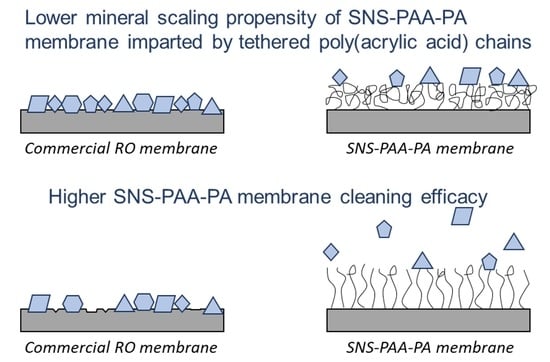
Calcium Sulfate and Calcium Carbonate Scaling of Thin-Film Composite Polyamide Reverse Osmosis Membranes with Surface-Tethered Polyacrylic Acid Chains
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Atmospheric-Pressure-Induced Graft Polymerization (APPIGP)
2.3. Surface Scanning Images
2.4. Membrane Performance Characterization
2.4.1. Permeability
2.4.2. Mineral Scaling Tests
3. Results and Discussion
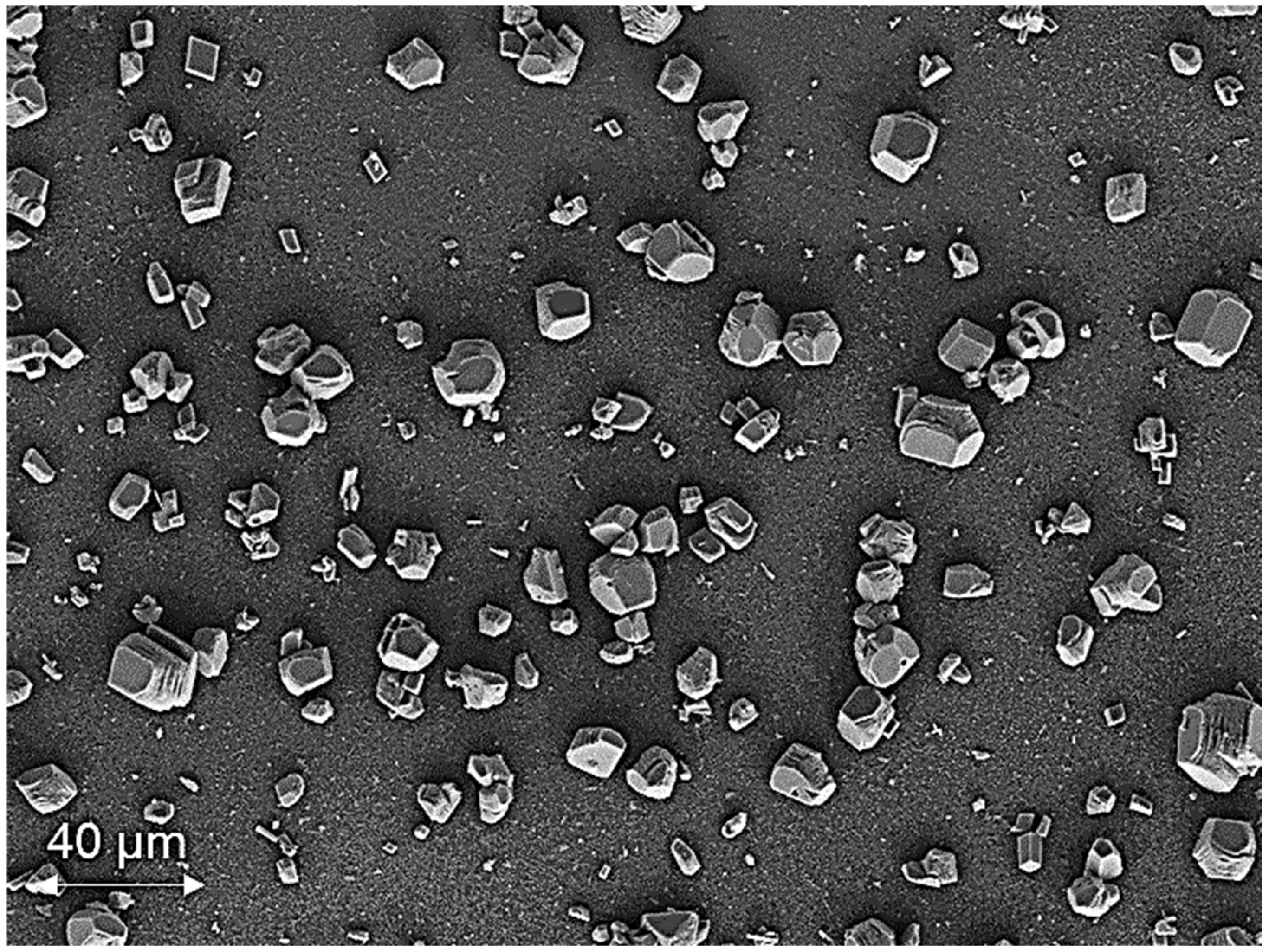
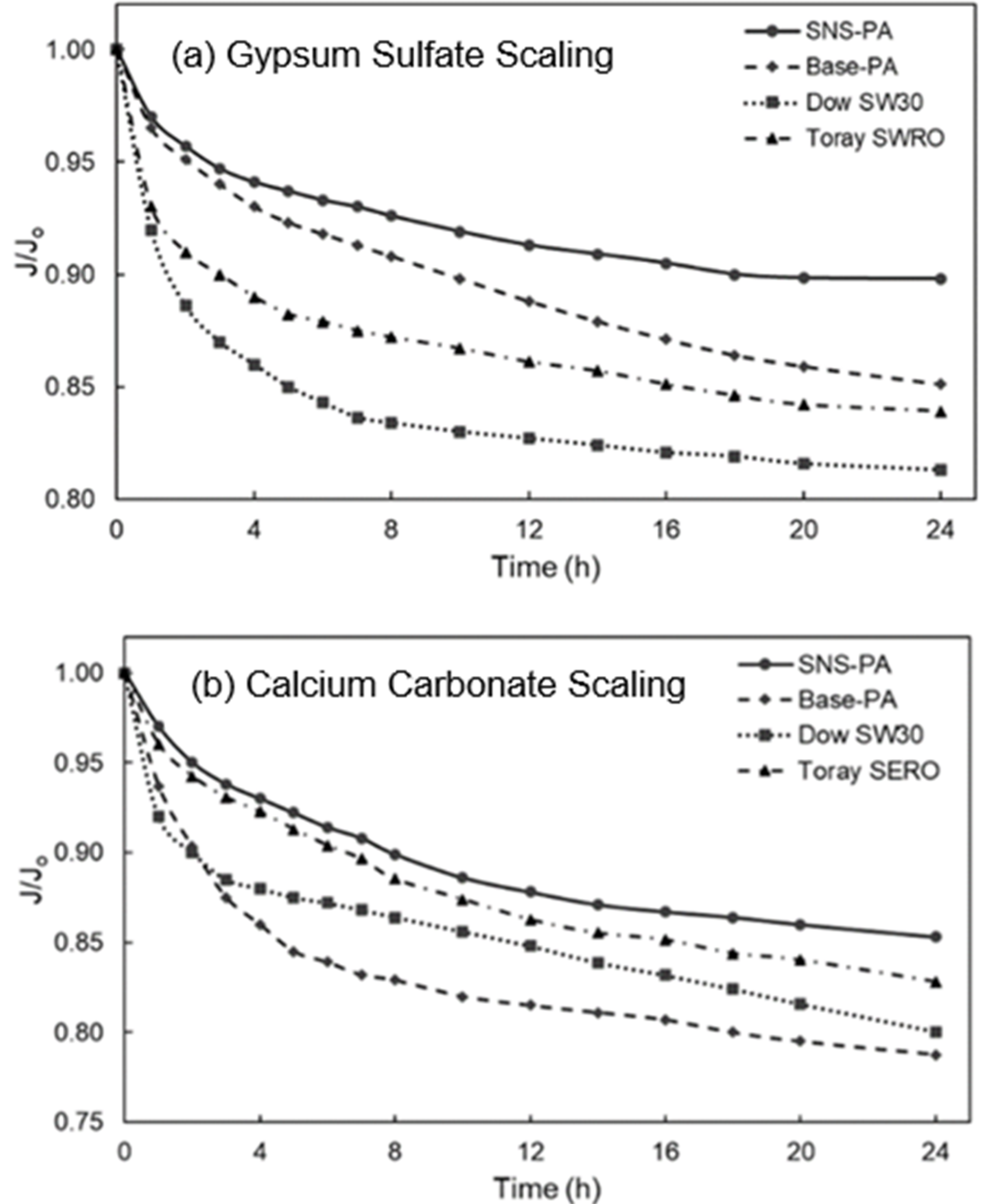
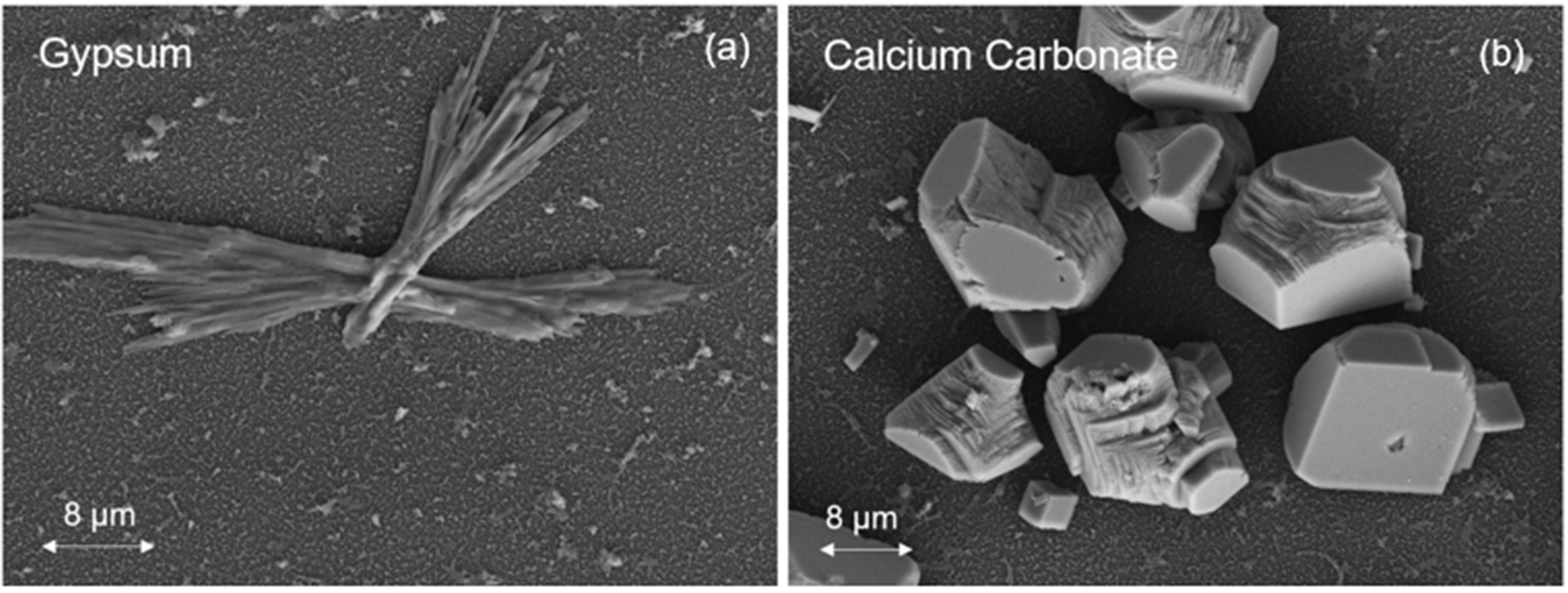
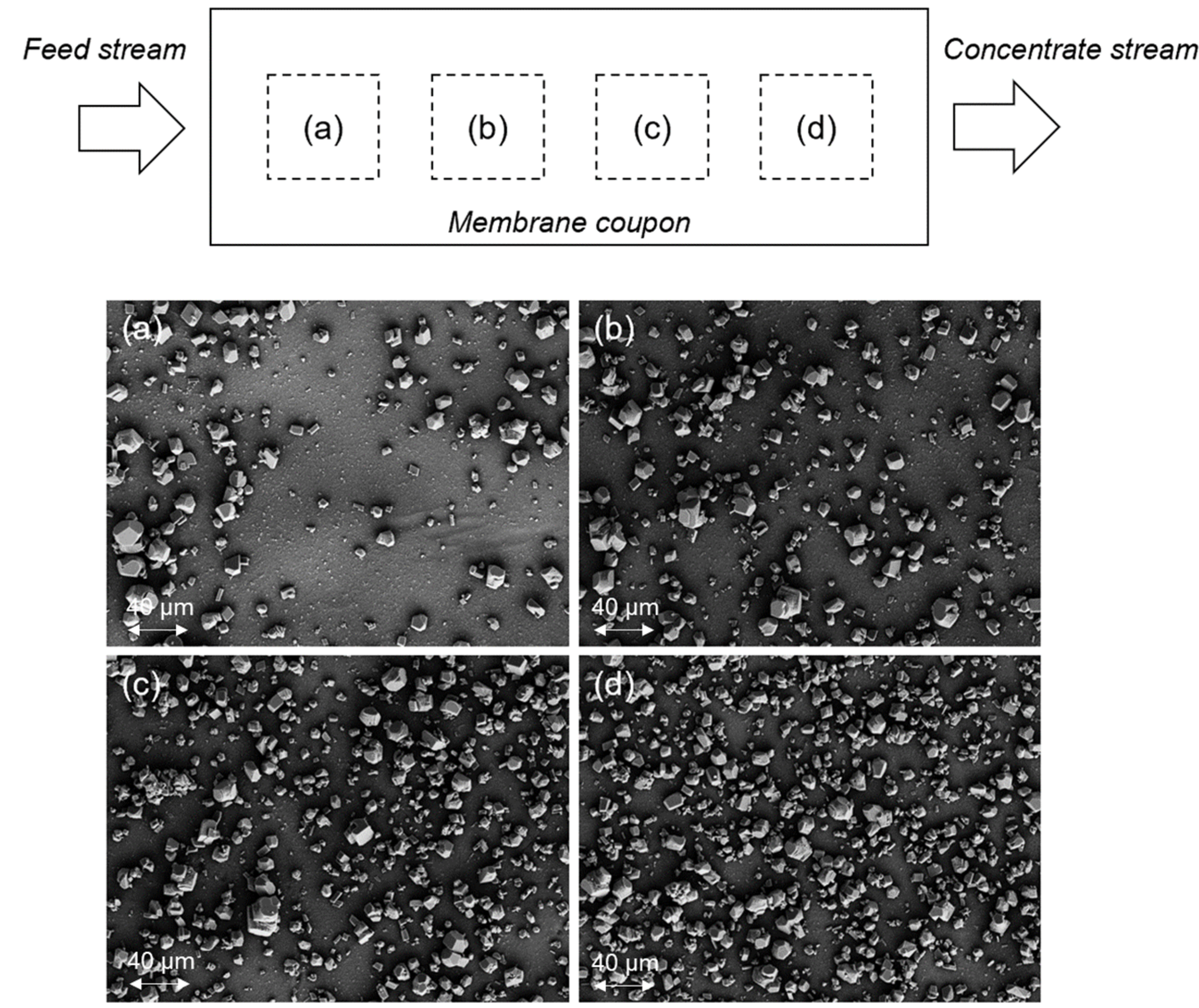
3.1. Membrane Scaling
3.2. Membrane Cleaning and Water Permeability Recovery
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Malaeb, L.; Ayoub, G.M. Reverse osmosis technology for water treatment: State of the art review. Desalination 2011, 267, 1–8. [Google Scholar] [CrossRef]
- Garud, R.; Kore, S.; Kore, V.; Kulkarni, G. A Short Review on Process and Applications of Reverse Osmosis. Univers. J. Environ. Res. Technol. 2011, 1, 233–238. [Google Scholar]
- Matin, A.; Rahman, F.; Shafi, H.Z.; Zubair, S.M. Scaling of reverse osmosis membranes used in water desalination: Phenomena, impact, and control; future directions. Desalination 2019, 455, 135–157. [Google Scholar] [CrossRef]
- Zhao, S.; Zou, L.; Mulcahy, D. Brackish water desalination by a hybrid forward osmosis–nanofiltration system using divalent draw solute. Desalination 2012, 284, 175–181. [Google Scholar] [CrossRef]
- Hegab, H.M.; Zou, L. Graphene oxide-assisted membranes: Fabrication and potential applications in desalination and water purification. J. Membr. Sci. 2015, 484, 95–106. [Google Scholar] [CrossRef]
- Rahardianto, A.; Shih, W.-Y.; Lee, R.-W.; Cohen, Y. Diagnostic characterization of gypsum scale formation and control in RO membrane desalination of brackish water. J. Membr. Sci. 2006, 279, 655–668. [Google Scholar] [CrossRef]
- Tong, T.; Wallace, A.F.; Zhao, S.; Wang, Z. Mineral scaling in membrane desalination: Mechanisms, mitigation strategies, and feasibility of scaling-resistant membranes. J. Membr. Sci. 2019, 579, 52–69. [Google Scholar] [CrossRef]
- Hasson, D.; Shemer, H.; Brook, I.; Zaslavschi, I.; Semiat, R.; Bartels, C.; Wilf, M. Scaling propensity of seawater in RO boron removal processes. J. Membr. Sci. 2011, 384, 198–204. [Google Scholar] [CrossRef]
- Rana, D.; Matsuura, T. Surface Modifications for Antifouling Membranes. Chem. Rev. 2010, 110, 2448–2471. [Google Scholar] [CrossRef]
- Asadollahi, M.; Bastani, D.; Musavi, S.A. Enhancement of surface properties and performance of reverse osmosis membranes after surface modification: A review. Desalination 2017, 420, 330–383. [Google Scholar] [CrossRef]
- Karanikola, V.; Boo, C.; Rolf, J.; Elimelech, M. Engineered Slippery Surface to Mitigate Gypsum Scaling in Membrane Distillation for Treatment of Hypersaline Industrial Wastewaters. Environ. Sci. Technol. 2018, 52, 14362–14370. [Google Scholar] [CrossRef]
- Kang, N.W.; Lee, S.; Kim, D.; Hong, S.; Kweon, J.H. Analyses of calcium carbonate scale deposition on four RO membranes under a seawater desalination condition. Water Sci. Technol. 2011, 64, 1573–1580. [Google Scholar] [CrossRef]
- Ashfaq, M.Y.; Al-Ghouti, M.A.; Zouari, N. Functionalization of reverse osmosis membrane with graphene oxide and polyacrylic acid to control biofouling and mineral scaling. Sci. Total. Environ. 2020, 736, 139500. [Google Scholar] [CrossRef]
- Ansari, A.; Peña-Bahamonde, J.; Wang, M.; Shaffer, D.L.; Hu, Y.; Rodrigues, D.F. Polyacrylic acid-brushes tethered to graphene oxide membrane coating for scaling and biofouling mitigation on reverse osmosis membranes. J. Membr. Sci. 2021, 630, 119308. [Google Scholar] [CrossRef]
- Cao, B.; Ansari, A.; Yi, X.; Rodrigues, D.F.; Hu, Y. Gypsum scale formation on graphene oxide modified reverse osmosis membrane. J. Membr. Sci. 2018, 552, 132–143. [Google Scholar] [CrossRef]
- Maruf, S.H.; Greenberg, A.R.; Pellegrino, J.; Ding, Y. Fabrication and characterization of a surface-patterned thin film composite membrane. J. Membr. Sci. 2014, 452, 11–19. [Google Scholar] [CrossRef]
- Choi, W.; Lee, C.; Lee, D.; Won, Y.J.; Lee, G.W.; Shin, M.G.; Chun, B.; Kim, T.-S.; Park, H.-D.; Jung, H.W.; et al. Sharkskin-mimetic desalination membranes with ultralow biofouling. J. Mater. Chem. A 2018, 6, 23034–23045. [Google Scholar] [CrossRef]
- Wiese, M.; Nir, O.; Wypysek, D.; Pokern, L.; Wessling, M. Fouling minimization at membranes having a 3D surface topology with microgels as soft model colloids. J. Membr. Sci. 2018, 569, 7–16. [Google Scholar] [CrossRef]
- Duan, W.; Dudchenko, A.; Mende, E.; Flyer, C.; Zhu, X.; Jassby, D. Electrochemical mineral scale prevention and removal on electrically conducting carbon nanotube—polyamide reverse osmosis membranes. Environ. Sci. Process. Impacts 2014, 16, 1300–1308. [Google Scholar] [CrossRef]
- Ray, J.R.; Wong, W.; Jun, Y.-S. Antiscaling efficacy of CaCO3and CaSO4on polyethylene glycol (PEG)-modified reverse osmosis membranes in the presence of humic acid: Interplay of membrane surface properties and water chemistry. Phys. Chem. Chem. Phys. 2017, 19, 5647–5657. [Google Scholar] [CrossRef]
- Kim, M.-M.; Lin, N.H.; Lewis, G.T.; Cohen, Y. Surface nano-structuring of reverse osmosis membranes via atmospheric pressure plasma-induced graft polymerization for reduction of mineral scaling propensity. J. Membr. Sci. 2010, 354, 142–149. [Google Scholar] [CrossRef]
- Lin, N.H.; Kim, M.-M.; Lewis, G.T.; Cohen, Y. Polymer surface nano-structuring of reverse osmosis membranes for fouling resistance and improved flux performance. J. Mater. Chem. 2010, 20, 4642–4652. [Google Scholar] [CrossRef]
- Jaramillo, H.; Boo, C.; Hashmi, S.M.; Elimelech, M. Zwitterionic coating on thin-film composite membranes to delay gypsum scaling in reverse osmosis. J. Membr. Sci. 2020, 618, 118568. [Google Scholar] [CrossRef]
- Kang, G.-D.; Cao, Y.-M. Development of antifouling reverse osmosis membranes for water treatment: A review. Water Res. 2012, 46, 584–600. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Rovira-Bru, M.; Giralt, F.; Cohen, Y. Hydraulic Resistance and Protein Fouling Resistance of a Zirconia Membrane with a Tethered PVP Layer. Water 2021, 13, 951. [Google Scholar] [CrossRef]
- Sheikholeslami, R.; Ong, H. Kinetics and thermodynamics of calcium carbonate and calcium sulfate at salinities up to 1.5 M. Desalination 2003, 157, 217–234. [Google Scholar] [CrossRef]
- Yin, Y.; Kalam, S.; Livingston, J.L.; Minjarez, R.; Lee, J.; Lin, S.; Tong, T. The use of anti-scalants in gypsum scaling mitigation: Comparison with membrane surface modification and efficiency in combined reverse osmosis and membrane distillation. J. Membr. Sci. 2021, 643, 120077. [Google Scholar] [CrossRef]
- Chen, Y.; Kim, S.; Kim, Y.; Walker, J.S.; Wolfe, T.; Coleman, K.; Cohen, Y. Scale up of polyamide reverse osmosis membranes surface modification with tethered poly(acrylic acid) for fabrication of low fouling spiral-wound elements. Desalination 2022, 536, 115762. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, J.; Cohen, Y. Fouling resistant and performance tunable ultrafiltration membranes via surface graft polymerization induced by atmospheric pressure air plasma. Sep. Purif. Technol. 2022, 286. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Zohuriaan-Mehr, M.J. Modification of carbohydrate polymers via grafting in air. 1. ceric-induced synthesis of starch-g-polyacrylonitrile in presence and absence of oxygen. Starch-Stärke 2002, 54, 140–147. [Google Scholar] [CrossRef]
- Kim, S.; Chen, Y.; Yoram, C. Surface Modified Reverse Osmosis Membranes. In The World Scientific Reference of Water Science; World Scientific Publishing: Hackensack, NJ, USA, 2022; pp. 307–376. [Google Scholar]
- Cohen, Y.; Lin, N.; Varin, K.J.; Chien, D.; Hicks, R.F. Membrane Surface Nanostructuring with Terminally Anchored Polymer Chains. In Functional Nanostructured Materials and Membranes for Water Treatment; Wiley: Hoboken, NJ, USA, 2013; pp. 85–124. [Google Scholar] [CrossRef]
- Yang, Z.; Guo, H.; Tang, C.Y. The upper bound of thin-film composite (TFC) polyamide membranes for desalination. J. Membr. Sci. 2019, 590, 117297. [Google Scholar] [CrossRef]
- Chen, Y.; Kim, S.; Cohen, Y. Tuning the hydraulic permeability and molecular weight cutoff (MWCO) of surface nano-structured ultrafiltration membranes. J. Membr. Sci. 2021, 629. [Google Scholar] [CrossRef]
- Rahardianto, A.; McCool, B.C.; Cohen, Y. Reverse Osmosis Desalting of Inland Brackish Water of High Gypsum Scaling Propensity: Kinetics and Mitigation of Membrane Mineral Scaling. Environ. Sci. Technol. 2008, 42, 4292–4297. [Google Scholar] [CrossRef]
- Gilron, J.; Hasson, D. Calcium sulphate fouling of reverse osmosis membranes: Flux decline mechanism. Chem. Eng. Sci. 1987, 42, 2351–2360. [Google Scholar] [CrossRef]
- le Gouellec, Y.A.; Elimelech, M. Calcium sulfate (gypsum) scaling in nanofiltration of agricultural drainage water. J. Membr. Sci. 2002, 205, 279–291. [Google Scholar] [CrossRef]
- Shih, W.-Y.; Rahardianto, A.; Lee, R.-W.; Cohen, Y. Morphometric characterization of calcium sulfate dihydrate (gypsum) scale on reverse osmosis membranes. J. Membr. Sci. 2005, 252, 253–263. [Google Scholar] [CrossRef]
- Lee, S.; Lee, C.-H. Effect of operating conditions on CaSO4 scale formation mechanism in nanofiltration for water softening. Water Res. 2000, 34, 3854–3866. [Google Scholar] [CrossRef]
- Supekar, O.D.; Park, D.J.; Greenberg, A.R.; Gopinath, J.T.; Bright, V.M. Real-time detection of early-stage calcium sulfate and calcium carbonate scaling using Raman spectroscopy. J. Membr. Sci. 2019, 596, 117603. [Google Scholar] [CrossRef]
- Thompson, J.; Lin, N.; Lyster, E.; Arbel, R.; Knoell, T.; Gilron, J.; Cohen, Y. RO membrane mineral scaling in the presence of a biofilm. J. Membr. Sci. 2012, 415-416, 181–191. [Google Scholar] [CrossRef]
- Lyster, E.; Cohen, Y. Numerical study of concentration polarization in a rectangular reverse osmosis membrane channel: Permeate flux variation and hydrodynamic end effects. J. Membr. Sci. 2007, 303, 140–153. [Google Scholar] [CrossRef]
- Lyster, E.; Au, J.; Rallo, R.; Giralt, F.; Cohen, Y. Coupled 3-D hydrodynamics and mass transfer analysis of mineral scaling-induced flux decline in a laboratory plate-and-frame reverse osmosis membrane module. J. Membr. Sci. 2009, 339, 39–48. [Google Scholar] [CrossRef]
- McCool, B.C.; Rahardianto, A.; Faria, J.; Kovac, K.; Lara, D.; Cohen, Y. Feasibility of reverse osmosis desalination of brackish agricultural drainage water in the San Joaquin Valley. Desalination 2010, 261, 240–250. [Google Scholar] [CrossRef]
- Cohen, Y.; Rahardianto, A.; Christofides, P.D.; Thompson, J.F.; Gao, L. Pilot-Scale Evaluation of High Recovery Desalination of Agricultural Drainage Water with Smart Integrated Membrane Systems (SIMS). Desalination and Water Purification Research and Development Program Final Report No. 173; U.S. Department of the Interior Bureau of Reclamation Technical Service Center: Denver, CO, USA, 2016. [Google Scholar]
- Varin, K.J.; Lin, N.H.; Cohen, Y. Biofouling and cleaning effectiveness of surface nanostructured reverse osmosis membranes. J. Membr. Sci. 2013, 446, 472–481. [Google Scholar] [CrossRef]
- Uchymiak, M.; Rahardianto, A.; Lyster, E.; Glater, J.; Cohen, Y. A novel RO ex situ scale observation detector (EXSOD) for mineral scale characterization and early detection. J. Membr. Sci. 2007, 291, 86–95. [Google Scholar] [CrossRef]
- Klimchouk, A. The dissolution and conversion of gypsum and anhydrite. Int. J. Speleol. 1996, 25, 21–36. [Google Scholar] [CrossRef]
- Zhang, X.; Tian, J.; Gao, S.; Shi, W.; Zhang, Z.; Cui, F.; Zhang, S.; Guo, S.; Yang, X.; Xie, H.; et al. Surface functionalization of TFC FO membranes with zwitterionic polymers: Improvement of antifouling and salt-responsive cleaning properties. J. Membr. Sci. 2017, 544, 368–377. [Google Scholar] [CrossRef]
- Darvishmanesh, S.; Qian, X.; Wickramasinghe, S.R. Responsive membranes for advanced separations. Curr. Opin. Chem. Eng. 2015, 8, 98–104. [Google Scholar] [CrossRef]
- Drechsler, A.; Elmahdy, M.M.; Uhlmann, P.; Stamm, M. pH and Salt Response of Mixed Brushes Made of Oppositely Charged Polyelectrolytes Studied by in Situ AFM Force Measurements and Imaging. Langmuir 2018, 34, 4739–4749. [Google Scholar] [CrossRef]
- Meng, J.; Cao, Z.; Ni, L.; Zhang, Y.; Wang, X.; Zhang, X.; Liu, E. A novel salt-responsive TFC RO membrane having superior antifouling and easy-cleaning properties. J. Membr. Sci. 2014, 461, 123–129. [Google Scholar] [CrossRef]
- You, M.; Wang, P.; Xu, M.; Yuan, T.; Meng, J. Fouling resistance and cleaning efficiency of stimuli-responsive reverse osmosis (RO) membranes. Polymer 2016, 103, 457–467. [Google Scholar] [CrossRef]
- Liu, H.; Yang, S.; Liu, Y.; Miao, M.; Zhao, Y.; Sotto, A.; Gao, C.; Shen, J. Fabricating a pH-responsive membrane through interfacial in-situ assembly of microgels for water gating and self-cleaning. J. Membr. Sci. 2019, 579, 230–239. [Google Scholar] [CrossRef]
- Sun, C.; Feng, X. Enhancing the performance of PVDF membranes by hydrophilic surface modification via amine treatment. Sep. Purif. Technol. 2017, 185, 94–102. [Google Scholar] [CrossRef]
- Israelachvili, J.N. Intermolecular and Surface Forces; Academic Press: Washington, DC, USA, 2015. [Google Scholar]
- Feast, W.J.; Munro, H.S. Polymer Surfaces and Interfaces; Wiley: New York, NY, USA, 1987. [Google Scholar]
- Marshall, A.; Munro, P.; Trägårdh, G. The effect of protein fouling in microfiltration and ultrafiltration on permeate flux, protein retention and selectivity: A literature review. Desalination 1993, 91, 65–108. [Google Scholar] [CrossRef]
- Fane, A.; Fell, C.; Suki, A. The effect of pH and ionic environment on the ultrafiltration of protein solutions with retentive membranes. J. Membr. Sci. 1983, 16, 195–210. [Google Scholar] [CrossRef]
- Larson, T.E.; Buswell, A.M.; Ludwig, H.F.; Langelier, W. Calcium carbonate saturation index and alkalinity interpretations [with discussion]. J. Am. Water Work. Assoc. 1942, 34, 1667–1684. [Google Scholar] [CrossRef]
- Warsinger, D.M.; Swaminathan, J.; Guillen-Burrieza, E.; Arafat, H.A.; Lienhard V, J.H. Scaling and fouling in membrane distillation for desalination applications: A review. Desalination 2015, 356, 294–313. [Google Scholar] [CrossRef]
- Wang, Y.-N.; Järvelä, E.; Wei, J.; Zhang, M.; Kyllönen, H.; Wang, R.; Tang, C.Y. Gypsum scaling and membrane integrity of osmotically driven membranes: The effect of membrane materials and operating conditions. Desalination 2016, 377, 1–10. [Google Scholar] [CrossRef]
- Peña, N.; Gallego, S.; Del Vigo, F.; Chesters, S. Evaluating impact of fouling on reverse osmosis membranes performance. Desalination Water Treat. 2013, 51, 958–968. [Google Scholar] [CrossRef]
- Sarker, N.; Cherukupally, P.; Gourevich, I.; Wilbur, J.; Jons, S.; Bilton, A. Multi-scale visualization of incipient CaCO3 scaling on the polyamide layer of reverse osmosis membranes. Desalination 2022, 539, 115956. [Google Scholar] [CrossRef]
- Benecke, J.; Haas, M.; Baur, F.; Ernst, M. Investigating the development and reproducibility of heterogeneous gypsum scaling on reverse osmosis membranes using real-time membrane surface imaging. Desalination 2018, 428, 161–171. [Google Scholar] [CrossRef]
- Tzotzi, C.; Pahiadaki, T.; Yiantsios, S.; Karabelas, A.; Andritsos, N. A study of CaCO3 scale formation and inhibition in RO and NF membrane processes. J. Membr. Sci. 2007, 296, 171–184. [Google Scholar] [CrossRef]
- Shmulevsky, M.; Li, X.; Shemer, H.; Hasson, D.; Semiat, R. Analysis of the onset of calcium sulfate scaling on RO membranes. J. Membr. Sci. 2017, 524, 299–304. [Google Scholar] [CrossRef]


| Membrane (b) | Permeability (c) (L·m−1·h−1·bar−1) | Rejection (d) (%) | |
|---|---|---|---|
| Observed | Intrinsic | ||
| SNS-PAA-PA | 1.69 | 99.3 | 99.5 |
| Toray SWRO | 1.33 | 99.0 | 99.2 |
| Base-PA | 2.91 | 99.0 | 99.3 |
| Dow SW30 | 1.60 | 89.9 | 99.2 |
| Ions | Model Solution Composition (a) | |
|---|---|---|
| Calcium Sulfate | Calcium Carbonate | |
| Ca2+ | 11.29 mM | 11.29 mM |
| Na+ | 127.95 mM | 160.33 mM |
| Cl− | 33.67 mM | 178.89 mM |
| SO42− | 58.43 mM | - |
| HCO3− | - | 4.02 mM |
| pH | 5.7 | 7.9 |
| TDS | 2611 mg/L | 2611 mg/L |
| Saturation index in solution (b) | SIg = 1.0 | SIc = 6.3 |
| Saturation index at membrane surface (c) | SIg,m = 1.7 | SIc,m = 10.6 |
| Membrane | Scalant | J/Jo (b) | Lp/Lp,o (c) |
|---|---|---|---|
| SNS-PAA-PA | Gypsum | 0.89 | 1.00 |
| Dow SW30 | Gypsum | 0.81 | 1.00 |
| Toray SWRO | Gypsum | 0.84 | 0.98 |
| Base-PA | Gypsum | 0.85 | 0.92 |
| SNS-PAA-PA | Calcite | 0.85 | 0.94 |
| Dow SW30 | Calcite | 0.80 | 1.00 |
| Toray SWRO | Calcite | 0.83 | 1.00 |
| Base-PA | Calcite | 0.79 | 1.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Cohen, Y. Calcium Sulfate and Calcium Carbonate Scaling of Thin-Film Composite Polyamide Reverse Osmosis Membranes with Surface-Tethered Polyacrylic Acid Chains. Membranes 2022, 12, 1287. https://doi.org/10.3390/membranes12121287
Chen Y, Cohen Y. Calcium Sulfate and Calcium Carbonate Scaling of Thin-Film Composite Polyamide Reverse Osmosis Membranes with Surface-Tethered Polyacrylic Acid Chains. Membranes. 2022; 12(12):1287. https://doi.org/10.3390/membranes12121287
Chicago/Turabian StyleChen, Yian, and Yoram Cohen. 2022. "Calcium Sulfate and Calcium Carbonate Scaling of Thin-Film Composite Polyamide Reverse Osmosis Membranes with Surface-Tethered Polyacrylic Acid Chains" Membranes 12, no. 12: 1287. https://doi.org/10.3390/membranes12121287
APA StyleChen, Y., & Cohen, Y. (2022). Calcium Sulfate and Calcium Carbonate Scaling of Thin-Film Composite Polyamide Reverse Osmosis Membranes with Surface-Tethered Polyacrylic Acid Chains. Membranes, 12(12), 1287. https://doi.org/10.3390/membranes12121287