Exergetic Performance Coefficient Analysis and Optimization of a High-Temperature Proton Exchange Membrane Fuel Cell
Abstract
:1. Introduction
2. Exergetic Performance Coefficient (EPC) of High-Temperature Proton Exchange Membrane Fuel Cell (HT-PEMFC)
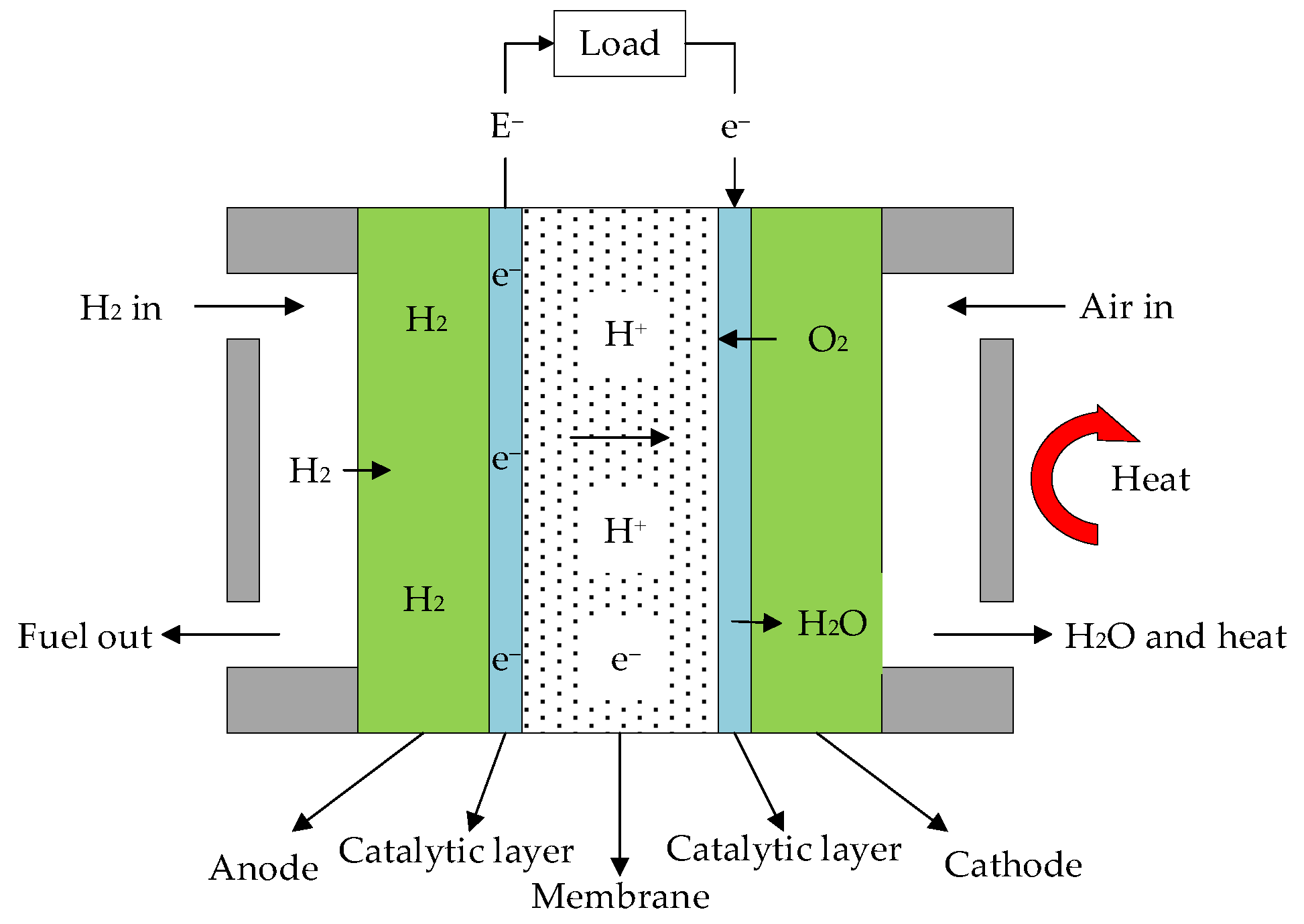
2.1. Working Principle of HT-PEMFC
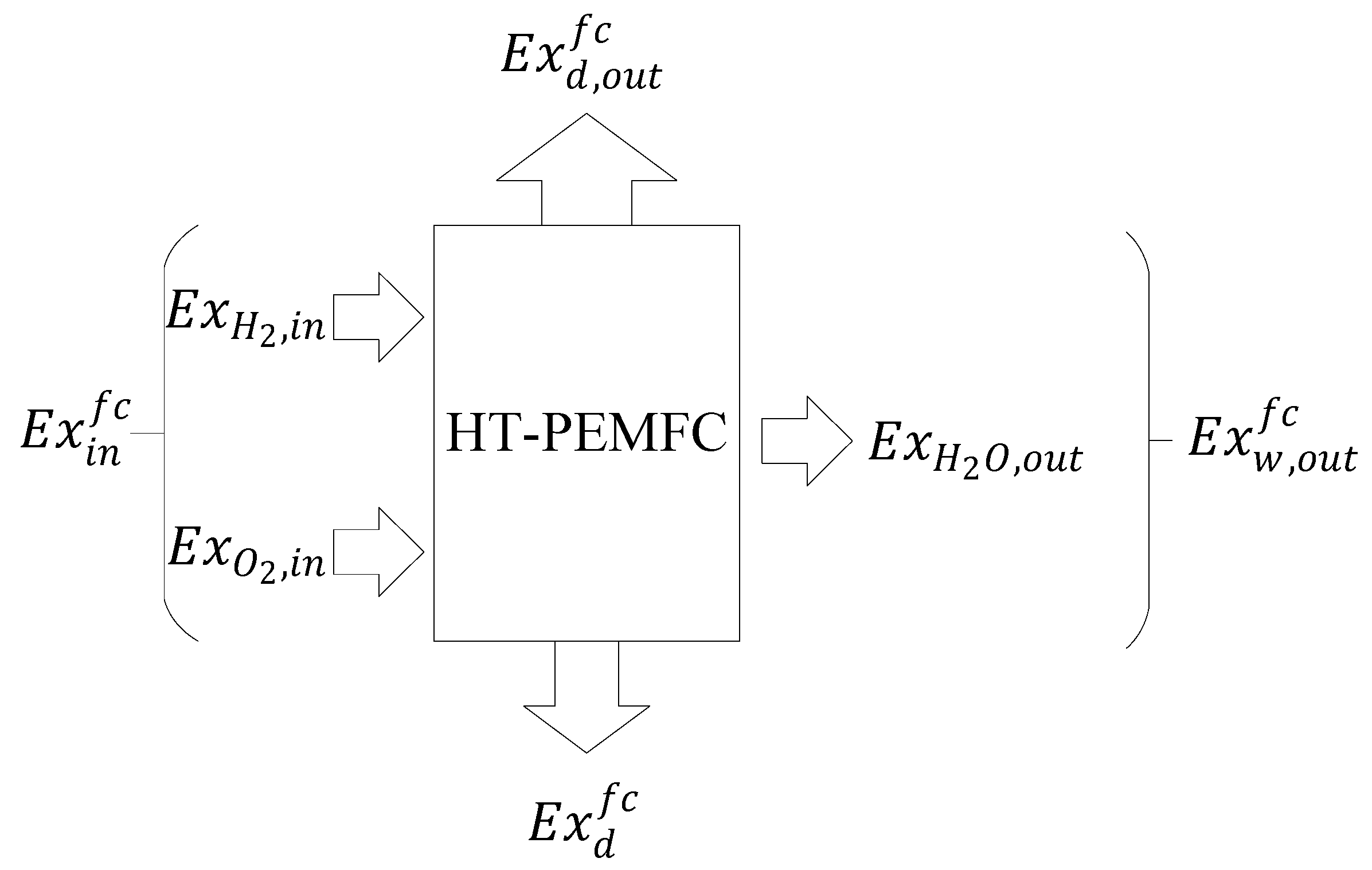
2.2. Thermodynamic Model of HT-PEMFC
- (1)
- The HT-PEMFC system is working under steady-state conditions;
- (2)
- Kinetic and potential energy are neglected;
- (3)
- All gases within the HT-PEMFC are assumed to be ideal gas;
- (4)
- The environment condition is 1.013 bar (1 atm) and 25 °C; air consists of 79% nitrogen and 21% oxygen;
- (5)
- Anode outlet temperature is equal to the operating temperature;
- (6)
- There is no leakage of hydrogen and oxygen within the HT-PEMFC structure.
2.3. Exergetic Performance Analysis
2.4. Optiaml Exergy Performance Coefficient
3. Results and Discussion
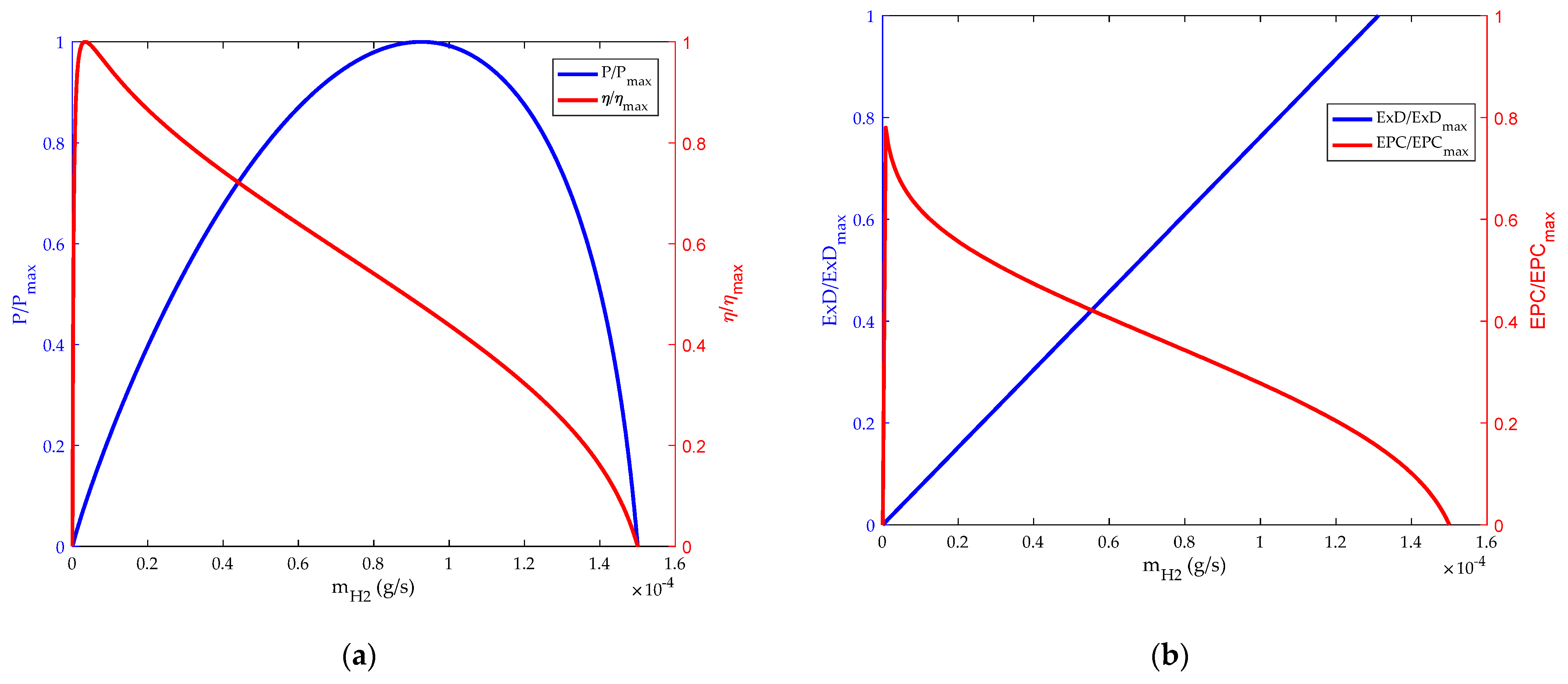
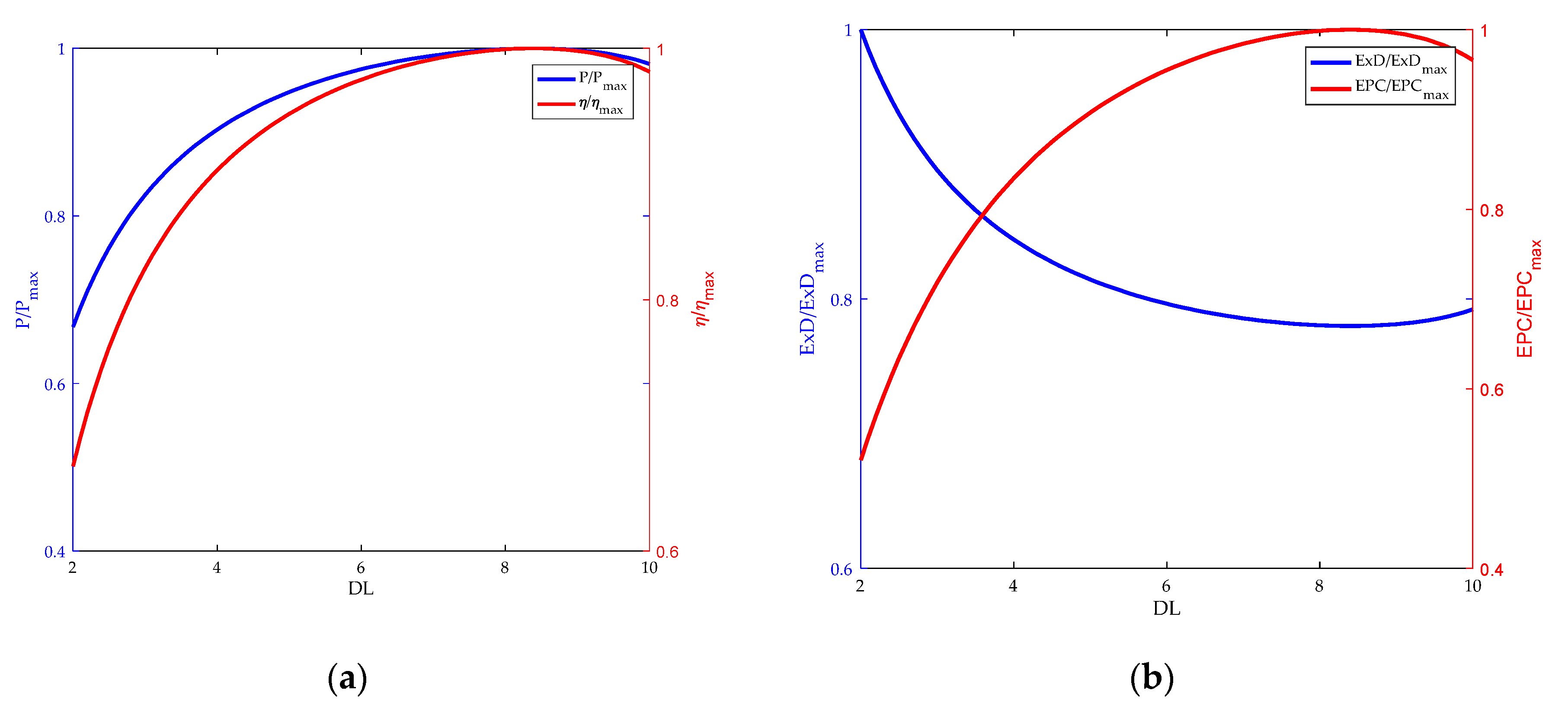
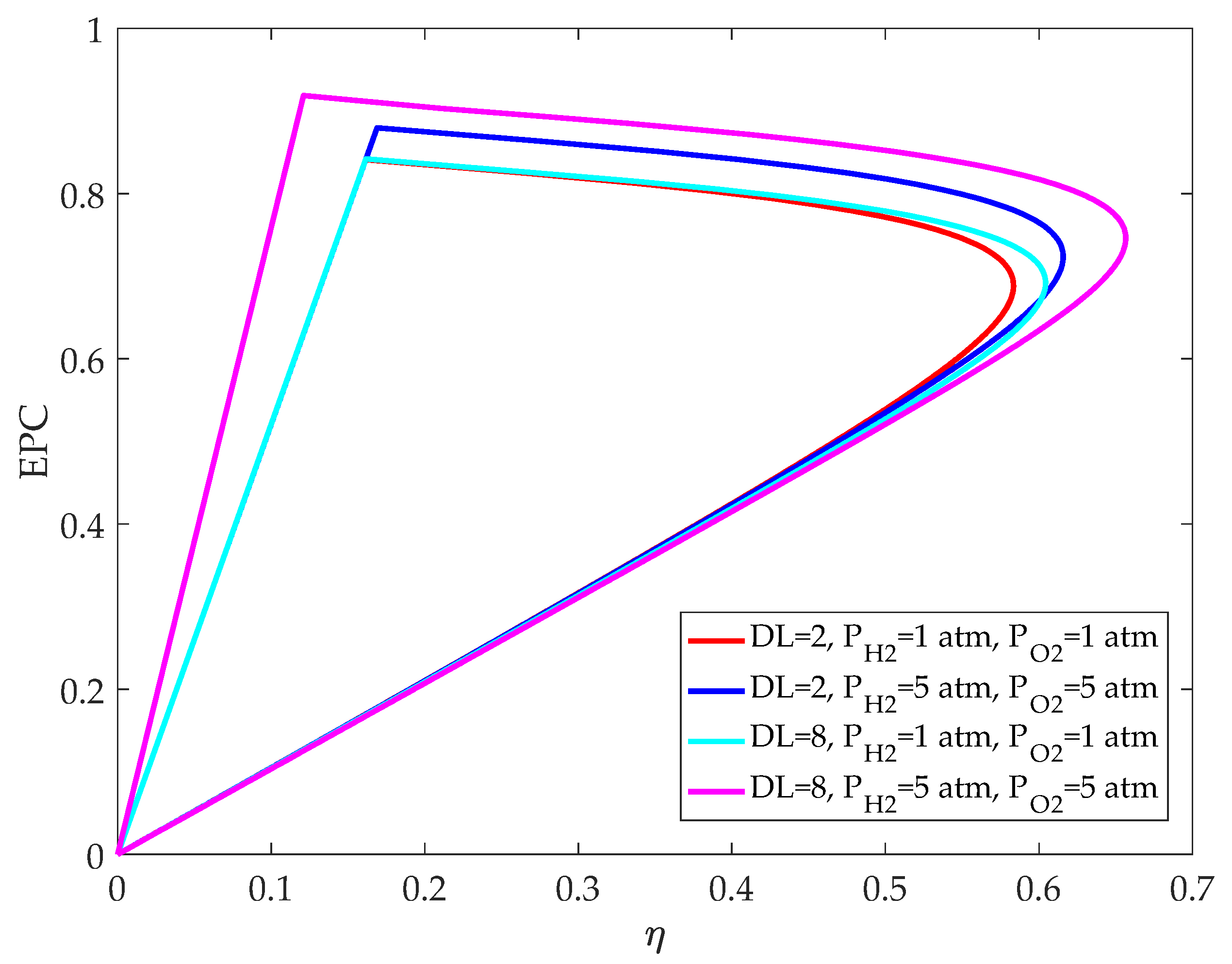
3.1. Comparsion of Relationship between and
3.2. Influence of Inlet Flow Rate on HT-PEMFC
3.3. Influence of Doping Level on HT-PEMFC
3.4. Influence of Inlet Pressure on HT-PEMFC
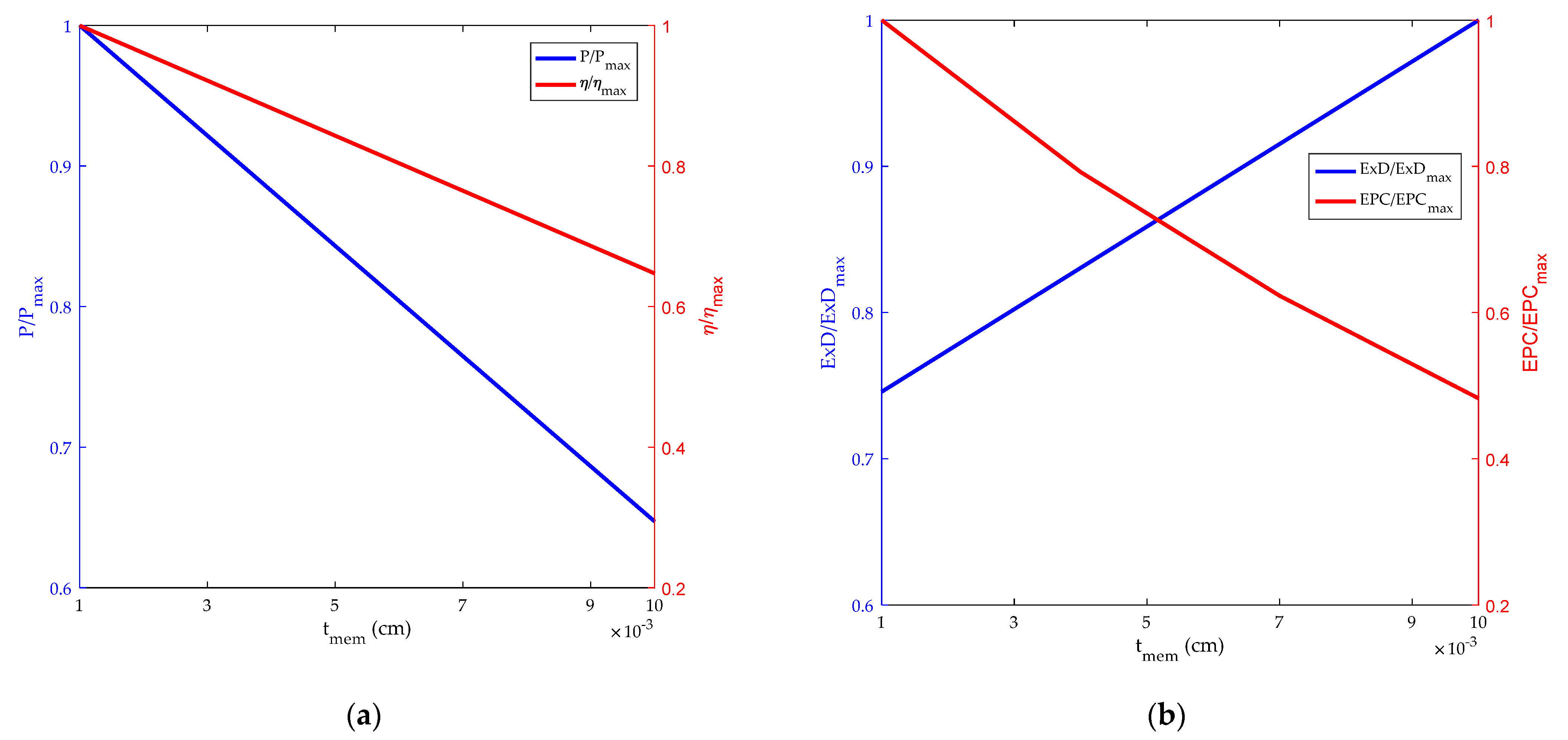
3.5. Influence of Film Thickness on HT-PEMFC
3.6. Relationship between EPC and Efficiency
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reddy, E.H.; Monder, D.S.; Jayanti, S. Parametric study of an external coolant system for a high temperature polymer electrolyte membrane fuel cell. Appl. Therm. Eng. 2013, 58, 155–164. [Google Scholar] [CrossRef]
- Alpaydin, G.U.; Devrim, Y.; Colpan, C.O. Performance of an HT-PEMFC having a catalyst with graphene and multiwalled carbon nanotube support. Int. J. Energy Res. 2019, 43, 3578–3589. [Google Scholar] [CrossRef]
- Reddy, E.H.; Jayanti, S. Thermal management strategies for a 1 kWe stack of a high temperature proton exchange membrane fuel cell. Appl. Therm. Eng. 2012, 48, 465–475. [Google Scholar] [CrossRef]
- Devrim, Y.; Arica, E.D. Multi-walled carbon nanotubes decorated by platinum catalyst for high temperature PEM fuel cell. Int. J. Hydrogen Energy 2019, 44, 18951–18966. [Google Scholar] [CrossRef]
- Oono, Y.; Sounai, A.; Hori, M. Influence of the phosphoric acid-doping level in a polybenzimidazole membrane on the cell performance of high-temperature proton exchange membrane fuel cells. J. Power Sources 2009, 189, 943–949. [Google Scholar] [CrossRef]
- Lee, D.; Lim, J.W.; Lee, D.G. Cathode/anode integrated composite bipolar plate for high-temperature PEMFC. Compos. Struct. 2017, 167, 144–151. [Google Scholar] [CrossRef]
- Muthuraja, P.; Prakash, S.; Shanmugam, V.M.; Radhakrsihnan, S.; Manisankar, P. Novel perovskite structured calcium titanate-PBI composite membranes for high-temperature PEM fuel cells: Synthesis and characterizations. Int. J. Hydrogen Energy 2018, 43, 4763–4772. [Google Scholar] [CrossRef]
- Li, Q.F.; Rudbeck, H.C.; Chromik, A.; Jensen, J.O.; Pan, C.; Steenberg, T.; Calverley, M.; Bjerrum, N.J.; Kerres, J. Properties, degradation and high temperature fuel cell test of different types of PBI and PBI blend membranes. J. Membr. Sci. 2010, 347, 260–270. [Google Scholar] [CrossRef]
- Selvakumar, K.; Prabhu, M.R. Investigation on meta-polybenzimidazole blend with sulfonated PVdF-HFP proton conducting polymer electrolytes for HT-PEM fuel cell application. J. Mater. Sci.-Mater. Electron. 2018, 29, 15163–15173. [Google Scholar] [CrossRef]
- Lobato, J.; Rodrigo, M.A.; Linares, J.J.; Scott, K. Effect of the catalytic ink preparation method on the performance of high temperature polymer electrolyte membrane fuel cells. J. Power Sources 2006, 157, 284–292. [Google Scholar] [CrossRef]
- Durmayaz, A.; Sogut, O.S.; Sahin, B.; Yavuz, H. Optimization of thermal systems based on finite-time thermodynamics and thermoeconomics. Prog. Energy Combust. 2004, 30, 175–217. [Google Scholar] [CrossRef]
- Qin, X.Y.; Chen, L.G.; Ge, Y.L.; Sun, F.R. Finite Time Thermodynamic Studies on Absorption Thermodynamic Cycles: A State-of-the-Art Review. Arab. J. Sci. Eng. 2013, 38, 405–419. [Google Scholar] [CrossRef]
- Ge, Y.L.; Chen, L.G.; Sun, F.R. Progress in Finite Time Thermodynamic Studies for Internal Combustion Engine Cycles. Entropy 2016, 18, 139. [Google Scholar] [CrossRef] [Green Version]
- Li, C.J.; Liu, Y.; Xu, B.; Ma, Z.S. Finite Time Thermodynamic Optimization of an Irreversible Proton Exchange Membrane Fuel Cell for Vehicle Use. Processes 2019, 7, 419. [Google Scholar] [CrossRef] [Green Version]
- He, S.; Lin, L.Y.; Wu, Z.X.; Chen, Z.M. Application of Finite Element Analysis in Properties Test of Finger-jointed Lumber. J. Bioresour. Bioprod. 2020, 5, 124. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Y.C.; Zhou, L.; Zhang, F.S.; Jing, Y.; Huang, M.Z.; Liu, H.B. Quality-related monitoring of papermaking wastewater treatment processes using dynamic multiblock partial least squares. J. Bioresour. Bioprod. 2021; in press. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Huang, Y.J.; Fu, H.Y.; Wang, Y.L.; Wang, Z. Deflection test and modal analysis of lightweight timber floors. J. Bioresour. Bioprod. 2021, 6, 266–278. [Google Scholar] [CrossRef]
- Hu, T.P.; Yu, Z.; Guo, L.; Xu, C.Y. Thermodynamic self-consistent dynamic model of wood dust explosion. J. For. Eng. 2019, 4, 29–34. [Google Scholar]
- Ji, X.L.; Yang, P. The exploration of the slope displacement with vegetation protection under different rainfall intensity. J. For. Eng. 2020, 5, 152–156. [Google Scholar]
- Li, X.S.; Deng, T.T.; Wang, M.H.; Ju, S.; Li, X.C.; Li, M. Linear positioning algorithm improvement of wood acoustic emission source based on wavelet and signal correlation analysis methods. J. For. Eng. 2020, 5, 138–143. [Google Scholar]
- Xu, B.; Li, D.X.; Ma, Z.S.; Zheng, M.; Li, Y.J. Thermodynamic Optimization of a High Temperature Proton Exchange Membrane Fuel Cell for Fuel Cell Vehicle Applications. Mathematics 2021, 9, 1792. [Google Scholar] [CrossRef]
- Guo, Y.H.; Guo, X.R.; Zhang, H.C.; Hou, S.J. Energetic, exergetic and ecological analyses of a high-temperature proton exchange membrane fuel cell based on a phosphoric-acid-doped polybenzimidazole membrane. Sustain. Energy Technol. Assesments 2020, 38, 100671. [Google Scholar] [CrossRef]
- Ye, L.; Jiao, K.; Du, Q.; Yin, Y. Exergy Analysis of High-Temperature Proton Exchange Membrane Fuel Cell Systems. Int. J. Green Energy 2015, 12, 917–929. [Google Scholar] [CrossRef]
- Guo, X.R.; Zhang, H.C.; Yuan, J.L.; Wang, J.T.; Zhao, J.P.; Wang, F.; Miao, H.; Hou, S.J. Energetic and exergetic analyses of a combined system consisting of a high-temperature polymer electrolyte membrane fuel cell and a thermoelectric generator with Thomson effect. Int. J. Hydrogen Energy 2019, 44, 16918–16932. [Google Scholar] [CrossRef]
- Liu, G.K.; Qin, Y.Z.; Yin, Y.F.; Bian, X.Z.; Kuang, C.C. Thermodynamic modeling and exergy analysis of proton exchange membrane fuel cell power system. Int. J. Hydrogen Energy 2020, 45, 29799–29811. [Google Scholar] [CrossRef]
- Li, D.; Li, S.; Ma, Z.; Xu, B.; Lu, Z.; Li, Y.; Zheng, M. Ecological Performance Optimization of a High Temperature Proton Exchange Membrane Fuel Cell. Mathematics 2021, 9, 1332. [Google Scholar] [CrossRef]
- Huang, Y.W.; Ding, H.; Zou, Y.F. Ecological Performance Analysis of an Integrated Proton Exchange Membrane Fuel Cell and Thermoelectric Devices. Int. J. Electrochem. Sci. 2020, 15, 2581–2593. [Google Scholar] [CrossRef]
- Li, C.J.; Xu, B.; Ma, Z.S. Ecological Performance of an Irreversible Proton Exchange Membrane Fuel Cell. Sci. Adv. Mater. 2020, 12, 1225–1235. [Google Scholar] [CrossRef]
- Peng, X.R.; Zhang, Z.K.; Zhao, L.Y. Analysis of Raman spectroscopy and XPS of plasma modified polypropylene decorative film. J. For. Eng. 2020, 5, 45–51. [Google Scholar]
- Yu, P.J.; Zhang, W.; Chen, M.Z.; Zhou, X.Y. Plasma-treated thermoplastic resin film as adhesive for preparing environmentally-friendly plywood. J. For. Eng. 2020, 5, 41–47. [Google Scholar]
- Liu, S.Q.; Jia, L.M. Review on sustainable development of forest-based biodiesel. J. Nanjing For. Univ. (Nat. Sci. Ed.) 2021, 44, 216–224. [Google Scholar]
- Xiong, G.K.; Li, Y.Q.; Xiong, Y.Q.; Duan, A.G.; Cao, D.C.; Sun, J.J.; Nie, L.Y.; Sheng, W.T. Effects of low stand density afforestation on the growth, stem-form and timber assortment structure of Cunninghamia lanceolata plantations. J. Nanjing For. Univ. (Nat. Sci. Ed.) 2021, 45, 165–173. [Google Scholar] [CrossRef]
- Yao, J.J.; Feng, X.Q.; Xiao, H.; Zheng, Y.; Zhang, C.L. Improvement effects of different solid waste and their disposal by products on saline-alkali soil in Huanghua Port. J. Nanjing For. Univ. (Nat. Sci. Ed.) 2021, 45, 45–52. [Google Scholar] [CrossRef]
- Zhang, Z.G. Researches on green features and category architecture of green strategies of renewable-resource-based enterprises: A case study of forestry enterprise. J. Nanjing For. Univ. (Nat. Sci. Ed.) 2020, 44, 1–8. [Google Scholar] [CrossRef]
- Zhou, S.J.; Wang, P.; Zhang, M.; Chen, S.Z.; Xu, W.; Zhu, L.T.; He, X.Q.; Gong, S.R. Effects of atmospheric acid deposition on root physiological characteristics of Pinus massoniana seedlings. J. Nanjing For. Univ. (Nat. Sci. Ed.) 2021, 44, 111–118. [Google Scholar] [CrossRef]
- Zhu, P.H.; Chen, Y.; Ji, K.S. A review of terpene synthases and genes in Pinaceae. J. Nanjing For. Univ. (Nat. Sci. Ed.) 2021, 45, 233–244. [Google Scholar] [CrossRef]
- Watowich, S.J.; Berry, R.S. Optimal current paths for model electrochemical systems. J. Phys. Chem. 1986, 90, 4624–4631. [Google Scholar] [CrossRef]
- Sieniutycz, S. Thermodynamics of Power Production in Fuel Cells. Chem. Process. Eng.-Inz. 2010, 31, 81–105. [Google Scholar]
- Sieniutycz, S.; Poswiata, A. Thermodynamic aspects of power production in thermal, chemical and electrochemical systems. Energy 2012, 45, 62–70. [Google Scholar] [CrossRef]
- Akkaya, A.V.; Sahin, B.; Erdem, H.H. Exergetic performance coefficient analysis of a simple fuel cell system. Int. J. Hydrogen Energy 2007, 32, 4600–4609. [Google Scholar] [CrossRef]
- Guo, X.R.; Zhang, H.C.; Zhao, J.P.; Wang, F.; Wang, J.T.; Miao, H.; Yuan, J.L. Performance evaluation of an integrated high-temperature proton exchange membrane fuel cell and absorption cycle system for power and heating/cooling cogeneration. Energy Convers. Manag. 2019, 181, 292–301. [Google Scholar] [CrossRef]
- Dincer, I.; Hussain, M.M.; Al-Zaharnah, I. Energy and exergy utilization in agricultural sector of Saudi Arabia. Energy Policy 2005, 33, 1461–1467. [Google Scholar] [CrossRef]
- Al-Sulaiman, F.A.; Dincer, I.; Hamdullahpur, F. Energy and exergy analyses of a biomass trigeneration system using an organic Rankine cycle. Energy 2012, 45, 975–985. [Google Scholar] [CrossRef]
- Xu, X.M.; Zhang, L.; Jiang, Y.P.; Chen, N. Active Control on Path Following and Lateral Stability for Truck-Trailer Combi-nations. Arab. J. Sci. Eng. 2019, 44, 1365–1377. [Google Scholar] [CrossRef]
- Zhou, W.L.; Zheng, Y.P.; Pan, Z.J.; Lu, Q. Review on the Battery Model and SOC Estimation Method. Processes 2021, 9, 1685. [Google Scholar] [CrossRef]
- Chang, C.C.; Zheng, Y.P.; Sun, W.M.; Ma, Z.S. LPV Estimation of SOC Based on Electricity Conversion and Hysteresis Characteristic. J. Energy Eng. 2019, 145, 04019026. [Google Scholar] [CrossRef]
- Tian, J.; Zeng, Q.K.; Wang, P.; Wang, X.Q. Active steering control based on preview theory for articulated heavy vehicles. PLoS ONE 2021, 16, e0252098. [Google Scholar] [CrossRef]
- Xu, X.M.; Lin, P. Parameter identification of sound absorption model of porous materials based on modified particle swarm optimization algorithm. PLoS ONE 2021, 16, e0250950. [Google Scholar] [CrossRef]
- Xu, X.M.; Chen, D.; Zhang, L.; Chen, N. Hopf Bifurcation Characteristics of the Vehicle with Rear Axle Compliance Steering. Shock. Vib. 2019, 2019, 3402084. [Google Scholar] [CrossRef] [Green Version]
- Ribeirinha, P.; Abdollahzadeh, M.; Pereira, A.; Relvas, F.; Boaventura, M.; Mendes, A. High temperature PEM fuel cell integrated with a cellular membrane methanol steam reformer: Experimental and modelling. Appl. Energy 2018, 215, 659–669. [Google Scholar] [CrossRef]




| Parameter | Value |
|---|---|
| Faraday constant, | 96,485 |
| Gas constant, | 8.314 |
| Number of electrons, | 2 |
| Operating temperature, | 438 [22] |
| Anode pressure () | 1 [22] |
| Cathode pressure () | 1 [22] |
| Anode gas compositions | 100% [22] |
| Cathode gas compositions | 21% [22] |
| The doping level, | 10 [26] |
| The relative humidity, | 3.8% [26] |
| Thickness of the electrolyte, | 0.005 [26] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Li, Y.; Ma, Z.; Zheng, M.; Lu, Z. Exergetic Performance Coefficient Analysis and Optimization of a High-Temperature Proton Exchange Membrane Fuel Cell. Membranes 2022, 12, 70. https://doi.org/10.3390/membranes12010070
Li D, Li Y, Ma Z, Zheng M, Lu Z. Exergetic Performance Coefficient Analysis and Optimization of a High-Temperature Proton Exchange Membrane Fuel Cell. Membranes. 2022; 12(1):70. https://doi.org/10.3390/membranes12010070
Chicago/Turabian StyleLi, Dongxu, Yanju Li, Zheshu Ma, Meng Zheng, and Zhanghao Lu. 2022. "Exergetic Performance Coefficient Analysis and Optimization of a High-Temperature Proton Exchange Membrane Fuel Cell" Membranes 12, no. 1: 70. https://doi.org/10.3390/membranes12010070
APA StyleLi, D., Li, Y., Ma, Z., Zheng, M., & Lu, Z. (2022). Exergetic Performance Coefficient Analysis and Optimization of a High-Temperature Proton Exchange Membrane Fuel Cell. Membranes, 12(1), 70. https://doi.org/10.3390/membranes12010070






