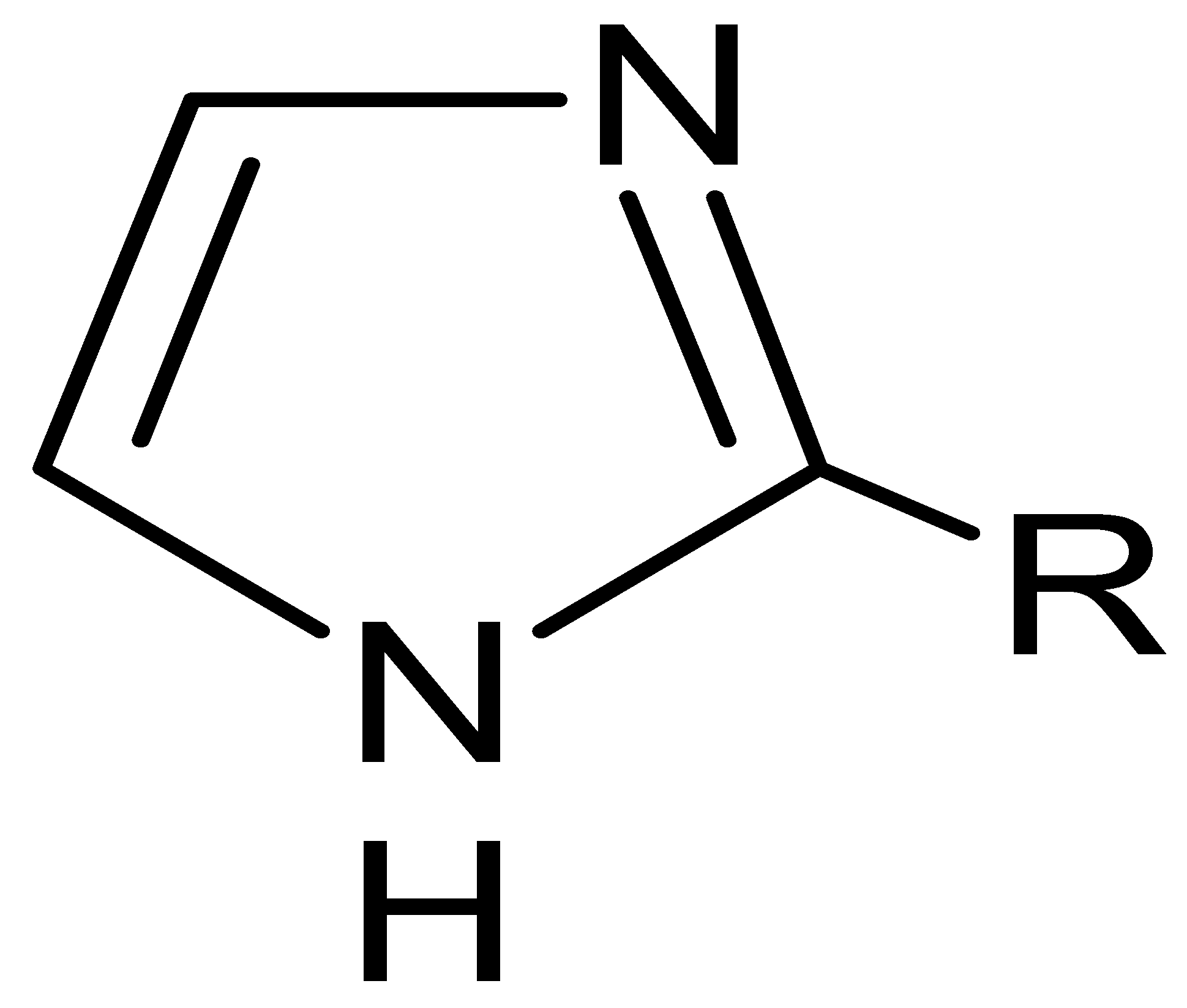
Abstract
Polymer inclusion membranes (PIMs) are an attractive approach to the separation of metals from an aqueous solution. This study is concerned with the use of 2-alkylimidazoles (alkyl = methyl, ethyl, propyl, butyl) as ion carriers in PIMs. It investigates the separation of copper (II), zinc (II), cobalt (II), and nickel (II) from aqueous solutions with the use of polymer inclusion membranes. PIMs are formed by casting a solution containing a carrier (extractant), a plasticizer (o-NPPE), and a base polymer such as cellulose triacetate (CTA) to form a thin, flexible, and stable film. The topics discussed include transport parameters, such as the type of carrier, initial fluxes, separation coefficients of copper in relation to other metals, as well as transport recovery of metal ions. The membrane was characterized using AFM and SEM to obtain information on its composition.
1. Introduction
The need for a more specific system for the recovery of non-ferrous metal, one that is more practical from both an economic and ecological standpoint, has led to the development of a new separation technique [1,2,3,4].
In recent years, it has been shown that membrane techniques have advantages over traditional metal compound removal and separation methods [5]. The use of liquid membranes—particularly polymer inclusion membranes (PIMs)—is especially distinguished in this respect [6,7]. PIMs have been proven to be a better alternative than ion exchange and solvent extraction methods. The advantage of using them is that the separation process is carried out in one step (unit process) and no toxic organic solvents are used because in PIMs both the extraction and back extraction processes occur simultaneously. For example, PIMs are successfully used in the recovery and separation of such metals as Cu [8,9], Co [10,11], Ni [10,12,13], Zn [12,14,15,16], Pb [12,17,18], Cd [10,12,19], Hg [20,21], Cr(III) [12,22], Cr(VI) [23], Mn [12], As [12,24], Fe [12], U [12,25], Ag [26,27], Au [28], as well as platinum group metals [29,30], lanthanides, and actinides [12,31,32].
A PIM is made from a base polymer, plasticizer, and carrier of metal ions. The polymer plays a key role in providing the membrane with mechanical strength, and its properties greatly affect the membrane’s permeability and durability. The solutions most often used as polymer matrices are CTA (cellulose triacetate) and PVC (polyvinyl chloride) [1,5,6,33]. The role of the plasticizer is to increase the flexibility and mechanical strength of the polymer matrix by penetrating between its polymer molecules and reducing the strength of the intermolecular forces, thereby increasing the distance between the polymer molecules. Some of the most commonly used plasticizers include o-nitrophenyl octyl and o-nitrophenyl pentyl ethers [6,34]. In principle, carriers used in PIMs are the same organic compounds as those used as extractants in solvent extraction [2,3,5,6,12]. It is crucial for a carrier to be stable on the feed phase side, as well as to readily decay at the membrane/receiving phase interface. Furthermore, a good carrier should be inexpensive, non-toxic, and soluble in the membrane [5,6,34,35]. The carrier’s primary function is to facilitate the transport of separated ions across the membrane. The following groups of carriers can be distinguished based on the chemical properties and the nature of interactions with metal ions:
- acidic carriers—capable of exchanging a proton for a metal ion; the most commonly used transporters of this group include organophosphorus acids (e.g., D2EHPA, Cyanex 272, Cyanex 303) [25,36,37,38], as well as hydroxyoximes (e.g., LIX-84 I) [39,40,41];
- alkaline carriers—organic compounds whose nature corresponds to Lewis bases; they form ionic pairs with metal ions. This group includes quaternary ammonium [42,43,44] and phosphonium salts [33,43], tertiary amines [44,45], pyridine and pyridine derivatives [6,46,47], and alkyl imidazole derivatives [8,27,48];
- inert carriers—capable of forming an inert complex with metal ions in the organic phase by replacing the water molecules in the metal aqua complex with their own molecules, which are more lyophilic. The group of these transporters includes such compounds as phosphoric acid esters [49,50] and phosphinic acid esters [51].
While commercial carriers of metal cations commonly used in the membrane technique enable the effective separation of ions, their selectivity is rather poor. Therefore, new complexing reagents are sought—ones that can selectively separate metal ions from aqueous solutions.
This paper presents a laboratory experiment in which polymer inclusion membranes doped with 2-alkylimidazole (alkyl = methyl, ethyl, propyl, butyl) were tested in order to assess their efficiency in the separation of copper, zinc, cobalt, and nickel ions from their equimolar mixtures. These metals are important for the development of the economy, especially for the development of modern technologies. The demand for them is still growing. In our previous work, alkyl imidazole derivatives were used as carriers in PIMs to separate non-ferrous metal ions [48]. It has been observed that the ion separation efficiency is affected by both the hydrophobic effect, which depends on the length of the alkyl substituent bound to the imidazole molecule [8,52], and the steric effect (steric hindrance) induced by the methyl substituent located in the imidazole molecule at position 2 or 4, i.e., at the donor nitrogen atom [53,54]. In this study, the influence of the length of the alkyl substituent at position 2 in the alkylimidazole molecule was investigated—i.e., the influence of the growth of the hindrance on the separation efficiency of copper, zinc, cobalt, and nickel ions.
2. Materials and Methods
2.1. Materials
All the reagents used were of analytical grade. Cellulose triacetate, 2-nitrophenyl pentyl ether (NPPE,) and dichloromethane were obtained from Fluka. All the stock solutions were prepared by dissolving the salts in distilled water. CoCl2 6H2O, NiCl2 6H2O, ZnCl2, and CuCl2 2H2O were purchased from POCh, Gliwice, Poland. The 2-alkylimidazole (alkyl = methyl, ethyl, propyl, butyl) were synthesized by A. Skrzypczak (Poznan University of Technology, Institute of Chemical Technology and Engineering) according to the procedure described in [55]. Table 1 shows certain physical and chemical properties of the 2-alkylimidazoles used in this study.

Table 1.
The general formula of the 2-alkylimidazoles used in the tests and some of their physicochemical properties.
2.2. Procedure
PIMs were made from CTA, NPPE, and 2-alkylimidazole and were prepared according to the procedure reported in the previous paper [8,52,53,54]. As shown in papers [8,48,52,53,54], the composition of a membrane containing alkylimidazoles as a carrier is optimal when such membrane contains 2.6 cm3 of NPPE per 1 g CTA and 1.0 mol/dm3 of alkylimidazole calculated on plasticizer.
Transport experiments were carried out at a temperature of 20 ± 0.2 °C, according to the procedure described in our previous paper [8,52,53,54]. The initial concentration (C0) of each metal ion in the feed phase was 10−3 mol/dm3. The feed aqueous phase was an aqueous solution with a pH of 6.0 (tetramethylammonium hydroxide) while the receiving phase was distilled water. In the feed phase, the metal concentration (Ct) was determined at appropriate time intervals using the atomic absorption spectroscopy method (AAS Spectrometer, AAS240FS, Agilent, Santa Clara, CA, USA).
The kinetics of the transport across PIMs was described as a first-order reaction in metal ion concentration (ln(C0/Ct) = −kt; where k is the rate constant, and t is the time of transport). The k values were calculated from the plots of ln(C0/Ct) vs. time. As expected, the relationship of ln(C0/Ct) vs. time was linear. The initial fluxes (Jo) were determined as Jo = (−kV/AC0), where V is the volume of the feed phase and A is the area of the membrane.
3. Results and Discussion
3.1. Membrane Characterization
As shown in numerous studies [10,15,48,50,51,52,53,54], the membrane’s physicochemical properties affect the selectivity of metal ion transport. Both scanning electron microscopy (SEM) (Hitachi SU3500 SEM/EDS Energy-Dispersive Spectroscopy Hitachi, Tokyo, Japan) and atomic-force MultiMode Scanning Probe Microscope IIIa (AFM) (Digital Instruments Veeco Metrology Group, Santa Barbara, CA, USA)—utilized in air and at room temperature—were used to characterize the PIM surfaces. Figure 1 shows AFM and SEM images of PIMs with carriers 1–4.
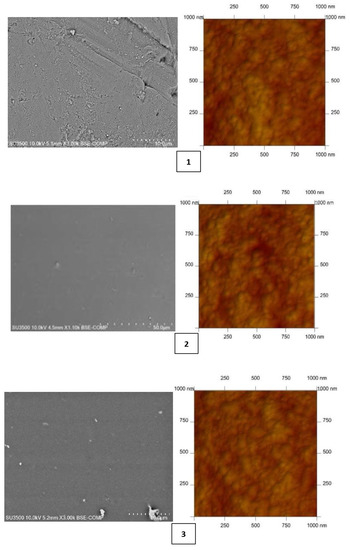
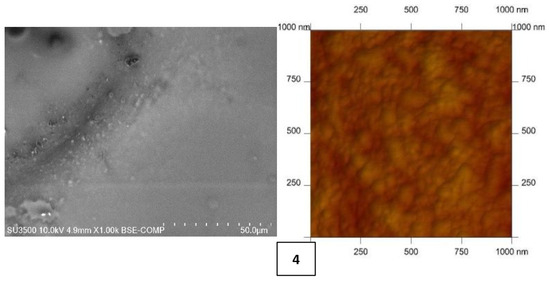
Figure 1.
SEM and 2D AFM images of PIMs doped 2-methylimidazole (1), 2-ethylimidazole (2), 2-propylimidazole (3), and 2-butylimidazole (4).
Both the SEM and AFM images in Figure 1 indicate that the carrier distribution in the investigated membranes is homogeneous throughout the entire surface after the solvent’s evaporation.
The average thickness of all membranes was measured using a Panametrics® Magna-Mike® 8500 (San Diego, CA, USA) manual precision thickness gauge. Mean roughness values of the membrane were calculated using AFM. Both the average thickness and roughness values are summarized in Table 2.

Table 2.
Average thickness and roughness of PIMs.
CTA-NPPE-2-alkylimidazole (1–4) membranes form thin films with a thickness of 26–30 µm. Many authors [57,58,59,60,61] have shown that microstructure of PIMs affects the transport of metal ions. Their roughness of PIMs 1–4 varies from 3.55 to 4.47 nm. These values are comparable to the values obtained for PIMs with other homologous series of alkylimidazoles (roughness of 2.2–7.2 nm) [48,57,58,59]; however, it is less than that of the CTA membrane obtained by Tor et al., which amounted to 14 nm [22]. The roughness values are also comparable with a CTA membrane doped with a thioazocrown derivative imidazole (a roughness of 3.3–5.3 nm) reported by Ulewicz [60] and with a membrane containing D2EHPA prepared by Salazar-Alvarez [61], whose roughness was 4.6 nm.
3.2. Membrane Transport
The transport of Co(II), Cu(II), Ni(II), and Zn(II) ions through PIMs from an equimolar mixture was studied using 2-alkylimidazole (alkyl = methyl, ethyl, propyl, butyl) as a carrier. Figure 2 shows changes in the ion concentration of the studied metals in the feed phase during this process.
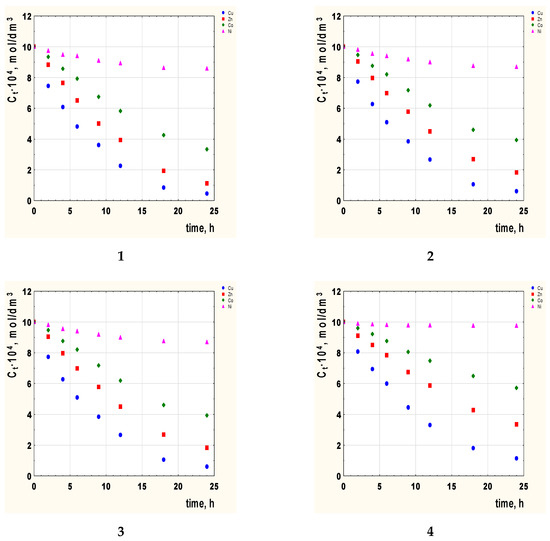
Figure 2.
The concentration Cu(II), Zn(II), Co(II), and Ni(II) ions vs. time for PIMs with 2-alkylimidazole as the carrier. 2-methylimidazole (1), 2-ethylimidazole (2), 2-propylimidazole (3), and 2-butylimidazole (4). Membrane: 2.6 cm3 o-NPPE/1 g CTA and 1.0 mol/dm3.
On the basis of Figure 2, it can be seen that regardless of the type of carrier used in the membrane, the changes in the concentrations of the tested metal ions can be arranged in the order Cu(II) > Zn(II) > Co(II) > Ni(II).
The optimal transport time is 24 h. After this time, the state of equilibrium is established and a further extension of the time does not change the concentrations of metals.
Table 3 shows the initial flux values (J0) of the studied metal ions, depending on the type of carrier used.

Table 3.
Kinetic parameters of transport by PIMs containing 2-alkylimidazoles and selectivity coefficient of Cu(II) ions.
The data in Table 3 show that the rate of transport of the studied metal ions decreases in the series Cu(II) > Zn(II) > Co(II) > Ni(II). As the length of the alkyl chain in the carrier molecule increases, the initial fluxes of all ions decrease. This phenomenon may be explained by kinetic factors in the formation of complexes of the studied metals at the membrane/feed phase interface. Ni(II) ion transport is particularly low, and in the case of membrane 4, it is completely inhibited. Ni(II) ions are virtually never transported across membranes containing 2-alkylimidazoles as carriers; they remain in the feed phase.
3.3. Complexation Mechanism
The transport process is similar for all carrier types, especially alkyl imidazole derivatives [8,27,48,52,53,54]. The alkyl substituent at position 2 hinders the formation of all metal complexes, as evidenced by the lower values of the stability constants (log β) exhibited by 2-alkylimidazole complexes compared to 1-alkylimidazoles (Table 4).

Table 4.
Comparison of the stability constant values (log β) of Cu(II), Zn(II), Co(II), and Ni(II) complexes.
The alkyl substituent located at position 2 in the alkylimidazole molecule is a steric hindrance to the binding of metal ions as it blocks access to the donor nitrogen atom. The longer the substituent, the more difficult the complexation reaction (Figure 3).
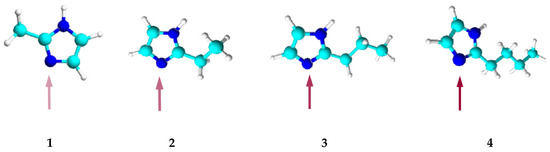
Figure 3.
Models of the 2-alkylimidazole molecule, 2-methylimidazole (1), 2-ethylimidazole (2), 2-propylimidazole (3), and 2-butylimidazole (4).
The steric effect represents a particular hindrance to the formation of Ni(II) as well as Zn(II) and Co(II) octahedral complexes. It is difficult for Ni(II) ions to bind with 2-alkylimidazoles, and in the case of 2-butylimidazole, a binding reaction does not occur at all (Table 4). Ni(II) ions mostly form 6-coordination complexes, because they have a rigid octahedral structure that is hard to deform. On the other hand, apart from the 6-coordinated ones, Zn(II) and Co(II) ions can also form tetrahedral complexes, which are more easily transferred by PIMs due to their smaller volume. Among the four cations studied, coordination sphere deformation is highly likely to occur only in the case of Cu(II) ions—due to the Jahn–Teller effect [70]—making the transport of such ions the easiest.
Due to differences in the complexation reaction using 2-alkylimidazoles, it is possible to selectively separate Cu(II) ions, particularly from Ni(II) ions (Table 3).
Figure 4 presents the proposed mechanism of the transport of M(II) ions across PIMs.
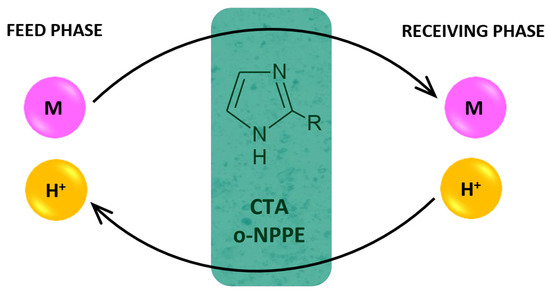
Figure 4.
Schematic transport of metal ions across PIM doped 2-alkylimidazoles.
In the transport mechanism shown in Figure 4, the complexation reaction takes place between the metal ion and 2-alkylimidazoles (L). Complex ions are formed in the membrane:
for Cu(II), Zn(II) Co(II) M2+ + 4 L ↔ [ML4]2+
for Ni(II) M2+ + 6 L ↔ [ML6]2+
The complex ion is then transferred across the membrane towards the receiving phase. Complex ions dissociate at the interface between the membrane and the receiving phase.
At the same time, the proton ions are transported from the receiving phase in the same way towards the feed phase.
3.4. Diffusion of Metal Ions across PIMs
The next step consisted in calculating the diffusion coefficient (Do) of the metal complex across the membrane doped with 2-alkylimidazole. Figure 5 shows the correlation graphs C0-Ct vs. time of transport for metal ions with the carriers 1–4 across PIMs.
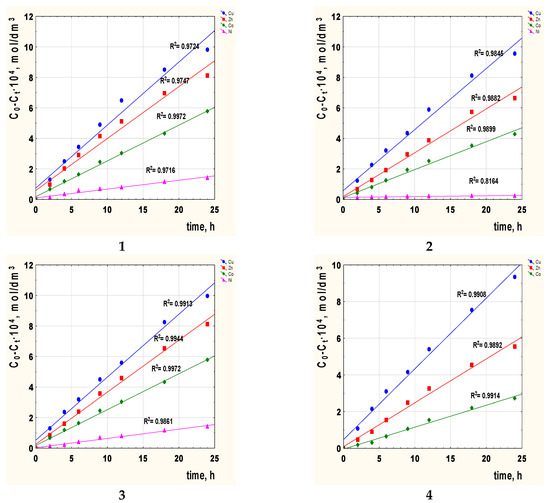
Figure 5.
The relationship C0-Ct vs. time of metal ions transport across PIMs with 2-alkylimidazole 1–4 as the carrier; 2-methylimidazole (1), 2-ethylimidazole (2), 2-propylimidazole (3), and 2-butylimidazole (4).
The diffusion coefficient of each metal ion was calculated, substituting Do = do/Δo, where do is the thickness of the membrane (Table 2), and Δo could be evaluated based on the angle of inclination of the lines presented in Figure 5. Table 5 shows the obtained values of diffusion coefficients.

Table 5.
Diffusion coefficients (Do) of Cu(II), Zn(II), Co(II), and Ni(II) complexes with 2-alkylimidazoles and membrane diffusion resistance values (Δo).
The value of the diffusion coefficient of M(II)-carrier species of 2.38 × 10−8–4.13 × 10−13 cm2/s (Table 5) is smaller than the value of 1.5 × 10−7 cm2/s reported for the Pb(II) complex with the D2EHPA in PIM by Salazar-Alvarez [61] and is within the range of 10−8–10−13 cm2/s, which indicates that the rate-determining step in the transport of metal ions is the passage across the membrane.
3.5. Transport Recovery
The transport recovery of Cu(II), Zn(II), Co(II), and Ni(II) ions from their equimolar solutions as a result of transport by PIMs doped with 2-alkylimidazoles 1–4 is shown in Figure 6.
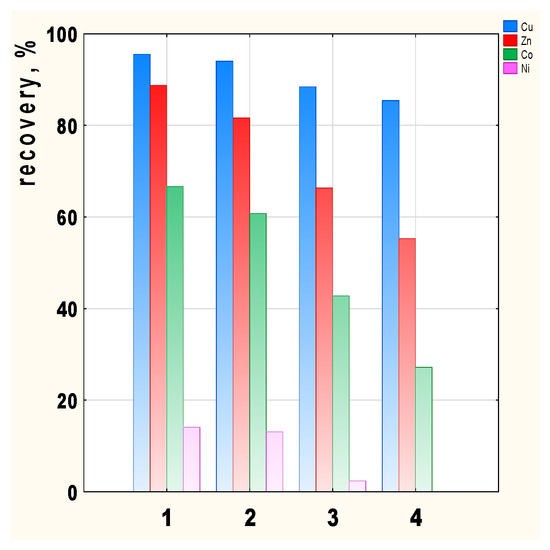
Figure 6.
Transport recovery of Cu(II), Zn(II), Co(II), and Ni(II) ions after 24 h depending on the carrier used; 2-methylimidazole (1), 2-ethylimidazole (2), 2-propylimidazole (3), and 2-butylimidazole (4).
Metal recovery depends on the carrier used in the membrane, with the highest recovery values achieved in the case of 2-methyl- (1) and 2-ethylimidazole (2) (Figure 6). Cu(II) recovery is the highest, ranging from 95.5 (membrane 1) to 85.4% (membrane 4) depending on the membrane used. Zn(II) and Co(II) recovery clearly decrease for carriers 3 and 4. There is little transport of Ni(II) ions across all the membranes tested, hence the Ni(II) recovery is 14% for membrane 1. In the case of the 2-butylimidazole membrane (4), Ni(II) ions remain in the feed phase.
3.6. Comparison of the Results with Previously Tested Alkyl Imidazole Derivatives
Table 6 shows the copper (II) separation coefficients in relation to Zn(II), Co(II), and Ni(II) from their equimolar solutions after a 24 h transport across PIMs with alkylimidazoles (1-alkylimidazoles, 1-alkyl-2-methylimidazoles, and 1-alkyl-4-methylimidazoles).

Table 6.
The copper (II) separation coefficients in relation to Zn(II), Co(II), and Ni(II) from their equimolar solutions after a 24 h transport across PIMs with alkylimidazoles.
In the case of PIM with 2-alkylimidazoles, the copper (II) separation coefficients in relation to Zn(II) and Co(II) are lower (Table 6). However, these membranes efficiently separate Ni(II) ions from Cu(II)-Zn(II)-Co(II)-Ni(II) mixture.
Table 7 contains the values of Cu(II) recovery in transport across PIMs doped with alkylimidazoles.

Table 7.
The values of Cu(II) recovery after a 24 h transport across PIMs doped with alkylimidazoles.
The data in Table 7 indicate that copper recovery after a 24-hour process of transport using alkylimidazole derivatives as carriers are high, which proves the efficiency of alkylimidazoles in the process of separation of Cu(II) ions. The highest Cu(II) recovery factors were achieved when using 1-hexylimidazole [48].
PIMs with 2-alkylimidazoles can be used both for the recovery of Cu(II) from Cu(II)-Zn(II)-Co(II)-Ni(II) mixtures, as well as for the separation of Cu (II) from Ni (II).
4. Conclusions
In the process of transport across polymer inclusion membranes using 2-alkylimidazole as a carrier, the rate of transport of metal ions studied decreases in the series Cu(II) > Zn(II) > Co(II) > Ni(II). The presence of a substituent at position 2 affects the value of all parameters characterizing transport. As the length of this substituent increases, the following changes can be observed:
- An increase in the initial fluxes of Cu(II) ions and a decrease in the initial fluxes of Zn(II), Co(II), and Ni(II) ions, up to a complete disappearance of the flux of Ni(II) ions when using 2-butylimidazole,
- An increase in the separation coefficients of Cu(II) ions relative to those of other metals. The highest separation coefficient values for Cu(II)/Zn(II) and Cu(II)/Co(II) are 2.6, 6.9 (membrane 4), and in the case of Cu(II)/Ni(II), 182 (membrane 3), respectively.
- A decrease in the recovery efficiency of each metal. Cu(II), Zn(II), Co(II), and Ni(II) recovery is the highest for the membrane with carrier 1 (2-methylimidazole) and amounts to 95.5%, 88.8%, 66.7%, and 14.1%, respectively.
The differences in ion transport and separation efficiency are caused by differences in the complexation reaction of Cu(II), Zn(II), Co(II), and Ni(II) with 2-alkylimidazoles.
Author Contributions
Conceptualization, E.R.-L.; methodology, E.R.-L. and W.U.; software, K.M. and E.R.-L.; validation, E.R.-L., K.M. and W.U.; formal analysis, E.R.-L. and K.M.; investigation, E.R.-L. and K.M. writing—original draft preparation, K.M.; writing—review and editing, E.R.-L. and K.M.; visualization, E.R.-L. and K.M. All authors have read and agreed to the published version of the manuscript.
Funding
Research financed by the Operational Program PL03 “Improvement of Environmental Monitoring and Inspection” under the EEA Funds 2009–2014, No. 309/2015/Wn15/MN-xn-03/D.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Almeida, M.I.; Cattrall, R.W.; Kolev, S.D. Polymer Inclusion Membranes: Concept and Applications. Procedia Eng. 2012, 44, 681–682. [Google Scholar] [CrossRef] [Green Version]
- Landaburu-Aguirre, J.; García, V.; Pongrácz, E.; Keiski, R. Applicability of Membrane Technologies for the Removal of Heavy Metals. Desalination 2006, 200, 272–273. [Google Scholar] [CrossRef]
- Keskin, B.; Zeytuncu-Gökoğlu, B.; Koyuncu, I. Polymer Inclusion Membrane Applications for Transport of Metal Ions: A Critical Review. Chemosphere 2021, 279, 130604. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.J.; Lee, S.M.; Wang, R.; Lee, J. Emerging Materials to Prepare Mixed Matrix Membranes for Pollutant Removal in Water. Membranes 2021, 11, 508. [Google Scholar] [CrossRef]
- Kislik, V.S. Liquid Membranes: Principles and Applications in Chemical Separations and Wastewater Treatment, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2009; ISBN 978-0-444-53218-3. [Google Scholar]
- Almeida, M.I.G.S.; Cattrall, R.W.; Kolev, S.D. Recent Trends in Extraction and Transport of Metal Ions Using Polymer Inclusion Membranes (PIMs). J. Membr. Sci. 2012, 415–416, 9–23. [Google Scholar] [CrossRef]
- Kuswandi, B.; Nitti, F.; Almeida, M.I.G.S.; Kolev, S.D. Water Monitoring Using Polymer Inclusion Membranes: A Review. Environ. Chem. Lett. 2020, 18, 129–150. [Google Scholar] [CrossRef]
- Radzyminska-Lenarcik, E.; Ulewicz, M. Selective Transport of Cu(II) across a Polymer Inclusion Membrane with 1-Alkylimidazole from Nitrate Solutions. Sep. Sci. Technol. 2012, 47, 1113–1118. [Google Scholar] [CrossRef]
- Palanivelu, K.; Kavitha, N. Recovery of Copper(II) through Polymer Inclusion Membrane with Di (2-Ethylhexyl) Phosphoric Acid as Carrier from e-Waste. J. Membr. Sci. 2012, 415–416, 663–669. [Google Scholar] [CrossRef]
- Pyszka, I.; Radzyminska-Lenarcik, E. New Polymer Inclusion Membrane in the Separation of Nonferrous Metal Ion from Aqueous Solutions. Membranes 2020, 10, 385. [Google Scholar] [CrossRef]
- Yıldız, Y.; Manzak, A.; Tutkun, O. Selective Extraction of Cobalt Ions through Polymer Inclusion Membrane Containing Aliquat 336 as a Carrier. Desalination Water Treat. 2016, 57, 4616–4623. [Google Scholar] [CrossRef]
- Zulkefeli, N.S.W.; Weng, S.K.; Abdul Halim, N.S. Removal of Heavy Metals by Polymer Inclusion Membranes. Curr. Pollut. Rep. 2018, 4, 84–92. [Google Scholar] [CrossRef]
- Macías, M.; Miguel, E. Optimization of Ni(II) Facilitated Transport from Aqueous Solutions Using a Polymer Inclusion Membrane. Water. Air. Soil Pollut. 2021, 232, 62. [Google Scholar] [CrossRef]
- Wang, D.; Liu, J.; Chen, J.; Liu, Q.; Zeng, H. New Insights into the Interfacial Behavior and Swelling of Polymer Inclusion Membrane (PIM) during Zn(II) Extraction Process. Chem. Eng. Sci. 2020, 220, 115620. [Google Scholar] [CrossRef]
- Baczyńska, M.; Waszak, M.; Nowicki, M.; Prządka, D.; Borysiak, S.; Regel-Rosocka, M. Characterization of Polymer Inclusion Membranes (PIMs) Containing Phosphonium Ionic Liquids as Zn (II) Carriers. Ind. Eng. Chem. Res. 2018, 57, 5070–5082. [Google Scholar] [CrossRef]
- Witt, K.; Radzyminska-Lenarcik, E.; Kosciuszko, A.; Gierszewska, M.; Ziuziakowski, K. The Influence of the Morphology and Mechanical Properties of Polymer Inclusion Membranes (PIMs) on Zinc Ion Separation from Aqueous Solutions. Polymers 2018, 10, 134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konczyk, J.; Ciesielski, W. Calixresorcin (4) Arene-Mediated Transport of Pb (II) Ions through Polymer Inclusion Membrane. Membranes 2021, 11, 285. [Google Scholar] [CrossRef]
- Sgarlata, C.; Arena, G.; Longo, E.; Zhang, D.; Yang, Y.; Bartsch, R. Heavy Metal Separation with Polymer Inclusion Membranes. J. Membr. Sci. 2008, 323, 444–451. [Google Scholar] [CrossRef]
- Kebiche-Senhadji, O.; Mansouri, L.; Tingry, S.; Seta, P.; Benamor, M. Facilitated Cd (II) Transport across CTA Polymer Inclusion Membrane Using Anion (Aliquat 336) and Cation (D2EHPA) Metal Carriers. J. Membr. Sci. 2008, 310, 438–445. [Google Scholar] [CrossRef]
- Elias, G.; Marguí, E.; Díez, S.; Fontàs, C. Polymer Inclusion Membrane as an Effective Sorbent to Facilitate Mercury Storage and Detection by X-Ray Fluorescence in Natural Waters. Anal. Chem. 2018, 90, 4756–4763. [Google Scholar] [CrossRef]
- Mercader-Trejo, F.E.; Rodríguez de San Miguel, E.; de Gyves, J. Mercury (II) Removal Using Polymer Inclusion Membranes Containing Cyanex 471X. J. Chem. Technol. Biotechnol. 2009, 84, 1323–1330. [Google Scholar] [CrossRef]
- Tor, A.; Arslan, G.; Muslu, H.; Celiktas, A.; Cengeloglu, Y.; Ersoz, M. Facilitated Transport of Cr (III) through Polymer Inclusion Membrane with Di(2-Ethylhexyl)Phosphoric Acid (DEHPA). J. Membr. Sci. 2009, 1–2, 169–174. [Google Scholar] [CrossRef]
- Gherasim, C.-V.; Bourceanu, G.; Olariu, R.-I.; Arsene, C. A Novel Polymer Inclusion Membrane Applied in Chromium (VI) Separation from Aqueous Solutions. J. Hazard. Mater. 2011, 197, 244–253. [Google Scholar] [CrossRef]
- Zawierucha, I.; Nowik-Zajac, A.; Malina, G. Selective Removal of As(V) Ions from Acid Mine Drainage Using Polymer Inclusion Membranes. Minerals 2020, 10, 909. [Google Scholar] [CrossRef]
- St John, A.M.; Cattrall, R.W.; Kolev, S.D. Transport and Separation of Uranium(VI) by a Polymer Inclusion Membrane Based on Di-(2-Ethylhexyl) Phosphoric Acid. J. Membr. Sci. 2012, 409–410, 242–250. [Google Scholar] [CrossRef]
- Nowik-Zając, A.; Zawierucha, I.; Kozłowski, C. Selective Removal of Silver(I) Using Polymer Inclusion Membranes Containing Calixpyrroles. RSC Adv. 2019, 9, 31122–31132. [Google Scholar] [CrossRef] [Green Version]
- Radzyminska-Lenarcik, E.; Ulewicz, M.; Pyszka, I. Application of Polymer Inclusion Membranes Doped with Alkylimidazole to Separation of Silver and Zinc Ions from Model Solutions and after Battery Leaching. Materials 2020, 13, 3103. [Google Scholar] [CrossRef]
- Kubota, F.; Kono, R.; Yoshida, W.; Sharaf, M.; Kolev, S.D.; Goto, M. Recovery of Gold Ions from Discarded Mobile Phone Leachate by Solvent Extraction and Polymer Inclusion Membrane (PIM) Based Separation Using an Amic Acid Extractant. Sep. Purif. Technol. 2019, 214, 156–161. [Google Scholar] [CrossRef]
- Fajar, A.T.N.; Hanada, T.; Firmansyah, M.L.; Kubota, F.; Goto, M. Selective Separation of Platinum Group Metals via Sequential Transport through Polymer Inclusion Membranes Containing an Ionic Liquid Carrier. ACS Sustain. Chem. Eng. 2020, 8, 11283–11291. [Google Scholar] [CrossRef]
- Fajar, A.T.N.; Hanada, T.; Goto, M. Recovery of Platinum Group Metals from a Spent Automotive Catalyst Using Polymer Inclusion Membranes Containing an Ionic Liquid Carrier. J. Membr. Sci. 2021, 629, 119296. [Google Scholar] [CrossRef]
- Croft, C.F.; Almeida, M.I.G.S.; Cattrall, R.W.; Kolev, S.D. Separation of Lanthanum(III), Gadolinium(III) and Ytterbium(III) from Sulfuric Acid Solutions by Using a Polymer Inclusion Membrane. J. Membr. Sci. 2018, 545, 259–265. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, L.; Yan, Y.; Chen, J.; Dai, J.; Dai, X. Separation of Adjacent Heavy Rare Earth Lutetium(III) and Ytterbium(III) by Task-Specific Ionic Liquid Cyphos IL 104 Embedded Polymer Inclusion Membrane. J. Membr. Sci. 2020, 610, 118263. [Google Scholar] [CrossRef]
- Baczyńska, M.; Regel-Rosocka, M.; Nowicki, M.; Wiśniewski, M. Effect of the Structure of Polymer Inclusion Membranes on Zn(II) Transport from Chloride Aqueous Solutions. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Nghiem, L.; Mornane, P.; Potter, I.; Perera, J.; Cattrall, R.; Kolev, S. Extraction and Transport of Metal Ions and Small Organic Compounds Using Polymer Inclusion Membranes (PIMs). J. Membr. Sci. 2006, 281, 7–41. [Google Scholar] [CrossRef]
- Zante, G.; Boltoeva, M.; Masmoudi, A.; Barillon, R.; Trébouet, D. Supported Ionic Liquid and Polymer Inclusion Membranes for Metal Separation. Sep. Purif. Rev. 2021, 232, 1–17. [Google Scholar] [CrossRef]
- Kolev, S.D.; Baba, Y.; Cattrall, R.W.; Tasaki, T.; Pereira, N.; Perera, J.M.; Stevens, G.W. Solid Phase Extraction of Zinc(II) Using a PVC-Based Polymer Inclusion Membrane with Di(2-Ethylhexyl)Phosphoric Acid (D2EHPA) as the Carrier. Talanta 2009, 78, 795–799. [Google Scholar] [CrossRef]
- Gega, J.; Otrembska, P. Separation of Ni(II) and Cd(II) Ions with Supported Liquid Membranes (SLM) Using D2EHPA as a Carrier. Sep. Sci. Technol. 2014, 49, 1756–1760. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Mohapatra, P.K.; Hassan, P.A.; Manchanda, V.K. Studies on the Selective Am3+ Transport, Irradiation Stability and Surface Morphology of Polymer Inclusion Membranes Containing Cyanex-301 as Carrier Extractant. J. Hazard. Mater. 2011, 192, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Gyves, J.; Hernández-Andaluz, A.; Rodríguez de San Miguel, E. LIX®-Loaded Polymer Inclusion Membrane for Copper(II) Transport: 2. Optimization of the Efficiency Factors (Permeability, Selectivity, and Stability) for LIX® 84-I. J. Membr. Sci. 2006, 268, 142–149. [Google Scholar] [CrossRef]
- Kagaya, S.; Cattrall, R.W.; Kolev, S.D. Solid-Phase Extraction of Cobalt(II) from Lithium Chloride Solutions Using a Poly(Vinyl Chloride)-Based Polymer Inclusion Membrane with Aliquat 336 as the Carrier. Anal. Sci. Int. J. Jpn. Soc. Anal. Chem. 2011, 27, 653–657. [Google Scholar] [CrossRef] [Green Version]
- Abdul-Halim, N.-S.; Whitten, P.; Nghiem, L. Characterising Poly (Vinyl Chloride)/Aliquat 336 Polymer Inclusion Membranes: Evidence of Phase Separation and Its Role in Metal Extraction. Fac. Eng. Inf. Sci. Pap. Part A 2013, 119, 14–18. [Google Scholar] [CrossRef] [Green Version]
- Annane, K.; Sahmoune, A.; Montels, P.; Tingry, S. Polymer Inclusion Membrane Extraction of Cadmium(II) with Aliquat 336 in Micro-Channel Cell. Chem. Eng. Res. Des. 2015, 94, 605–610. [Google Scholar] [CrossRef]
- Kogelnig, D.; Regelsberger, A.; Stojanovic, A.; Jirsa, F.; Krachler, R.; Keppler, B. A Polymer Inclusion Membrane Based on the Ionic Liquid Trihexyl(Tetradecyl)Phosphonium Chloride and PVC for Solid–Liquid Extraction of Zn(II) from Hydrochloric Acid Solution. Monatshefte Chem. 2011, 142, 769–772. [Google Scholar] [CrossRef]
- Kozlowski, C.A. Kinetics of Chromium(VI) Transport from Mineral Acids across Cellulose Triacetate (CTA) Plasticized Membranes Immobilized by Tri-n-Octylamine. Ind. Eng. Chem. Res. 2007, 46, 5420–5428. [Google Scholar] [CrossRef]
- Pospiech, B. Selective Recovery of Cobalt(II) towards Lithium(I) from Chloride Media by Transport across Polymer Inclusion Membrane with Triisooctylamine. Pol. J. Chem. Technol. 2014, 16, 15–20. [Google Scholar] [CrossRef] [Green Version]
- Djunaidi, M.; Cahyono, H.; Ismiyarto, I.; Siswanta, D.; Jumina, J. Synthesis of Poly Pyridine-2-Ylmethyl 2-(Eugenoxy) Acetate (PMEOA) as a Metal Mixture Carrier. IOP Conf. Ser. Mater. Sci. Eng. 2020, 959, 012033. [Google Scholar] [CrossRef]
- Tasaki, T.; Oshima, T.; Baba, Y. Selective Extraction and Transport of Copper(II) with New Alkylated Pyridinecarboxylic Acid Derivatives. Talanta 2007, 73, 387–393. [Google Scholar] [CrossRef]
- Ulewicz, M.; Radzyminska-Lenarcik, E. Application of Hydrophobic Alkylimidazoles in the Separation of Non-Ferrous Metal Ions across Plasticised Membranes—A Review. Membranes 2020, 10, 331. [Google Scholar] [CrossRef] [PubMed]
- Pospiech, B.; Walkowiak, W.; Woźniak, M.J. Application of TBP in Selective Removal of Iron(III) in Solvent Extraction and Transport through Polymer Inclusion Membranes Processes. Physicochem. Probl. Miner. Process. 2005, 39, 89–98. [Google Scholar]
- Arous, O.; Amara, M.; Trari, M.; Bouguelia, A.; Kerdjoudj, H. Cadmium(II) and Lead(II) Transport in a Polymer Inclusion Membrane Using Tributyl Phosphate as Mobile Carrier and CuFeO(2) as a Polarized Photo Electrode. J. Hazard. Mater. 2010, 180, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Kusumocahyo, S.P.; Kanamori, T.; Sumaru, K.; Aomatsu, S.; Matsuyama, H.; Teramoto, M.; Shinbo, T. Development of Polymer Inclusion Membranes Based on Cellulose Triacetate: Carrier-Mediated Transport of Cerium(III). J. Membr. Sci. 2004, 244, 251–257. [Google Scholar] [CrossRef]
- Radzyminska-Lenarcik, E.; Ulewicz, M. The Application of Polymer Inclusion Membranes Based on CTA with 1-Alkylimidazole for the Separation of Zinc(II) and Manganese(II) Ions from Aqueous Solutions. Polymers 2019, 11, 242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radzyminska-Lenarcik, E.; Ulewicz, M. Polymer Inclusion Membranes (PIMs) Doped with Alkylimidazole and Their Application in the Separation of Non-Ferrous Metal Ions. Polymers 2019, 11, 1780. [Google Scholar] [CrossRef] [Green Version]
- Radzyminska-Lenarcik, E.; Ulewicz, M. Application of Polymer Inclusion and Membranes Supported with 1-Alkyl-2-Methylimidazoles for Separation of Selected Transition Metal Ions. Desalination Water Treat. 2017, 64, 425–431. [Google Scholar] [CrossRef]
- Pernak, J.; Krysinski, J.; Skrzypczak, A. Bakterizide Wirkung von Iminiumverbindungen. Tenside Surfactants Deterg. 1987, 24, 276–279. [Google Scholar] [CrossRef]
- Lenarcik, B.; Ojczenasz, P. The Influence of the Size and Position of the Alkyl Groups in Alkylimidazole Molecules on Their Acid—Base Properties. J. Heterocycl. Chem. 2002, 39, 287–290. [Google Scholar] [CrossRef]
- Ulewicz, M.; Radzymińska-Lenarcik, E. Transport of Metal Ions across Polymer Inclusion Membrane with 1-Alkylimidazole. Physicochem. Probl. Miner. Process. 2011, 46, 119–130. [Google Scholar]
- Ulewicz, M.; Radzyminska-Lenarcik, E. Supported Liquid (SLM) and Polymer Inclusion (PIM) Membranes Pertraction of Copper(II) from Aqueous Nitrate Solutions by 1-Hexyl-2-Methylimidazole. Sep. Sci. Technol. 2012, 47, 1383–1389. [Google Scholar] [CrossRef]
- Ulewicz, M.; Radzyminska-Lenarcik, E. Application of Polymer and Supported Membranes with 1-Decyl-4-Methylimidazole for Pertraction of Transition Metal Ions. Sep. Sci. Technol. 2014, 49, 1713–1721. [Google Scholar] [CrossRef]
- Ulewicz, M.; Szczygelska-Tao, J.; Biernat, J.F. Selectivity of Pb(II) Transport across Polymer Inclusion Membranes Doped with Imidazole Azothiacrown Ethers. J. Membr. Sci. 2009, 344, 32–38. [Google Scholar] [CrossRef]
- Salazar-Alvarez, G.; Bautista-Flores, A.N.; Miguel, E.R. Transport Characterisation of a PIM System Used for the Extraction of Pb(II) Using D2ehpa as Carrier. J. Membr. Sci. 2005, 250, 247–257. [Google Scholar] [CrossRef]
- Radzyminska-Lenarcik, E. The Influence of the Alkyl Chain Length on Extraction Equilibrium of Cu(II) Complexes with 1-Alkylimidazoles in Aqueous Solution/Organic Solvent Systems. Solvent Extr. Ion Exch. 2007, 25, 53–64. [Google Scholar] [CrossRef]
- Lenarcik, B.; Kierzkowska, A. The Influence of Alkyl Chain Length on Stability Constants of Zn(II) Complexes with 1-Alkylimidazoles in Aqueous Solutions and Their Partition Between Aqueous Phase and Organic Solvent. Solvent Extr. Ion Exch. 2004, 22, 449–471. [Google Scholar] [CrossRef]
- Lenarcik, B.; Ojczenasz, P. Investigation of the Stability of Co(II) Complexes with a Homologous Series of 1-Alkylimidazoles in Aqueous Solution by Using a Partition Method with Several Solvents. Sep. Sci. Technol. 2005, 39, 199–226. [Google Scholar] [CrossRef]
- Lenarcik, B.; Rauckyte, T. The Influence of Alkyl Chain Length on Extraction Equilibria of Ni(II) Complexes with 1-Alkylimidazoles in Aqueous Solution/Organic Solvent Systems. Sep. Sci. Technol. 2004, 39, 3353–3372. [Google Scholar] [CrossRef]
- Lenarcik, B.; Kulig, J.; Laider, P. Stability and Structure of Transition Metal Complexes with Azoles in Aqueous Solutions. Part II. 2-Methylimidazole Complexes of Co(II), Cu(II) and Zn(II). Rocz. Chem. 1974, 48, 1151–1158. [Google Scholar] [CrossRef]
- Lenarcik, B.; Kurdziel, K. Stability and Structure of Transition Metal Complexes with Azoles in Aqueous Solutions Part 25 The Effect of the Size and Position of an Alkyl Substituent on the Stability and Structure of Alkilimidazole Complexes. Pol. J. Chem. 1982, 56, 3–14. [Google Scholar]
- Lenarcik, B.; Kurdziel, K.; Czopek, R. Stability and Structure of Transition Metal Complexes with Azoles in Aqueous Solutions. Part XXVIII. The Steric Effect in the Complex Formation of 2-n-Propylimidazole and 2-Isopropylimidazole. Pol. J. Chem. 1991, 65, 815–820. [Google Scholar]
- Lenarcik, B.; Kurdziel, K.; Czopek, R. Stability and Structure of Transition Metal Complexes with Azoles in Aqueous Solutions. Part XXIX. The Influence of the Size and Structure of the Alkyl Group on the Formation of 2-Alkylimidazole Complexes. Pol. J. Chem. 1991, 65, 1235–1241. [Google Scholar]
- Gaǎzo, J.; Bersuker, I.B.; Garaj, J.; Kabešová, M.; Kohout, J.; Langfelderová, H.; Melník, M.; Serator, M.; Valach, F. Plasticity of the Coordination Sphere of Copper(II) Complexes, Its Manifestation and Causes. Coord. Chem. Rev. 1976, 19, 253–297. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).