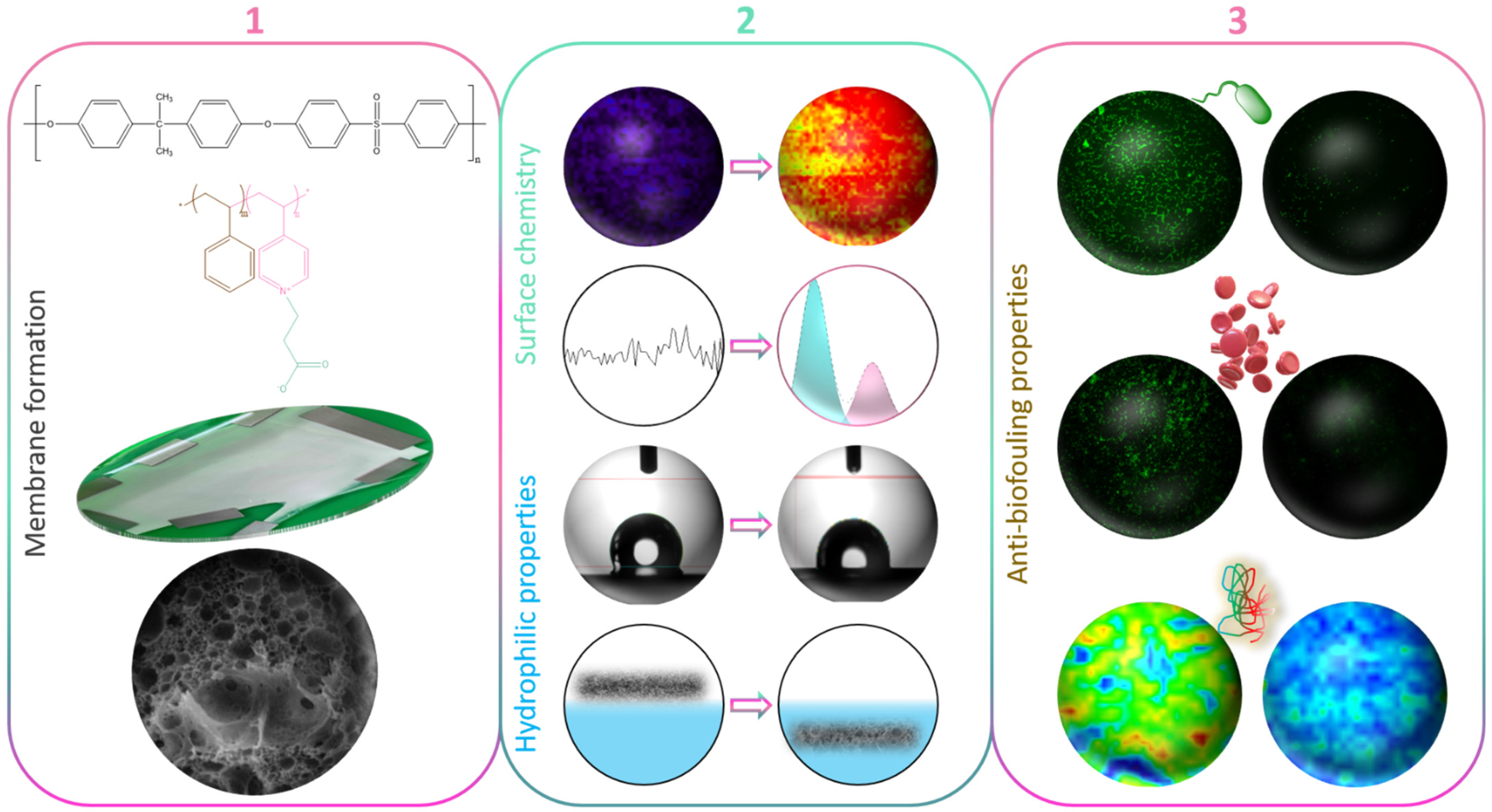
A Biofouling Resistant Zwitterionic Polysulfone Membrane Prepared by a Dual-Bath Procedure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
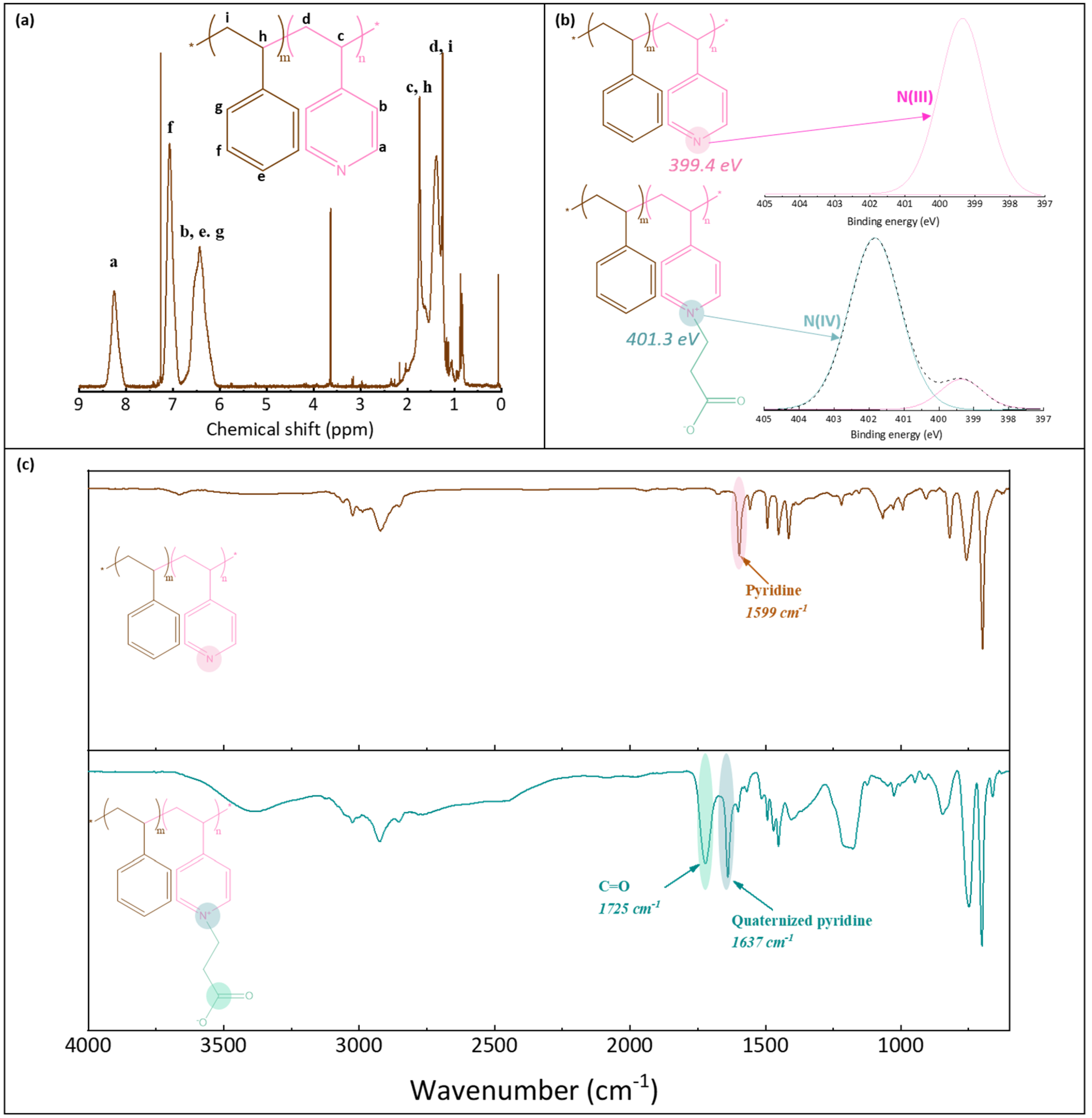
2.2. Synthesis and Characterization of Random Copolymers
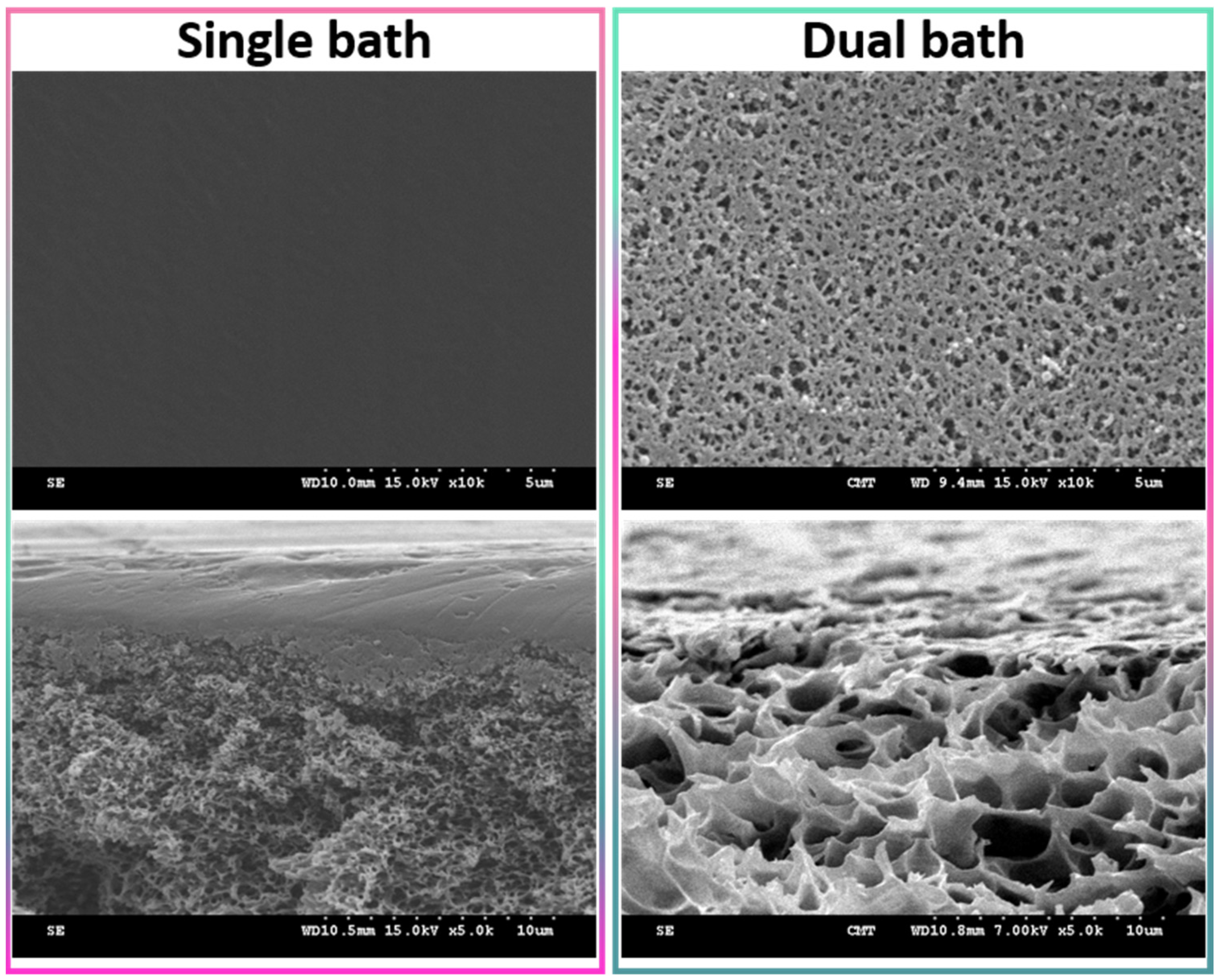
2.3. Casting Solution and Membrane Preparation
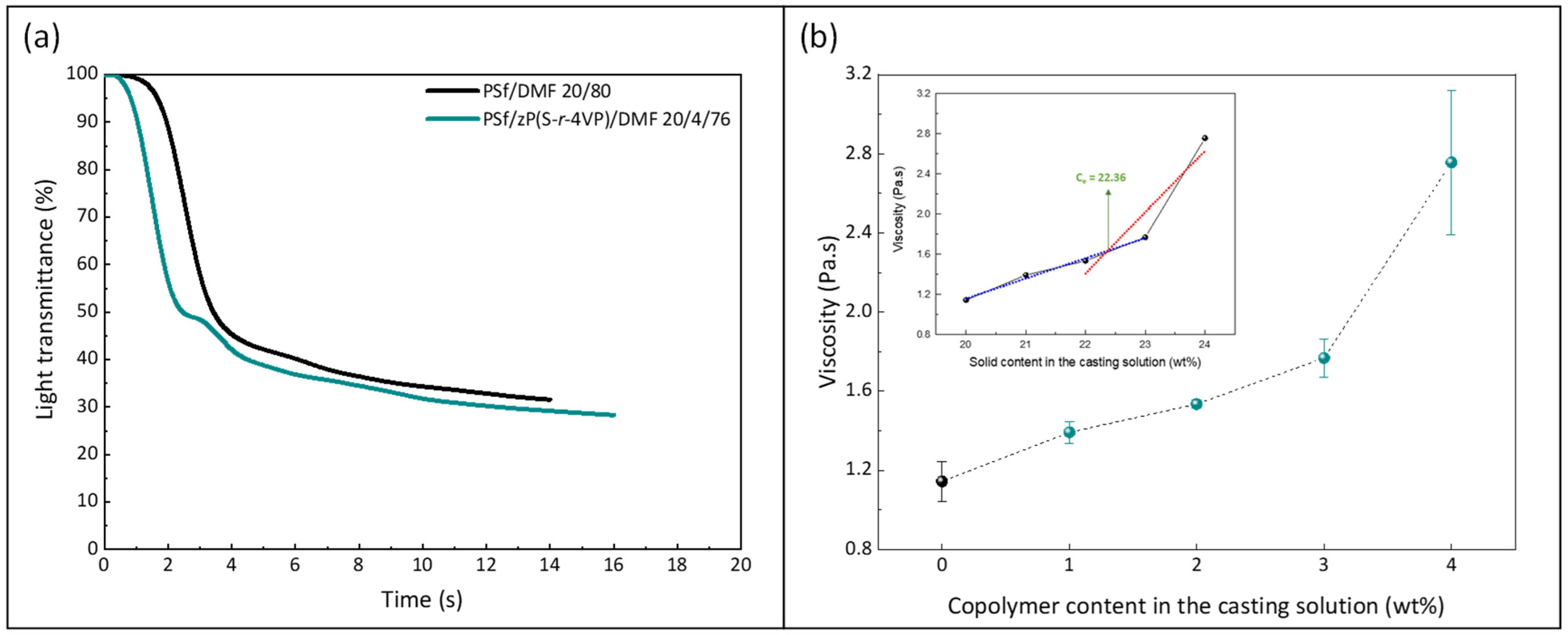
2.4. Light Transmittance Tests
2.5. Physical Characterization of Membranes
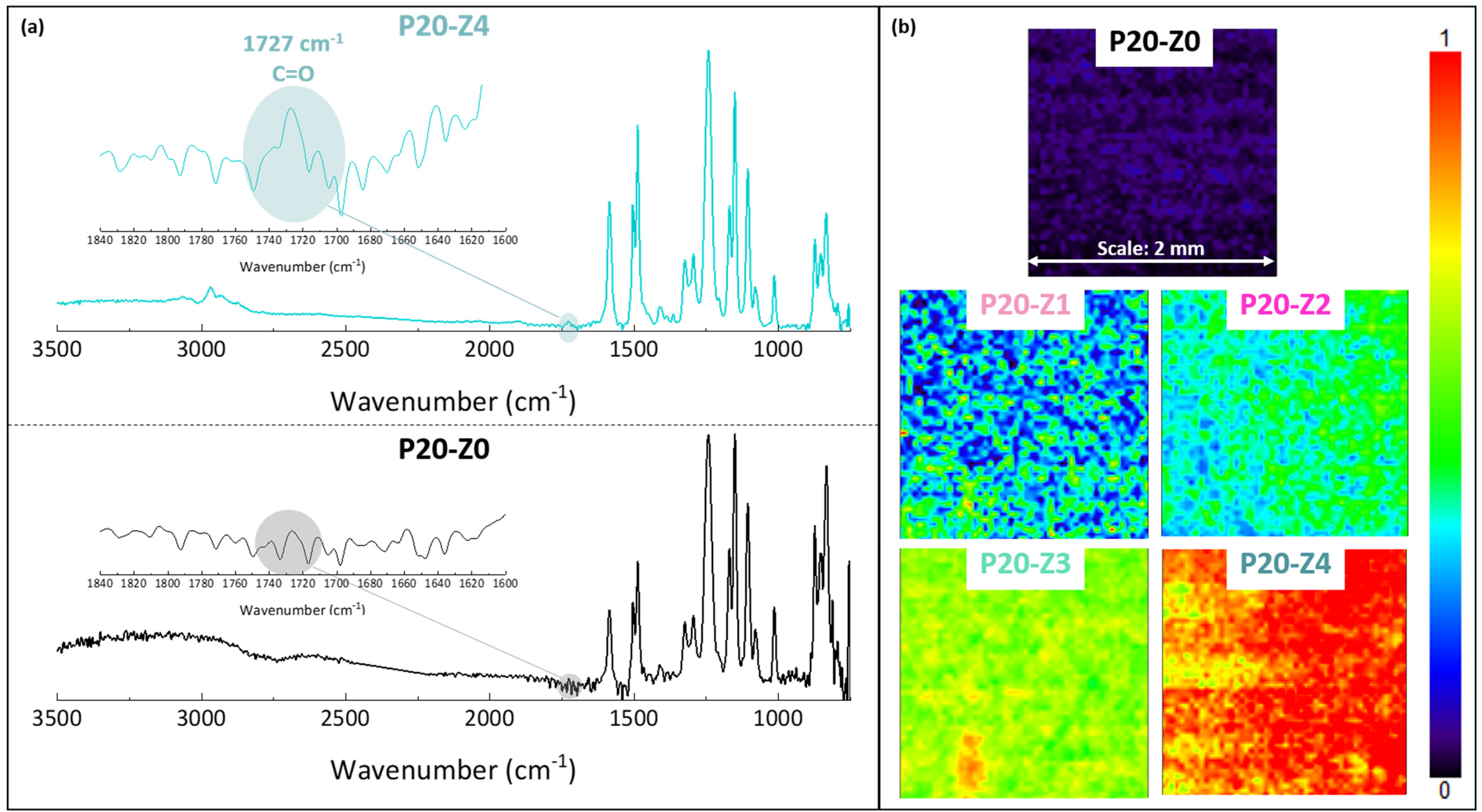
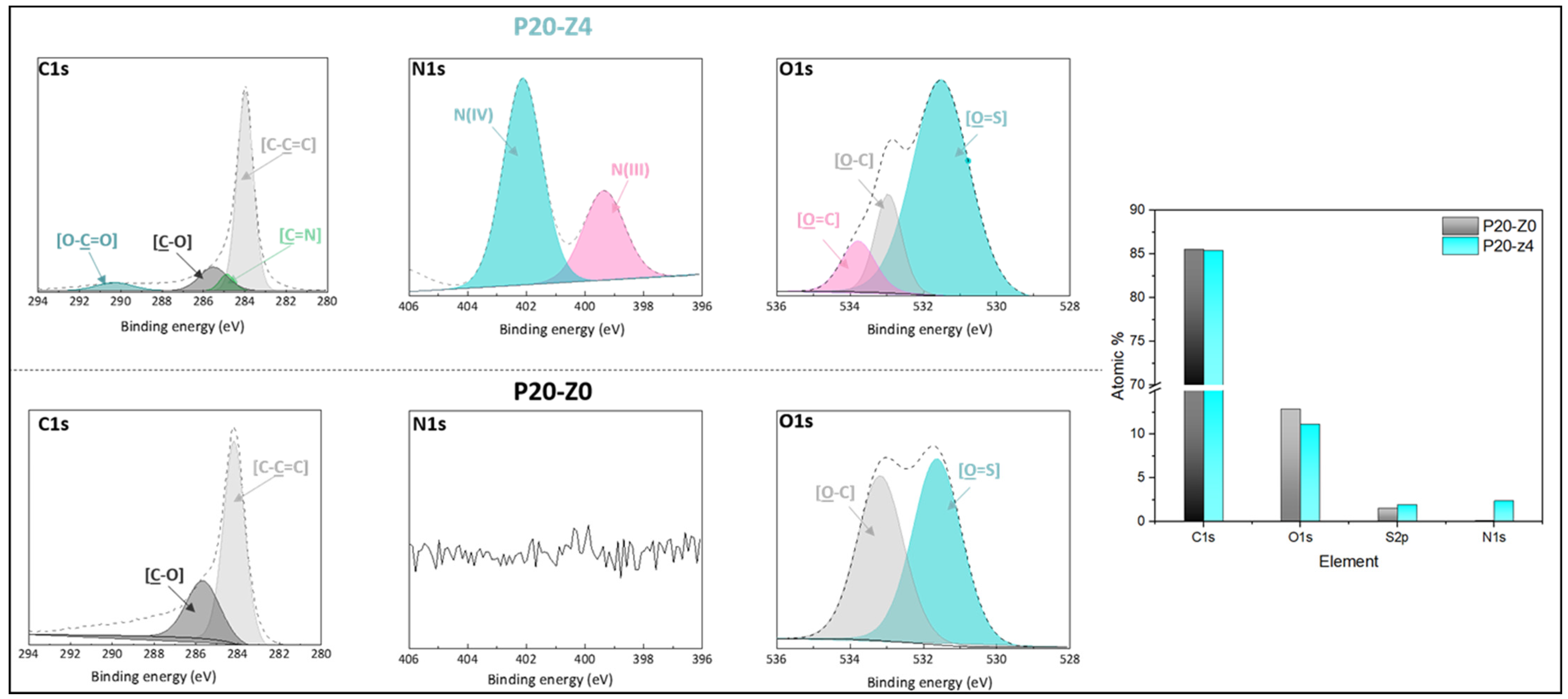
2.6. Chemical Characterization of Membranes
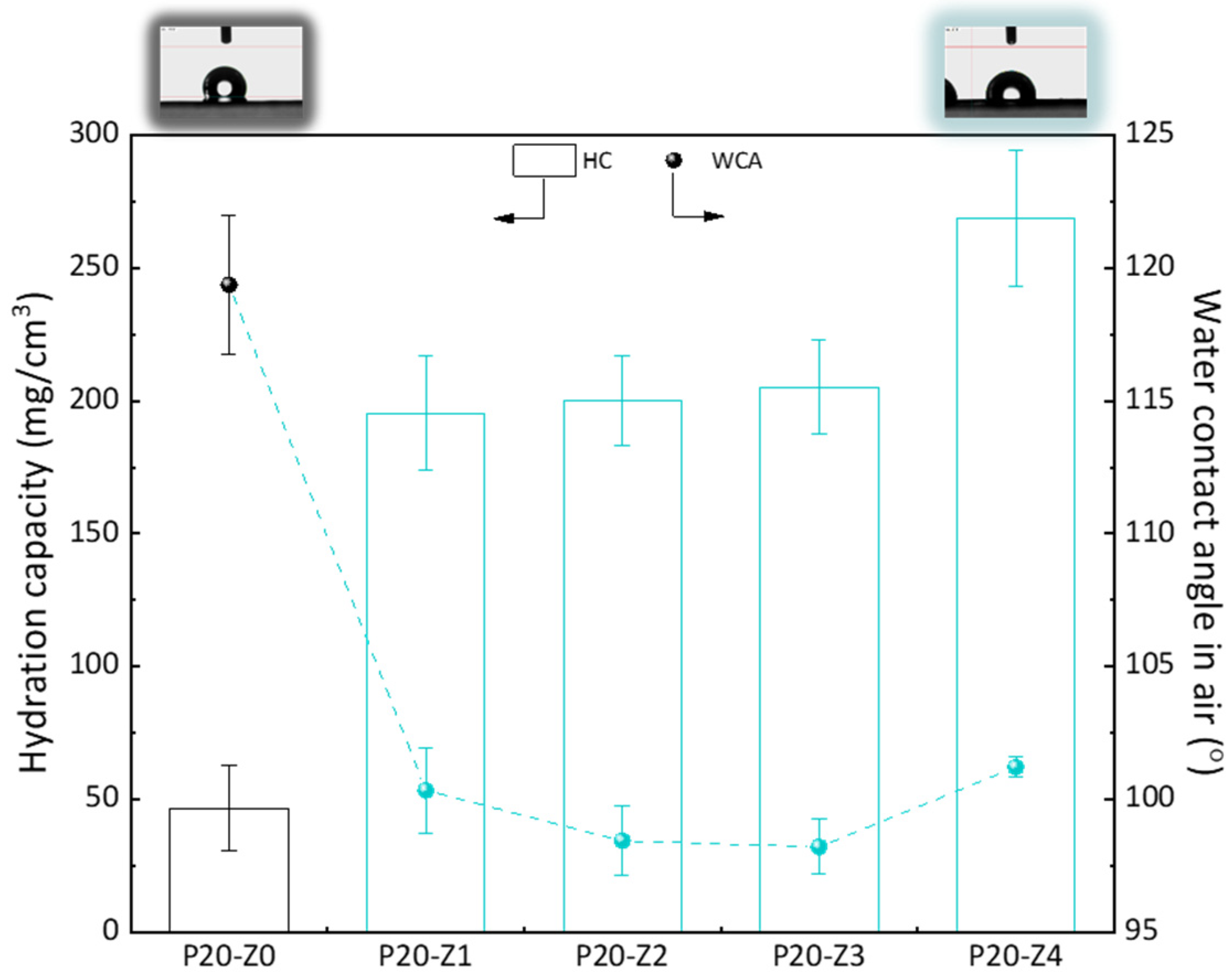
2.7. Characterization of Membranes’ Hydrophilic Properties
2.8. Biofouling Tests
3. Results and Discussion
3.1. Physical Properties of Membranes
3.2. Chemical Properties of Membranes
3.3. Hydrophilic Properties of Membranes
3.4. Effect of the Zwitterionic Copolymer on Resistance to Biofouling by Escherichia coli Bacteria
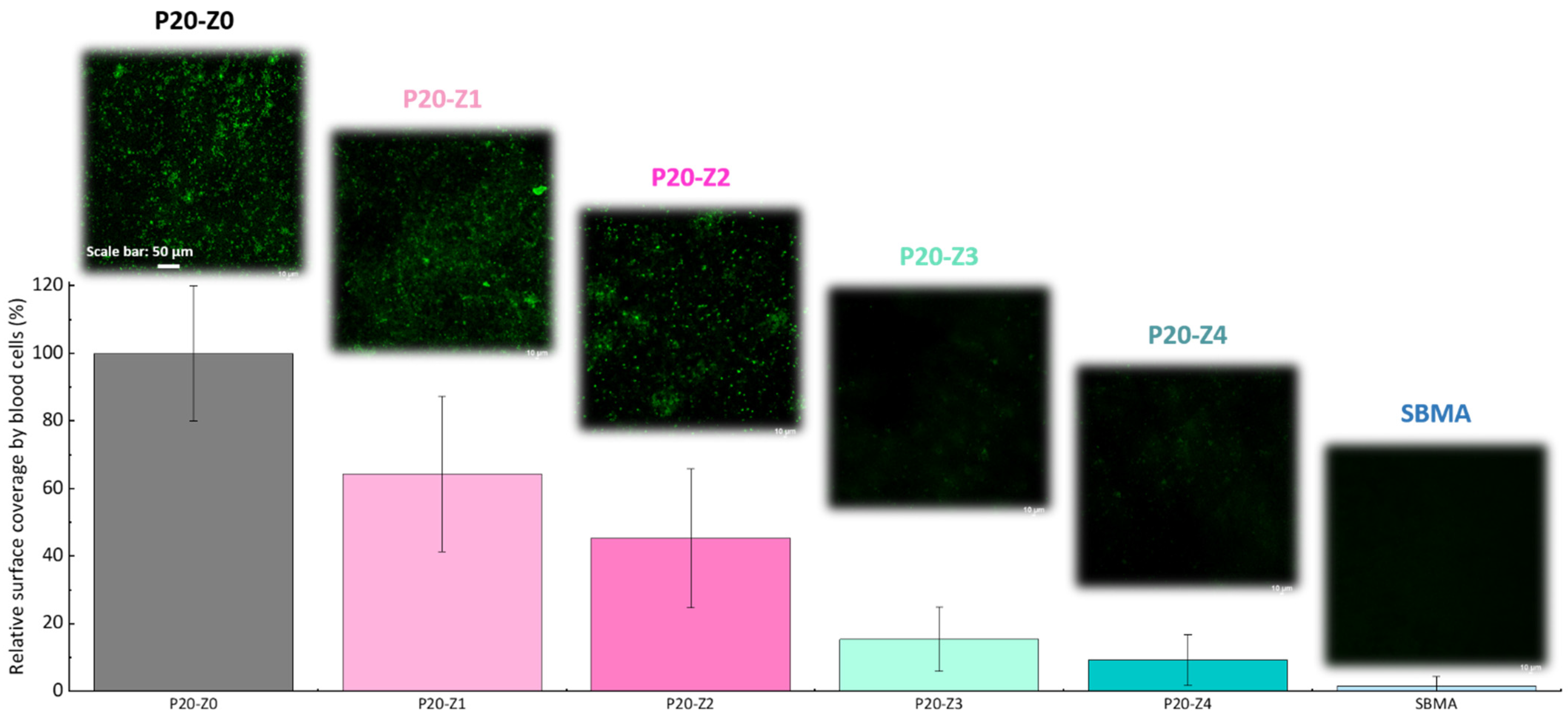
3.5. Effect of the Zwitterionic Copolymer on Resistance to Biofouling by Whole Blood
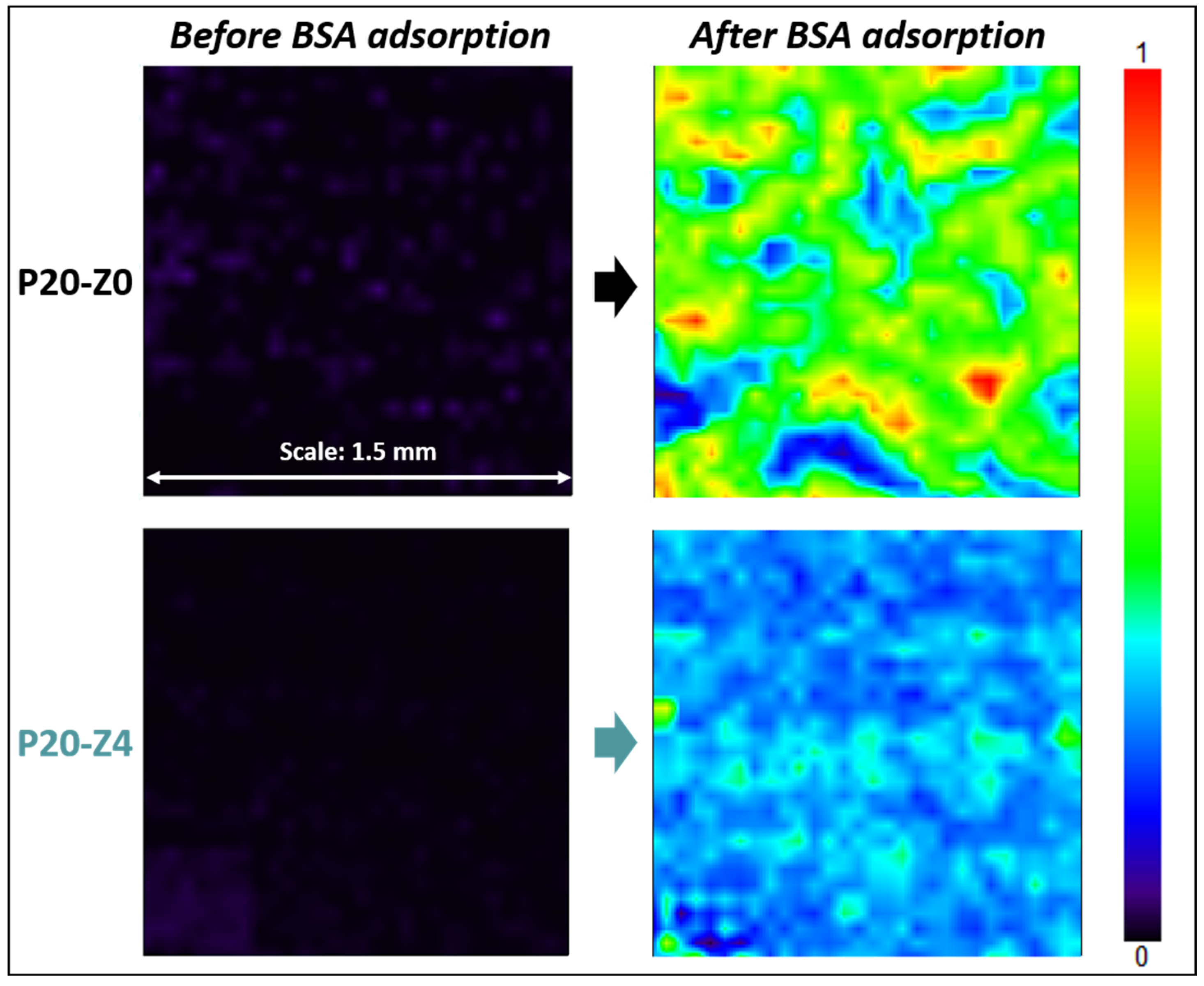
3.6. Effect of the Zwitterionic Copolymer on Resistance to the Adsorption of Bovine Serum Albumin Protein
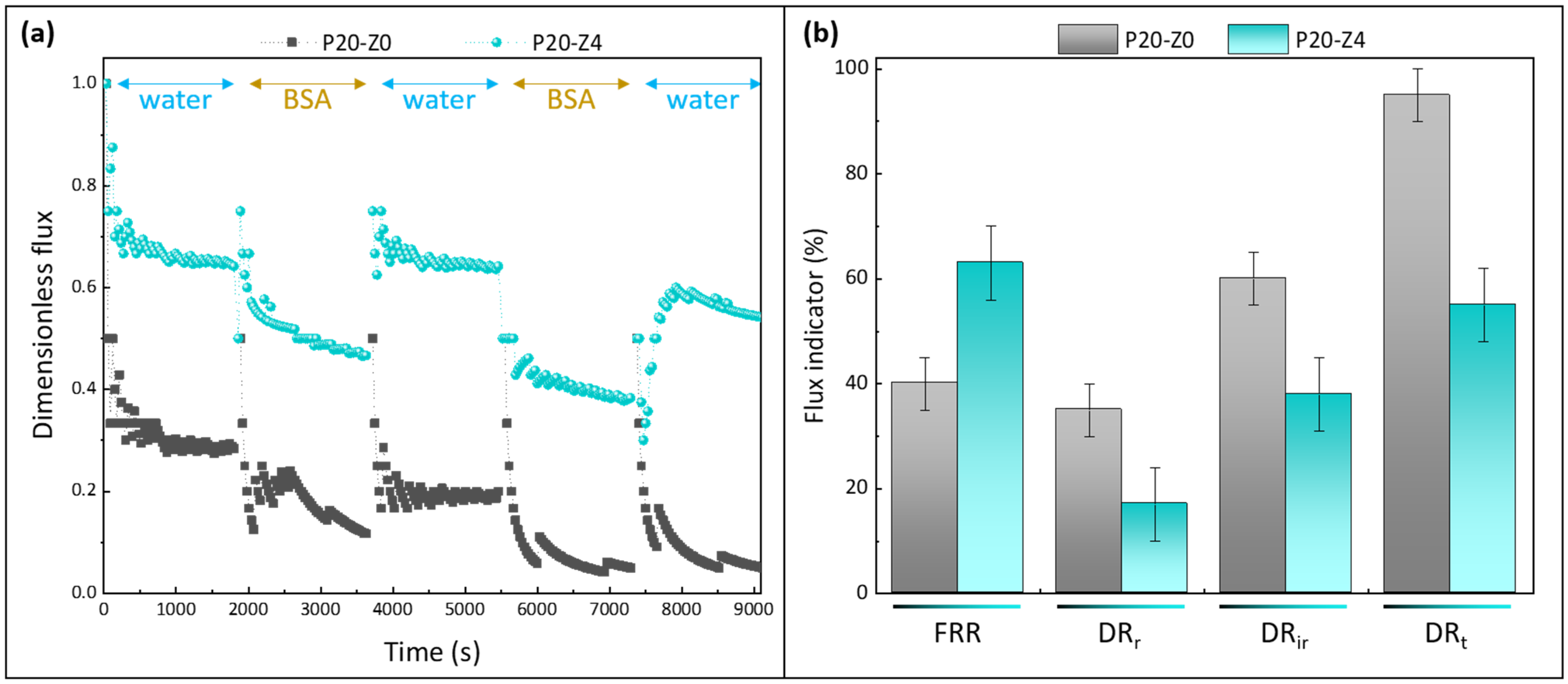
3.7. Effect of the Zwitterionic Copolymer on Resistance to Biofouling in Dynamic Conditions
3.8. Assessment of the Modified Membranes’ Stability—Directions to Explore to Improve the Design
- Increasing the length of the hydrophobic segments, i.e., augmenting the relative proportion of styrene units, would be the most evident method as it would permit the strengthening of the stabilizing hydrophobic interactions (and in the meantime weaken the hydrophilic interactions).
- Reducing the zwitterionic degree of the 4VP units (from 78% as in this work to a lower value) may provide a good tradeoff between the reduction of destabilizing hydrophilic interactions and the preservation of antifouling properties.
- One could also consider changing the copolymer configuration. While we worked here with a random copolymer (it can be readily synthesized at relatively low costs), a block copolymer may lead to better stability and antifouling performances, although it would be more challenging to synthesize. In block configuration, all hydrophobic units would be entangled in the matrix, while most hydrophilic units would be found at the interface between the membrane and the surrounding environment. In other words, each unit would fulfill the function it was originally intended for. In random configuration, some isolated hydrophobic units surrounded by numerous hydrophilic units may not be entangled in the membrane and conversely, some hydrophilic units may be found trapped in the main polymer matrix.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tan, X.; Rodrigue, D. A Review on Porous Polymeric Membrane Preparation. Part I: Production Techniques with Polysulfone and Poly (Vinylidene Fluoride). Polymers 2019, 11, 1160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zodrow, K.; Brunet, L.; Mahendra, S.; Li, D.; Zhang, A.; Li, Q.; Alvarez, P.J. Polysulfone ultrafiltration membranes impregnated with silver nanoparticles show improved biofouling resistance and virus removal. Water Res. 2009, 43, 715–723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbari, T.; Datwani, S. Gas separation properties of polysulfone membranes treated with molecular bromine. J. Membr. Sci. 1995, 107, 263–266. [Google Scholar] [CrossRef]
- Peng, Y.; Dong, Y.; Fan, H.; Chen, P.; Li, Z.; Jiang, Q. Preparation of polysulfone membranes via vapor-induced phase separation and simulation of direct-contact membrane distillation by measuring hydrophobic layer thickness. Desalination 2013, 316, 53–66. [Google Scholar] [CrossRef]
- Koga, Y.; Fujieda, H.; Meguro, H.; Ueno, Y.; Aoki, T.; Miwa, K.; Kainoh, M. Biocompatibility of Polysulfone Hemodialysis Membranes and Its Mechanisms: Involvement of Fibrinogen and Its Integrin Receptors in Activation of Platelets and Neutrophils. Artif. Organs 2018, 42, E246–E258. [Google Scholar] [CrossRef]
- Urducea, C.B.; Nechifor, A.C.; Dimulescu, I.A.; Oprea, O.; Nechifor, G.; Totu, E.E.; Isildak, I.; Albu, P.C.; Bungău, S.G. Control of Nanostructured Polysulfone Membrane Preparation by Phase Inversion Method. Nanomaterials 2020, 10, 2349. [Google Scholar] [CrossRef]
- Han, M.-J. Thermodynamic and rheological variation in polysulfone solution by PVP and its effect in the preparation of phase inversion membrane. J. Membr. Sci. 2001, 202, 55–61. [Google Scholar] [CrossRef]
- Kim, I.-C.; Lee, K.-H. Effect of various additives on pore size of polysulfone membrane by phase-inversion process. J. Appl. Polym. Sci. 2003, 89, 2562–2566. [Google Scholar] [CrossRef]
- Park, H.C.; Kim, Y.P.; Kim, H.Y.; Kang, Y.S. Membrane formation by water vapor induced phase inversion. J. Membr. Sci. 1999, 156, 169–178. [Google Scholar] [CrossRef]
- Pesek, S.; Koros, W. Aqueous quenched asymmetric polysulfone membranes prepared by dry/wet phase separation. J. Membr. Sci. 1993, 81, 71–88. [Google Scholar] [CrossRef]
- Pinnau, I.; Koros, W.J. Structures and gas separation properties of asymmetric polysulfone membranes made by dry, wet, and dry/wet phase inversion. J. Appl. Polym. Sci. 1991, 43, 1491–1502. [Google Scholar] [CrossRef]
- Hilal, N.; Ogunbiyi, O.O.; Miles, N.; Nigmatullin, R. Methods Employed for Control of Fouling in MF and UF Membranes: A Comprehensive Review. Sep. Sci. Technol. 2005, 40, 1957–2005. [Google Scholar] [CrossRef]
- Chapman, R.G.; Ostuni, E.; Takayama, S.; Holmlin, R.E.; Yan, A.L.; Whitesides, G.M. Surveying for Surfaces that Resist the Adsorption of Proteins. J. Am. Chem. Soc. 2000, 122, 8303–8304. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, Y.; He, M.; Su, Y.; Zhao, X.; Elimelech, M.; Jiang, Z. Antifouling membranes for sustainable water purification: Strategies and mechanisms. Chem. Soc. Rev. 2016, 45, 5888–5924. [Google Scholar] [CrossRef] [PubMed]
- Lowe, S.; O’Brien-Simpson, N.M.; Connal, L.A. Antibiofouling polymer interfaces: Poly(ethylene glycol) and other promising candidates. Polym. Chem. 2015, 6, 198–212. [Google Scholar] [CrossRef] [Green Version]
- Leng, C.; Hung, H.-C.; Sun, S.; Wang, D.; Li, Y.; Jiang, S.; Chen, Z. Probing the Surface Hydration of Nonfouling Zwitterionic and PEG Materials in Contact with Proteins. ACS Appl. Mater. Interfaces 2015, 7, 16881–16888. [Google Scholar] [CrossRef]
- Chiag, Y.-C.; Chang, Y.; Chen, W.-Y.; Ruaan, R.-C. Biofouling Resistance of Ultrafiltration Membranes Controlled by Surface Self-Assembled Coating with PEGylated Copolymers. Langmuir 2012, 28, 1399–1407. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhu, J.; Qiu, M.; He, C. Antifouling PVDF membrane grafted with zwitterionic poly(lysine methacrylamide) brushes. RSC Adv. 2016, 6, 61434–61442. [Google Scholar] [CrossRef]
- Fang, L.-F.; Jeon, S.; Kakihana, Y.; Kakehi, J.-I.; Zhu, B.-K.; Matsuyama, H.; Zhao, S. Improved antifouling properties of polyvinyl chloride blend membranes by novel phosphate based-zwitterionic polymer additive. J. Membr. Sci. 2017, 528, 326–335. [Google Scholar] [CrossRef]
- Hou, S.; Wang, X.; Dong, X.; Zheng, J.; Li, S. Renewable antibacterial and antifouling polysulfone membranes incorporating a PEO-grafted amphiphilic polymer and N-chloramine functional groups. J. Colloid Interface Sci. 2019, 554, 658–667. [Google Scholar] [CrossRef]
- Zhong, D.; Wang, Z.; Zhou, J.; Wang, Y. Additive-free preparation of hemodialysis membranes from block copolymers of polysulfone and polyethylene glycol. J. Membr. Sci. 2021, 618, 118690. [Google Scholar] [CrossRef]
- Yu, H.; Cao, Y.; Kang, G.; Liu, J.; Li, M.; Yuan, Q. Enhancing antifouling property of polysulfone ultrafiltration membrane by grafting zwitterionic copolymer via UV-initiated polymerization. J. Membr. Sci. 2009, 342, 6–13. [Google Scholar] [CrossRef]
- Yue, W.-W.; Li, H.-J.; Xiang, T.; Qin, H.; Sun, S.-D.; Zhao, C.-S. Grafting of zwitterion from polysulfone membrane via surface-initiated ATRP with enhanced antifouling property and biocompatibility. J. Membr. Sci. 2013, 446, 79–91. [Google Scholar] [CrossRef]
- Xiang, T.; Lu, T.; Xie, Y.; Zhao, W.-F.; Sun, S.-D.; Zhao, C.-S. Zwitterionic polymer functionalization of polysulfone membrane with improved antifouling property and blood compatibility by combination of ATRP and click chemistry. Acta Biomater. 2016, 40, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Shahkaramipour, N.; Jafari, A.; Tran, T.; Stafford, C.M.; Cheng, C.; Lin, H. Maximizing the grafting of zwitterions onto the surface of ultrafiltration membranes to improve antifouling properties. J. Membr. Sci. 2020, 601, 117909. [Google Scholar] [CrossRef]
- Ali, F.A.A.; Alam, J.; Shukla, A.K.; Alhoshan, M.; Ansari, M.A.; Al-Masry, W.A.; Rehman, S.; Alam, M. Evaluation of antibacterial and antifouling properties of silver-loaded GO polysulfone nanocomposite membrane against Escherichia coli, Staphylococcus aureus, and BSA protein. React. Funct. Polym. 2019, 140, 136–147. [Google Scholar] [CrossRef]
- Khan, A.; Sherazi, T.A.; Khan, Y.; Li, S.; Naqvi, S.A.R.; Cui, Z. Fabrication and characterization of polysulfone/modified nanocarbon black composite antifouling ultrafiltration membranes. J. Membr. Sci. 2018, 554, 71–82. [Google Scholar] [CrossRef]
- Li, X.; Janke, A.; Formanek, P.; Fery, A.; Stamm, M.; Tripathi, B.P. High permeation and antifouling polysulfone ultrafiltration membranes with in situ synthesized silica nanoparticles. Mater. Today Commun. 2020, 22, 100784. [Google Scholar] [CrossRef]
- Nair, A.K.; Isloor, A.M.; Kumar, R.; Ismail, A.F. Antifouling and performance enhancement of polysulfone ultrafiltration membranes using CaCO3 nanoparticles. Desalination 2013, 322, 69–75. [Google Scholar] [CrossRef]
- Dizon, G.V.; Venault, A. Direct in-situ modification of PVDF membranes with a zwitterionic copolymer to form bi-continuous and fouling resistant membranes. J. Membr. Sci. 2018, 550, 45–58. [Google Scholar] [CrossRef]
- Chakrabarty, B.; Ghoshal, A.; Purkait, M.K. Preparation, characterization and performance studies of polysulfone membranes using PVP as an additive. J. Membr. Sci. 2008, 315, 36–47. [Google Scholar] [CrossRef]
- Ma, Y.; Shi, F.; Ma, J.; Wu, M.; Zhang, J.; Gao, C. Effect of PEG additive on the morphology and performance of polysulfone ultrafiltration membranes. Desalination 2011, 272, 51–58. [Google Scholar] [CrossRef]
- Xu, Z.; Liao, J.; Tang, H.; Li, N. Antifouling polysulfone ultrafiltration membranes with pendent sulfonamide groups. J. Membr. Sci. 2018, 548, 481–489. [Google Scholar] [CrossRef]
- Tang, S.-H.; Venault, A.; Hsieh, C.; Dizon, G.V.; Lo, C.-T.; Chang, Y. A bio-inert and thermostable zwitterionic copolymer for the surface modification of PVDF membranes. J. Membr. Sci. 2020, 598, 117655. [Google Scholar] [CrossRef]
- Osadchii, D.Y.; Olivos-Suarez, A.I.; Bavykina, A.V.; Gascon, J. Revisiting Nitrogen Species in Covalent Triazine Frameworks. Langmuir 2017, 33, 14278–14285. [Google Scholar] [CrossRef] [Green Version]
- Hsiao, S.-W.; Venault, A.; Yang, H.-S.; Chang, Y. Bacterial resistance of self-assembled surfaces using PPOm-b-PSBMAn zwitterionic copolymer—Concomitant effects of surface topography and surface chemistry on attachment of live bacteria. Colloids Surfaces B: Biointerfaces 2014, 118, 254–260. [Google Scholar] [CrossRef]
- Bokhorst, H.; Altena, F.; Smolders, C. Formation of asymmetric cellulose acetate membranes. Desalination 1981, 38, 349–360. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.; Yang, F.; Xiang, M. Phase separation in a PSf/DMF/water system: A proposed mechanism for macrovoid formation. RSC Adv. 2014, 4, 42391–42402. [Google Scholar] [CrossRef]
- Hung, W.-L.; Wang, D.-M.; Lai, J.-Y.; Chou, S.-C. On the initiation of macrovoids in polymeric membranes—Effect of polymer chain entanglement. J. Membr. Sci. 2016, 505, 70–81. [Google Scholar] [CrossRef]
- Loh, C.H.; Wang, R. Insight into the role of amphiphilic pluronic block copolymer as pore-forming additive in PVDF membrane formation. J. Membr. Sci. 2013, 446, 492–503. [Google Scholar] [CrossRef]
- Venault, A.; Liu, Y.-H.; Wu, J.-R.; Yang, H.-S.; Chang, Y.; Lai, J.-Y.; Aimar, P. Low-biofouling membranes prepared by liquid-induced phase separation of the PVDF/polystyrene-b-poly (ethylene glycol) methacrylate blend. J. Membr. Sci. 2014, 450, 340–350. [Google Scholar] [CrossRef] [Green Version]
- Tian, X.; Qiu, Y.-R. 2-methoxyethylacrylate modified polysulfone membrane and its blood compatibility. Arch. Biochem. Biophys. 2017, 631, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Tu, M.-M.; Xu, J.-J.; Qiu, Y.-R. Surface hemocompatible modification of polysulfone membrane via covalently grafting acrylic acid and sulfonated hydroxypropyl chitosan. RSC Adv. 2019, 9, 6254–6266. [Google Scholar] [CrossRef] [Green Version]
- Ostuni, E.; Chapman, R.G.; Holmlin, R.E.; Takayama, A.S.; Whitesides, G.M. A Survey of Structure−Property Relationships of Surfaces that Resist the Adsorption of Protein. Langmuir 2001, 17, 5605–5620. [Google Scholar] [CrossRef]
- Hester, J.F.; Banerjee, A.P.; Mayes, A.M. Preparation of Protein-Resistant Surfaces on Poly(vinylidene fluoride) Membranes via Surface Segregation. Macromol. 1999, 32, 1643–1650. [Google Scholar] [CrossRef]
- Frigon, D.; Biswal, B.K.; Mazza, A.; Masson, L.; Gehr, R. Biological and Physicochemical Wastewater Treatment Processes Reduce the Prevalence of Virulent Escherichia coli. Appl. Environ. Microbiol. 2013, 79, 835–844. [Google Scholar] [CrossRef] [Green Version]
- Anastasi, E.M.; Matthews, B.; Stratton, H.; Katouli, M. Pathogenic Escherichia coli Found in Sewage Treatment Plants and Environmental Waters. Appl. Environ. Microbiol. 2012, 78, 5536–5541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, T.; Roddick, F.A.; Fan, L. Biofouling of Water Treatment Membranes: A Review of the Underlying Causes, Monitoring Techniques and Control Measures. Membr. 2012, 2, 804–840. [Google Scholar] [CrossRef] [Green Version]
- Tang, L.; Huynh, K.A.; Fleming, M.L.; Larronde-Larretche, M.; Chen, K.L. Imparting antimicrobial and anti-adhesive properties to polysulfone membranes through modification with silver nanoparticles and polyelectrolyte multilayers. J. Colloid Interface Sci. 2015, 451, 125–133. [Google Scholar] [CrossRef]
- Lee, S.Y.; Lee, Y.; Le Thi, P.; Oh, D.H.; Park, K.D. Sulfobetaine methacrylate hydrogel-coated anti-fouling surfaces for implantable biomedical devices. Biomater. Res. 2018, 22, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Shen, J.; Du, M.; Wu, Z.; Song, Y.; Zheng, Q. Strategy to construct polyzwitterionic hydrogel coating with antifouling, drag-reducing and weak swelling performance. RSC Adv. 2019, 9, 2081–2091. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; He, C.; He, H.; Cheng, C.; Zhu, J.; Xiao, Z.; Zhang, H.; Li, X.; Zheng, J.; Xiao, J. Importance of zwitterionic incorporation into polymethacrylate-based hydrogels for simultaneously improving optical transparency, oxygen permeability, and antifouling properties. J. Mater. Chem. B 2017, 5, 4595–4606. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Li, L.; Zhao, C.; Zheng, J. Surface hydration: Principles and applications toward low-fouling/nonfouling biomaterials. Polymers 2010, 51, 5283–5293. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Lin, W.; Wang, Z.; Chen, S.; Chang, Y. Investigation of the Hydration of Nonfouling Material Poly(sulfobetaine methacrylate) by Low-Field Nuclear Magnetic Resonance. Langmuir 2012, 28, 7436–7441. [Google Scholar] [CrossRef] [PubMed]
- Koga, Y.; Meguro, H.; Fujieda, H.; Ueno, Y.; Miwa, K.; Kainoh, M. A new hydrophilic polysulfone hemodialysis membrane can prevent platelet–neutrophil interactions and successive neutrophil activation. Int. J. Artif. Organs 2019, 42, 175–181. [Google Scholar] [CrossRef] [Green Version]
- Wenten, I.; Aryanti, P.; Khoiruddin, K.; Hakim, A.; Himma, N. Advances in Polysulfone-Based Membranes for Hemodialysis. J. Membr. Sci. Res. 2016, 2, 78–89. [Google Scholar]
- Ishihara, K.; Fukumoto, K.; Iwasaki, Y.; Nakabayashi, N. Modification of polysulfone with phospholipid polymer for improvement of the blood compatibility. Part 2. Protein adsorption and platelet adhesion. Biomaterials 1999, 20, 1553–1559. [Google Scholar] [CrossRef]
- Pape, A.C.H.; Ippel, B.D.; Dankers, P.Y.W. Cell and Protein Fouling Properties of Polymeric Mixtures Containing Supramolecular Poly(ethylene glycol) Additives. Langmuir 2017, 33, 4076–4082. [Google Scholar] [CrossRef]
- Benavente, L.; Coetsier, C.; Venault, A.; Chang, Y.; Causserand, C.; Bacchin, P.; Aimar, P. FTIR mapping as a simple and powerful approach to study membrane coating and fouling. J. Membr. Sci. 2016, 520, 477–489. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Ye, L.; Zhao, W.; Chen, L.; Zhang, M.; Yang, G.; Zhang, H. Antifouling mechanism of the additive-free β-PVDF membrane in water purification process: Relating the surface electron donor monopolarity to membrane-foulant interactions. J. Membr. Sci. 2020, 601, 117873. [Google Scholar] [CrossRef]
- Le, T.-N.; Au-Duong, A.-N.; Lee, C.-K. Facile coating on microporous polypropylene membrane for antifouling microfiltration using comb-shaped poly(N-vinylpyrrolidone) with multivalent catechol. J. Membr. Sci. 2019, 574, 164–173. [Google Scholar] [CrossRef]
- Astaraee, R.S.; Mohammadi, T.; Kasiri, N. Analysis of BSA, dextran and humic acid fouling during microfiltration, experimental and modeling. Food Bioprod. Process. 2015, 94, 331–341. [Google Scholar] [CrossRef]
- Velasco, C.; Calvo, J.; Palacio, L.; Carmona, J.; Prádanos, P.; Hernández, A. Flux kinetics, limit and critical fluxes for low pressure dead-end microfiltration. The case of BSA filtration through a positively charged membrane. Chem. Eng. Sci. 2015, 129, 58–68. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Wang, Z.; Zhang, Q.; Xi, X.; Zhang, J.; Yang, W. Equilibrium and thermodynamic studies on adsorption of BSA using PVDF microfiltration membrane. Desalination 2012, 307, 61–67. [Google Scholar] [CrossRef]
- Kanagaraj, P.; Neelakandan, S.; Nagendran, A.; Rana, D.; Matsuura, T.; Muthumeenal, A. Performance studies of PEI/SPEI blend ultra-filtration membranes via surface modification using cSMM additives. RSC Adv. 2015, 5, 27594–27602. [Google Scholar] [CrossRef]
- Zin, G.; Penha, F.M.; Rezzadori, K.; Silva, F.L.; Guizoni, K.; Petrus, J.C.C.; Oliveira, J.V.; Di Luccio, M. Fouling control in ultrafiltration of bovine serum albumin and milk by the use of permanent magnetic field. J. Food Eng. 2016, 168, 154–159. [Google Scholar] [CrossRef] [Green Version]
- Madaeni, S.S.; Rahimpour, A. Effect of type of solvent and non-solvents on morphology and performance of polysulfone and polyethersulfone ultrafiltration membranes for milk concentration. Polym. Adv. Technol. 2005, 16, 717–724. [Google Scholar] [CrossRef]
- Sinha, M.K.; Purkait, M.K. Increase in hydrophilicity of polysulfone membrane using polyethylene glycol methyl ether. J. Membr. Sci. 2013, 437, 7–16. [Google Scholar] [CrossRef]
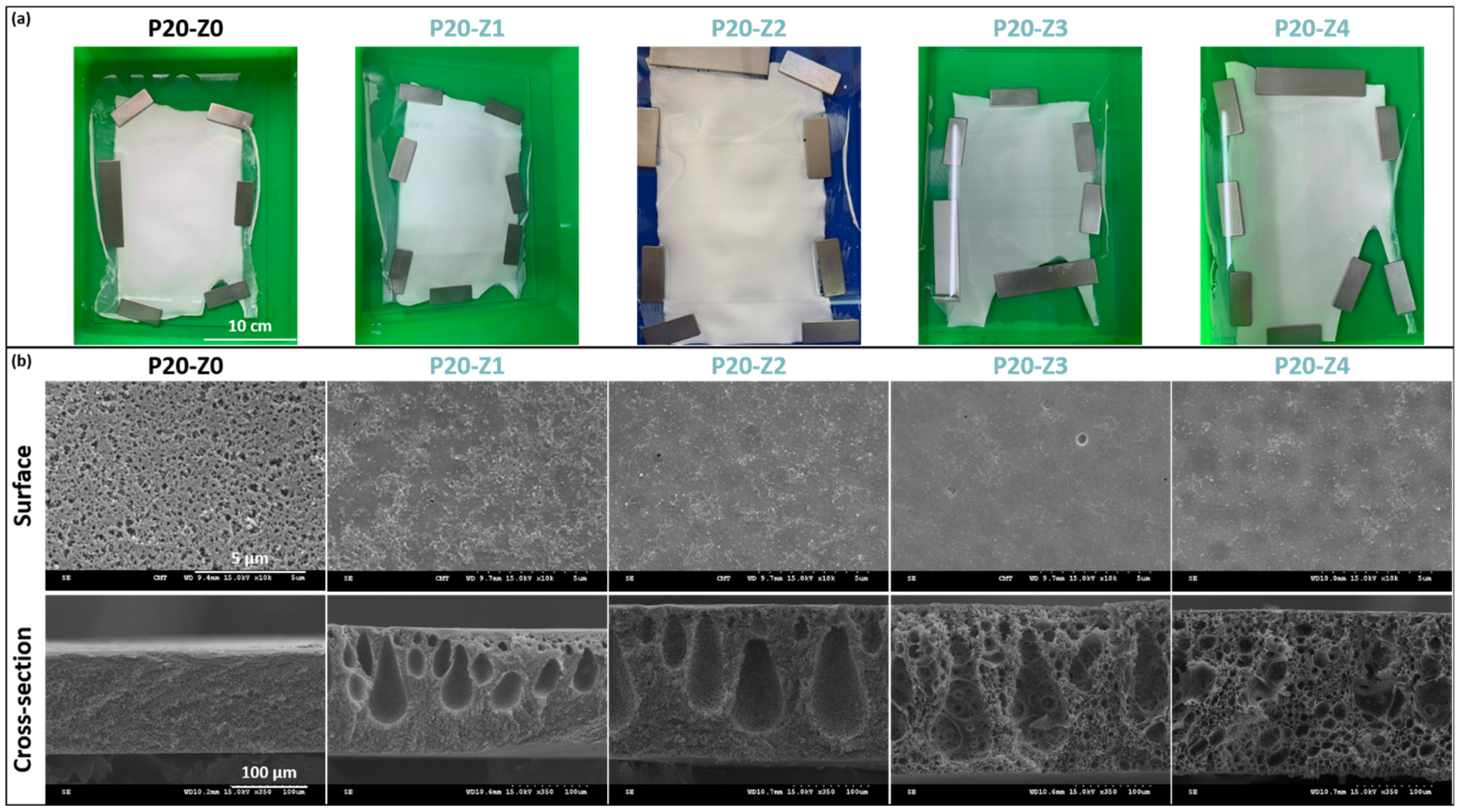


| Membrane ID | Porosity (%) | Surface Pore Size (μm) (1) | Mean Pore Size (nm) (2) |
|---|---|---|---|
| P20-Z0 | 73.2 ± 1.4 | 0.9 ± 0.3 | 6.3 |
| P20-Z1 | 77.0 ± 1.5 | / | 9.1 |
| P20-Z2 | 80.2 ± 0.5 | / | 10.4 |
| P20-Z3 | 79.2 ± 0.5 | / | 10.3 |
| P20-Z4 | 74.1 ± 1.5 | / | 10.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maggay, I.V.B.; Aini, H.N.; Lagman, M.M.G.; Tang, S.-H.; Aquino, R.R.; Chang, Y.; Venault, A. A Biofouling Resistant Zwitterionic Polysulfone Membrane Prepared by a Dual-Bath Procedure. Membranes 2022, 12, 69. https://doi.org/10.3390/membranes12010069
Maggay IVB, Aini HN, Lagman MMG, Tang S-H, Aquino RR, Chang Y, Venault A. A Biofouling Resistant Zwitterionic Polysulfone Membrane Prepared by a Dual-Bath Procedure. Membranes. 2022; 12(1):69. https://doi.org/10.3390/membranes12010069
Chicago/Turabian StyleMaggay, Irish Valerie B., Hana Nur Aini, Mary Madelaine G. Lagman, Shuo-Hsi Tang, Ruth R. Aquino, Yung Chang, and Antoine Venault. 2022. "A Biofouling Resistant Zwitterionic Polysulfone Membrane Prepared by a Dual-Bath Procedure" Membranes 12, no. 1: 69. https://doi.org/10.3390/membranes12010069
APA StyleMaggay, I. V. B., Aini, H. N., Lagman, M. M. G., Tang, S.-H., Aquino, R. R., Chang, Y., & Venault, A. (2022). A Biofouling Resistant Zwitterionic Polysulfone Membrane Prepared by a Dual-Bath Procedure. Membranes, 12(1), 69. https://doi.org/10.3390/membranes12010069








