived Bifunctional Air Electrodes with Ultrahigh Electrochemical Properties for Rechargeable Zinc-Air Batteries. Adv. Funct. Mater. 2018, 28, 1705048. [Google Scholar] [CrossRef]
- Zhang, Q.; Xiao, W.; Guo, W.H.; Yang, Y.X.; Lei, J.L.; Luo, H.Q.; Li, N.B. Macroporous Array Induced Multiscale Modulation at the Surface/Interface of Co(OH)(2)/NiMo Self-Supporting Electrode for Effective Overall Water Splitting. Adv. Funct. Mater. 2021, 31, 2102117. [Google Scholar] [CrossRef]
- Yang, H.Y.; Driess, M.; Menezes, P.W. Self-Supported Electrocatalysts for Practical Water Electrolysis. Adv. Energy Mater. 2021, 11, 2102074. [Google Scholar] [CrossRef]
- Weng, C.-C.; Ren, J.-T.; Wang, H.-Y.; Lv, X.-W.; Song, Y.-J.; Wang, Y.-S.; Chen, L.; Tian, W.-W.; Yuan, Z.-Y. Triple-phase oxygen electrocatalysis of hollow spherical structures for rechargeable Zn-Air batteries. Appl. Catal. B Environ. 2022, 307, 121190. [Google Scholar] [CrossRef]
- Ji, D.X.; Fan, L.; Li, L.L.; Mao, N.; Qin, X.H.; Peng, S.J.; Ramakrishna, S. Hierarchical catalytic electrodes of cobalt-embedded carbon nanotubeicarbon flakes arrays for flexible solid-state zinc-air batteries. Carbon 2019, 142, 379–387. [Google Scholar] [CrossRef]
- Jin, Q.Y.; Ren, B.W.; Cui, H.; Wang, C.X. Nitrogen and cobalt co-doped carbon nanotube films as binder-free trifunctional electrode for flexible zinc-air battery and self-powered overall water splitting. Appl. Catal. B Environ. 2021, 283, 119643. [Google Scholar] [CrossRef]
- Xu, N.N.; Wilson, J.A.; Wang, Y.D.; Su, T.S.; Wei, Y.N.; Qiao, J.L.; Zhou, X.D.; Zhang, Y.X.; Sun, S.H. Flexible self-supported bi-metal electrode as a highly stable carbon- and binder-free cathode for large-scale solid-state zinc-air batteries. Appl. Catal. B Environ. 2020, 272, 118953. [Google Scholar] [CrossRef]
- Manawi, Y.M.; Ihsanullah; Samara, A.; Al-Ansari, T.; Atieh, M.A. Review of Carbon Nanomaterials’ Synthesis via the Chemical Vapor Deposition (CVD) Method. Materials 2018, 11, 822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, M.; Ando, Y. Chemical Vapor Deposition of Carbon Nanotubes: A Review on Growth Mechanism and Mass Production. J. Nanosci. Nanotechnol. 2010, 10, 3739–3758. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Zhao, C.; Qu, Y.; Zhou, H.; Zhou, F.; Wang, J.; Wu, Y.; Li, Y. Trifunctional Self-Supporting Cobalt-Embedded Carbon Nanotube Films for ORR, OER, and HER Triggered by Solid Diffusion from Bulk Metal. Adv. Mater. 2019, 31, 1808043. [Google Scholar] [CrossRef]
- Sultan, S.; Tiwari, J.N.; Singh, A.N.; Zhumagali, S.; Ha, M.; Myung, C.W.; Thangavel, P.; Kim, K.S. Single Atoms and Clusters Based Nanomaterials for Hydrogen Evolution, Oxygen Evolution Reactions, and Full Water Splitting. Adv. Energy Mater. 2019, 9, 1900624. [Google Scholar] [CrossRef]
- Han, X.P.; Ling, X.F.; Wang, Y.; Ma, T.Y.; Zhong, C.; Hu, W.B.; Deng, Y.D. Generation of Nanoparticle, Atomic-Cluster, and Single-Atom Cobalt Catalysts from Zeolitic Imidazole Frameworks by Spatial Isolation and Their Use in Zinc-Air Batteries. Angew. Chem. Int. Ed. 2019, 58, 5359–5364. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, X.; Yu, L.; Hou, J.; Zhou, Z.; Lv, R. Atomic Fe–N4/C in Flexible Carbon Fiber Membrane as Binder-Free Air Cathode for Zn–Air Batteries with Stable Cycling over 1000 h. Adv. Mater. 2022, 34, 2105410. [Google Scholar] [CrossRef]
- Son, H.J.; Cho, Y.R.; Park, Y.-E.; Ahn, S.H. Flexible, compressible, versatile biomass-derived freestanding carbon monoliths as binder- and substrate-free tri-functional electrodes for solid-state zinc-air batteries and overall water splitting. Appl. Catal. B-Environ. 2022, 304, 120977. [Google Scholar] [CrossRef]
- Son, H.J.; Kim, M.J.; Ahn, S.H. Monolithic Co-N-C Membrane Integrating Co Atoms and Clusters as a Self-Supporting Multi-Functional Electrode for Solid-State Zinc–Air Batteries and Self-Powered Water Splitting. Chem. Eng. J. 2021, 414, 128739. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, X.; Xi, S.; Zhang, L.; Chen, Z.; Zeng, Z.; Huang, M.; Yang, H.; Liu, B.; Pennycook, S.J.; et al. Atomically Dispersed Cobalt Trifunctional Electrocatalysts with Tailored Coordination Environment for Flexible Rechargeable Zn–Air Battery and Self-Driven Water Splitting. Adv. Energy Mater. 2020, 10, 2002896. [Google Scholar] [CrossRef]
- Ge, H.Y.; Li, G.D.; Shen, J.X.; Ma, W.Q.; Meng, X.G.; Xu, L.Q. Co4N nanoparticles encapsulated in N-doped carbon box as tri-functional catalyst for Zn-air battery and overall water splitting. Appl. Catal. B Environ. 2020, 275, 119104. [Google Scholar] [CrossRef]
- Guo, H.; Feng, Q.C.; Zhu, J.X.; Xu, J.S.; Li, Q.Q.; Liu, S.L.; Xu, K.W.; Zhang, C.; Liu, T.X. Cobalt nanoparticle-embedded nitrogen-doped carbon/carbon nanotube frameworks derived from a metal-organic framework for tri-functional ORR, OER and HER electrocatalysis. J. Mater. Chem. A 2019, 7, 3664–3672. [Google Scholar] [CrossRef]
- Zhou, C.; Zhao, S.; Meng, H.; Han, Y.; Jiang, Q.; Wang, B.; Shi, X.; Zhang, W.; Zhang, L.; Zhang, R. RuCoOx Nanofoam as a High-Performance Trifunctional Electrocatalyst for Rechargeable Zinc–Air Batteries and Water Splitting. Nano Lett. 2021, 21, 9633–9641. [Google Scholar] [CrossRef]
- Yan, L.; Wang, H.; Shen, J.; Ning, J.; Zhong, Y.; Hu, Y. Formation of mesoporous Co/CoS/Metal-N-C@S, N-codoped hairy carbon polyhedrons as an efficient trifunctional electrocatalyst for Zn-air batteries and water splitting. Chem. Eng. J. 2021, 403, 126385. [Google Scholar] [CrossRef]
- Logeshwaran, N.; Ramakrishnan, S.; Chandrasekaran, S.S.; Vinothkannan, M.; Kim, A.R.; Sengodan, S.; Velusamy, D.B.; Varadhan, P.; He, J.-H.; Yoo, D.J. An efficient and durable trifunctional electrocatalyst for zinc–air batteries driven overall water splitting. Appl. Catal. B Environ. 2021, 297, 120405. [Google Scholar] [CrossRef]
- Wang, N.; Ning, S.; Yu, X.; Chen, D.; Li, Z.; Xu, J.; Meng, H.; Zhao, D.; Li, L.; Liu, Q.; et al. Graphene composites with Ru-RuO2 heterostructures: Highly efficient Mott–Schottky-type electrocatalysts for pH-universal water splitting and flexible zinc–air batteries. Appl. Catal. B Environ. 2022, 302, 120838. [Google Scholar] [CrossRef]
- Ramakrishnan, S.; Velusamy, D.B.; Sengodan, S.; Nagaraju, G.; Kim, D.H.; Kim, A.R.; Yoo, D.J. Rational design of multifunctional electrocatalyst: An approach towards efficient overall water splitting and rechargeable flexible solid-state zinc–air battery. Appl. Catal. B Environ. 2022, 300, 120752. [Google Scholar] [CrossRef]
- Zhao, M.; Liu, H.; Zhang, H.; Chen, W.; Sun, H.; Wang, Z.; Zhang, B.; Song, L.; Yang, Y.; Ma, C.; et al. A pH-universal ORR catalyst with single-atom iron sites derived from a double-layer MOF for superior flexible quasi-solid-state rechargeable Zn–air batteries. Energy Environ. Sci. 2021, 14, 6455–6463. [Google Scholar] [CrossRef]
- Ma, L.; Chen, S.; Wang, D.; Yang, Q.; Mo, F.; Liang, G.; Li, N.; Zhang, H.; Zapien, J.A.; Zhi, C. Super-Stretchable Zinc–Air Batteries Based on an Alkaline-Tolerant Dual-Network Hydrogel Electrolyte. Adv. Energy Mater. 2019, 9, 1803046. [Google Scholar] [CrossRef]
- Fan, X.; Liu, J.; Song, Z.; Han, X.; Deng, Y.; Zhong, C.; Hu, W. Porous nanocomposite gel polymer electrolyte with high ionic conductivity and superior electrolyte retention capability for long-cycle-life flexible zinc–air batteries. Nano Energy 2019, 56, 454–462. [Google Scholar] [CrossRef]
- Yang, L.; Shi, L.; Wang, D.; Lv, Y.; Cao, D. Single-atom cobalt electrocatalysts for foldable solid-state Zn-air battery. Nano Energy 2018, 50, 691–698. [Google Scholar] [CrossRef]
- Huang, Y.; Li, Z.; Pei, Z.; Liu, Z.; Li, H.; Zhu, M.; Fan, J.; Dai, Q.; Zhang, M.; Dai, L.; et al. Solid-State Rechargeable Zn//NiCo and Zn–Air Batteries with Ultralong Lifetime and High Capacity: The Role of a Sodium Polyacrylate Hydrogel Electrolyte. Adv. Energy Mater. 2018, 8, 1802288. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, H.-R.; Park, S.-H.; Ahn, S.H. All-in-One Process for Mass Production of Membrane-Type Carbon Aerogel Electrodes for Solid-State Rechargeable Zinc-Air Batteries. Membranes 2022, 12, 1243. https://doi.org/10.3390/membranes12121243
Jo H-R, Park S-H, Ahn SH. All-in-One Process for Mass Production of Membrane-Type Carbon Aerogel Electrodes for Solid-State Rechargeable Zinc-Air Batteries. Membranes. 2022; 12(12):1243. https://doi.org/10.3390/membranes12121243
Chicago/Turabian StyleJo, Hye-Rin, Seung-Hee Park, and Sung Hoon Ahn. 2022. "All-in-One Process for Mass Production of Membrane-Type Carbon Aerogel Electrodes for Solid-State Rechargeable Zinc-Air Batteries" Membranes 12, no. 12: 1243. https://doi.org/10.3390/membranes12121243
APA StyleJo, H.-R., Park, S.-H., & Ahn, S. H. (2022). All-in-One Process for Mass Production of Membrane-Type Carbon Aerogel Electrodes for Solid-State Rechargeable Zinc-Air Batteries. Membranes, 12(12), 1243. https://doi.org/10.3390/membranes12121243






