Novel Mixed Matrix Membranes Based on Polymer of Intrinsic Microporosity PIM-1 Modified with Metal-Organic Frameworks for Removal of Heavy Metal Ions and Food Dyes by Nanofiltration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Membrane Preparation
2.3. Preparation of Samples of Model Mixture for Analysis
2.3.1. Solutions of Heavy Metal Ions in Water
2.3.2. Solutions of Food Dyes in Ethanol
2.4. Preparation of Samples of Real Mixture for Analysis
2.4.1. Untreated Wastewater Samples from Galvanic Production
2.4.2. Caramel Samples Containing Dyes
2.5. Scanning Electron Microscopy
2.6. Infrared Spectroscopy
2.7. Atomic Force Microscopy
2.8. Investigation of Contact Angles
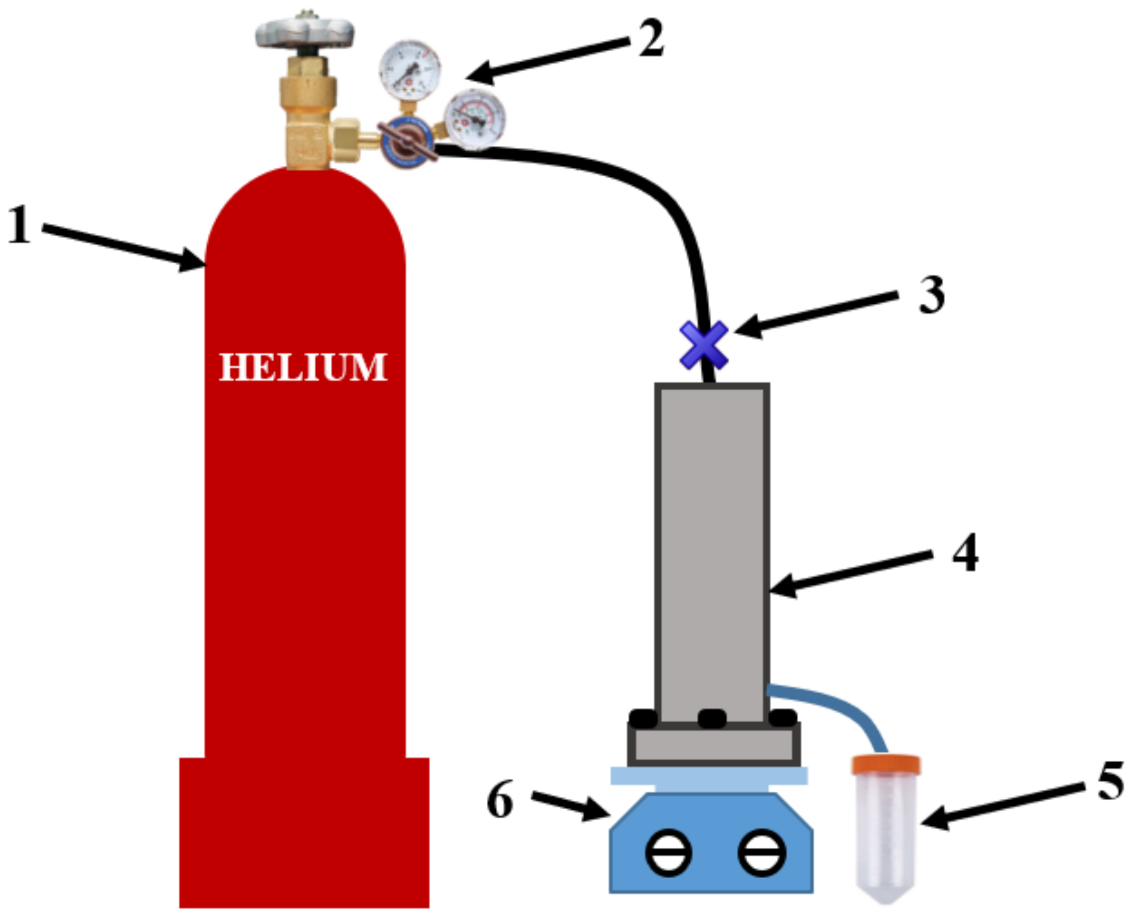
2.9. Nanofiltration Experiment
2.10. Spectrophotometric Analysis
2.11. Stripping Voltammetry
2.12. Inductively Coupled Plasma Atomic Emission Spectrometry
3. Results
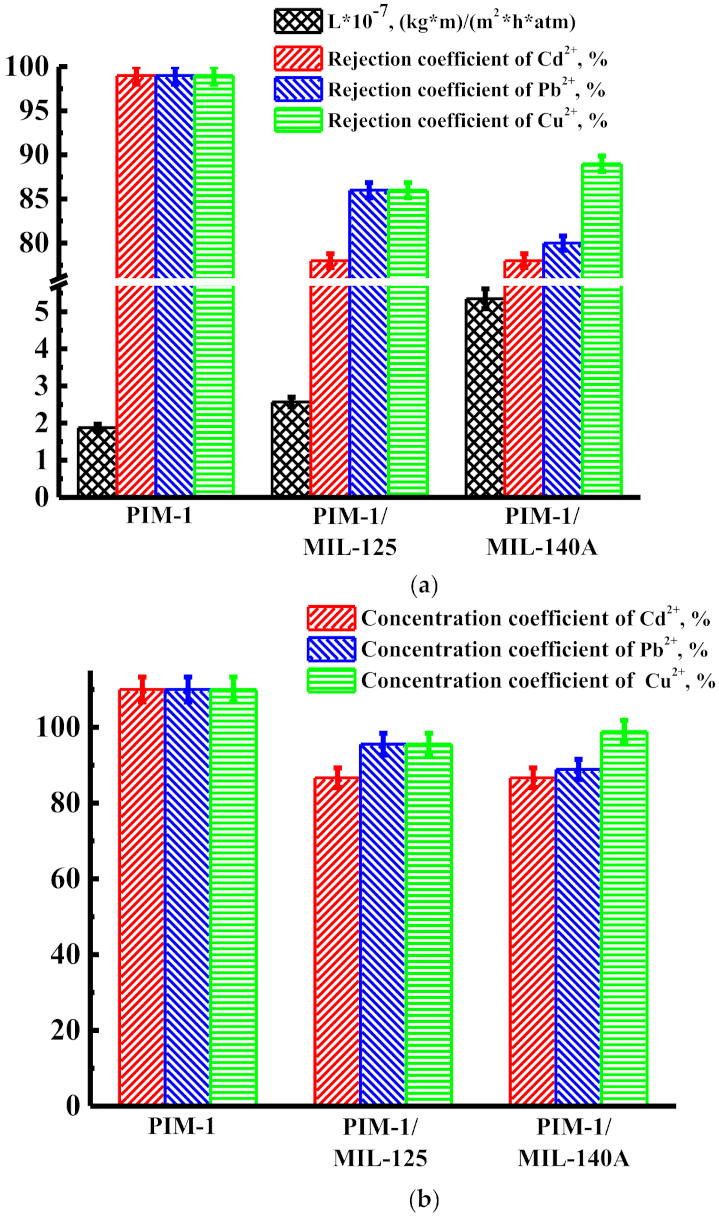
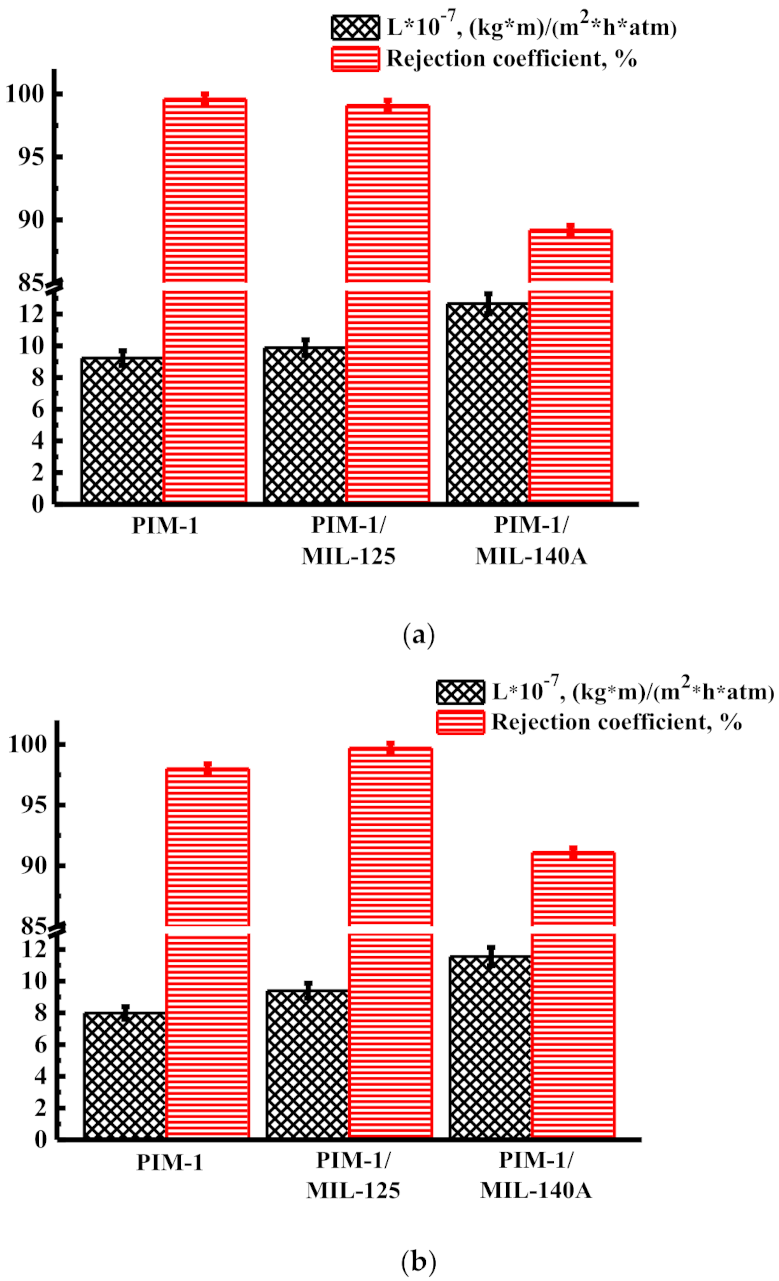
3.1. Transport Properties of Nanofiltration Membranes
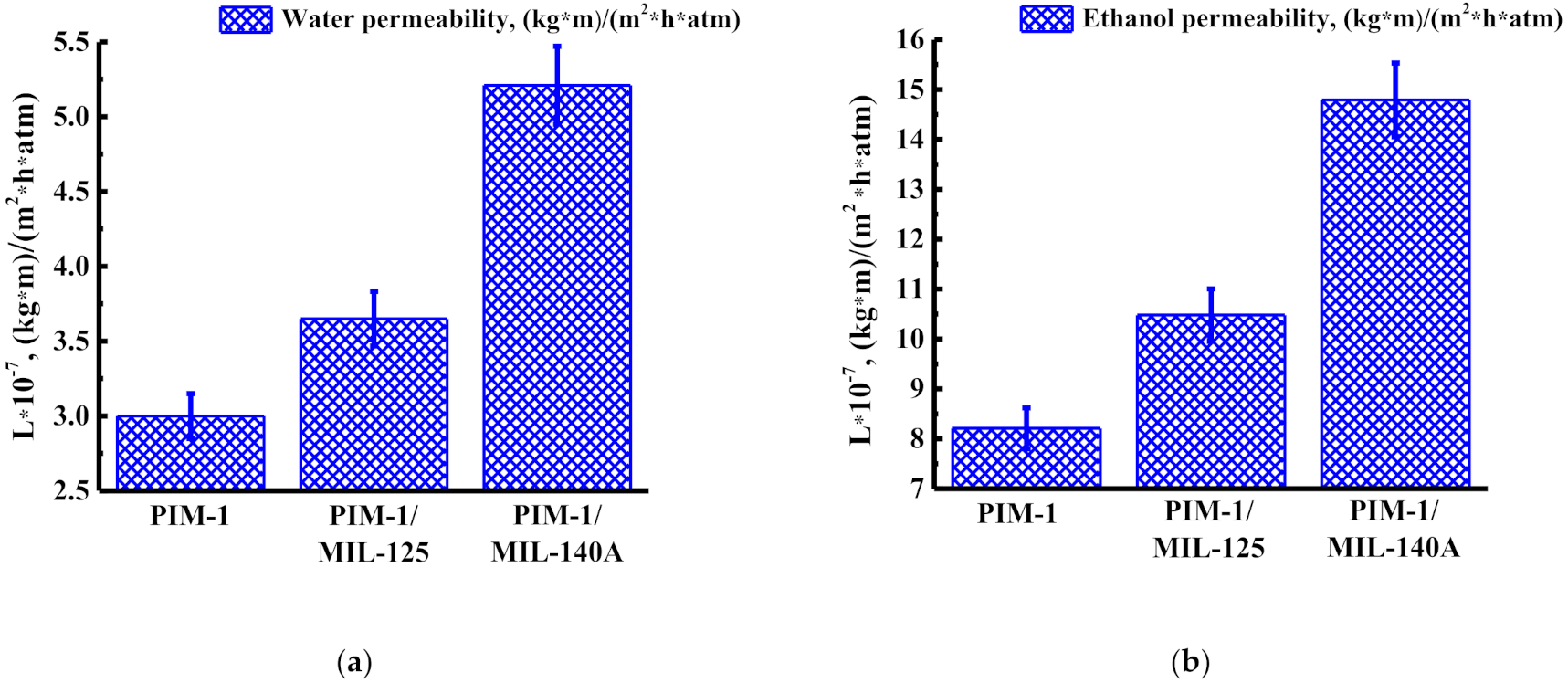
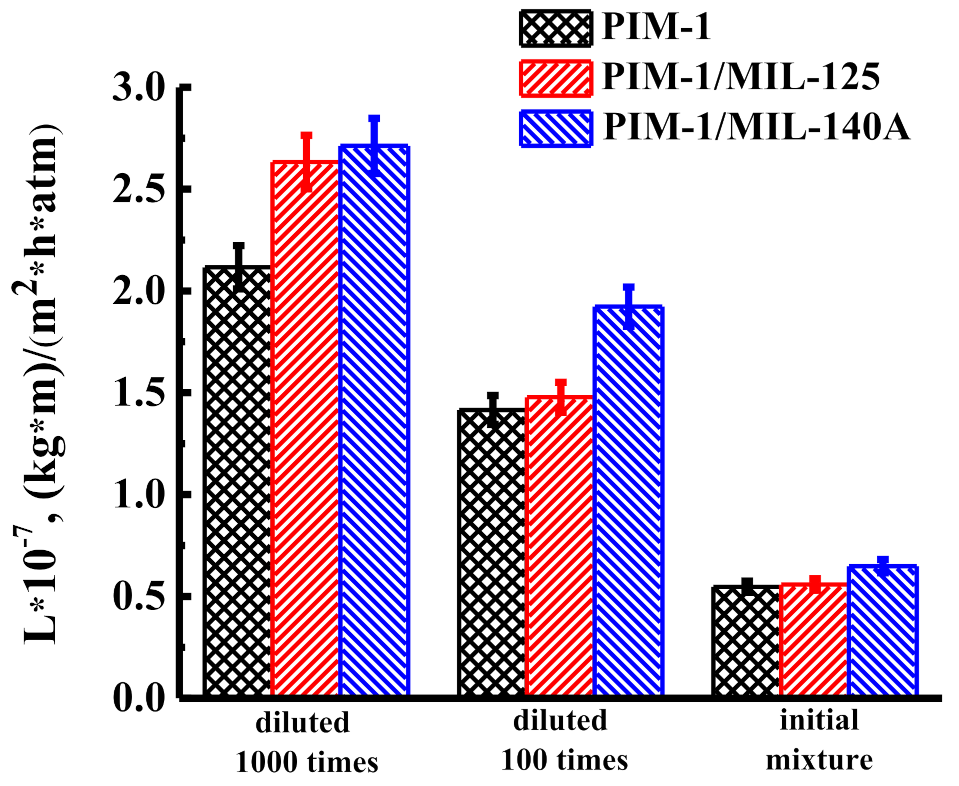
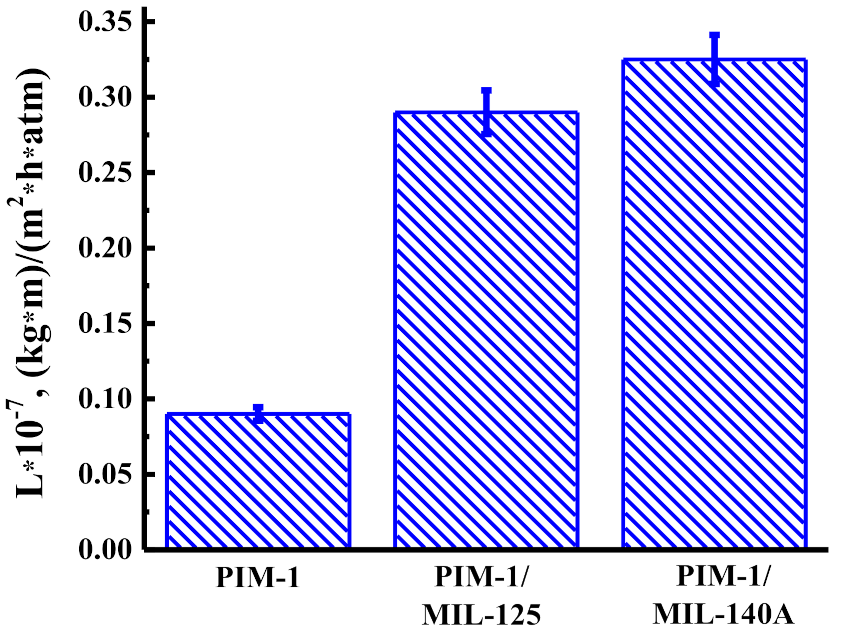
3.1.1. Solvent Permeability
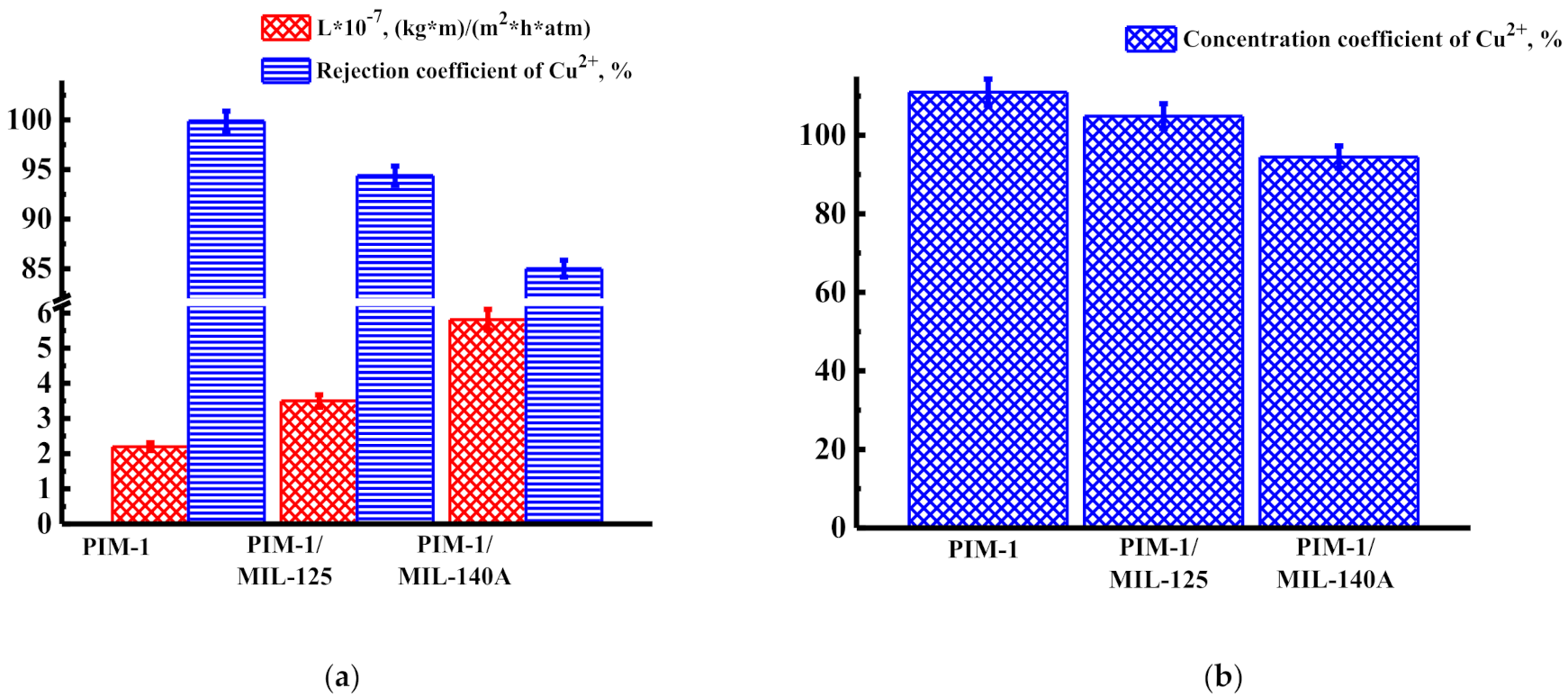
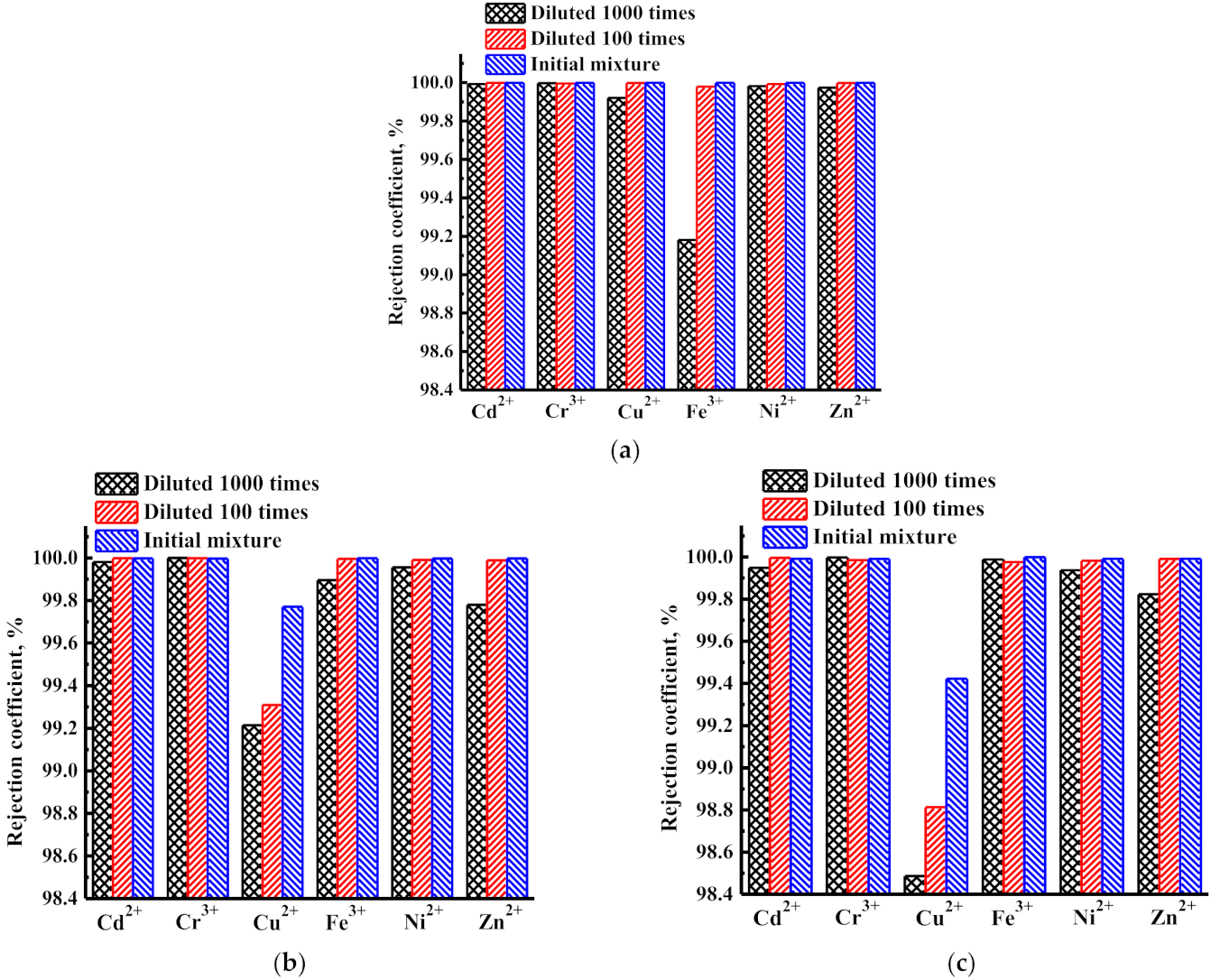
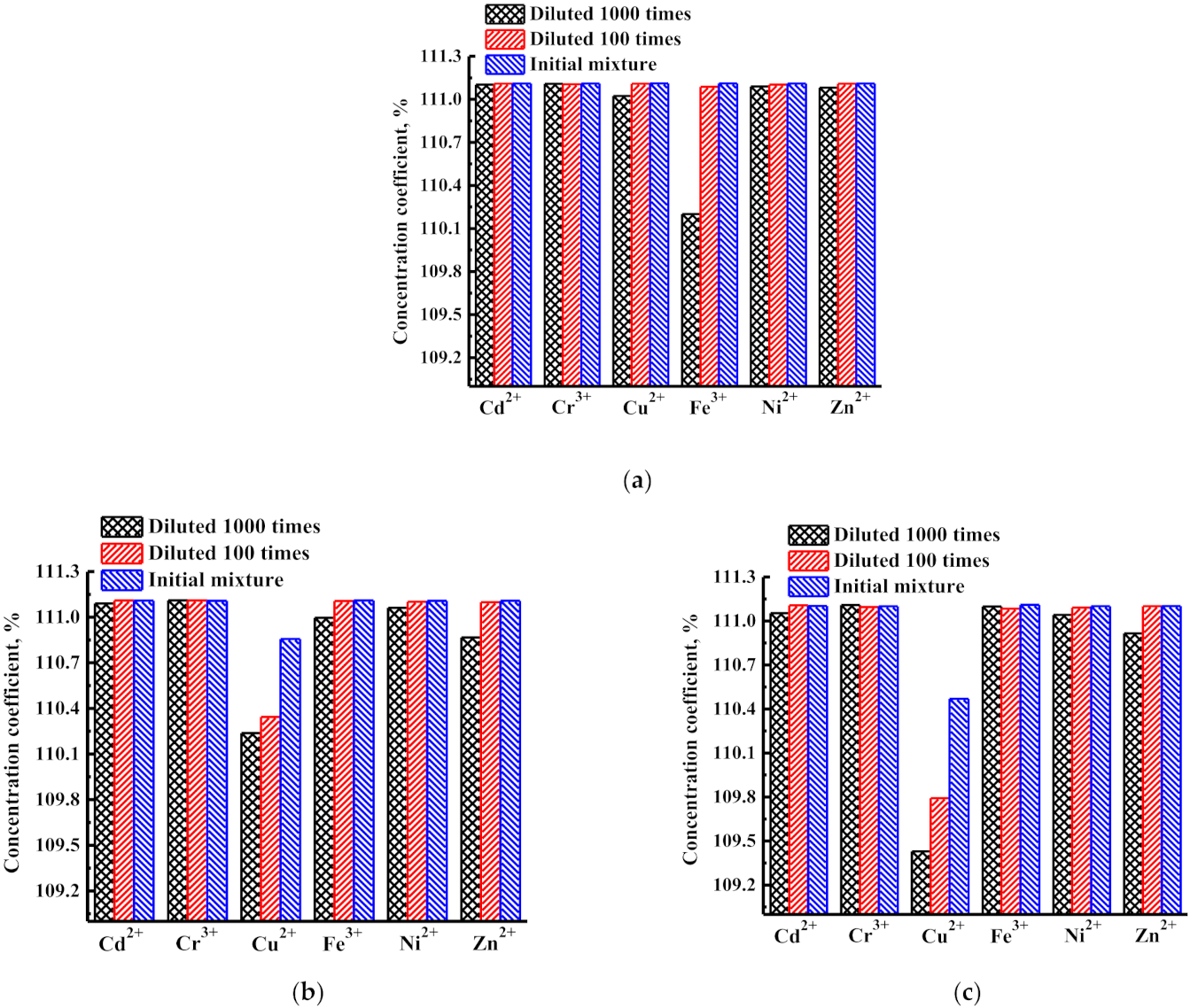
3.1.2. Concentration of Heavy Metal Ions
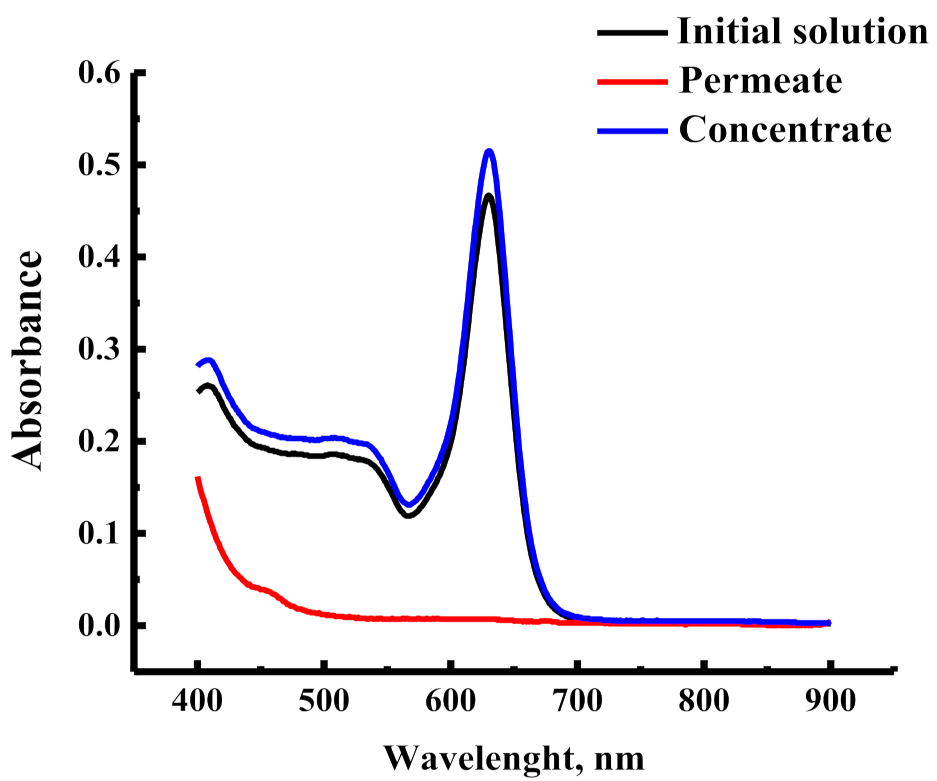
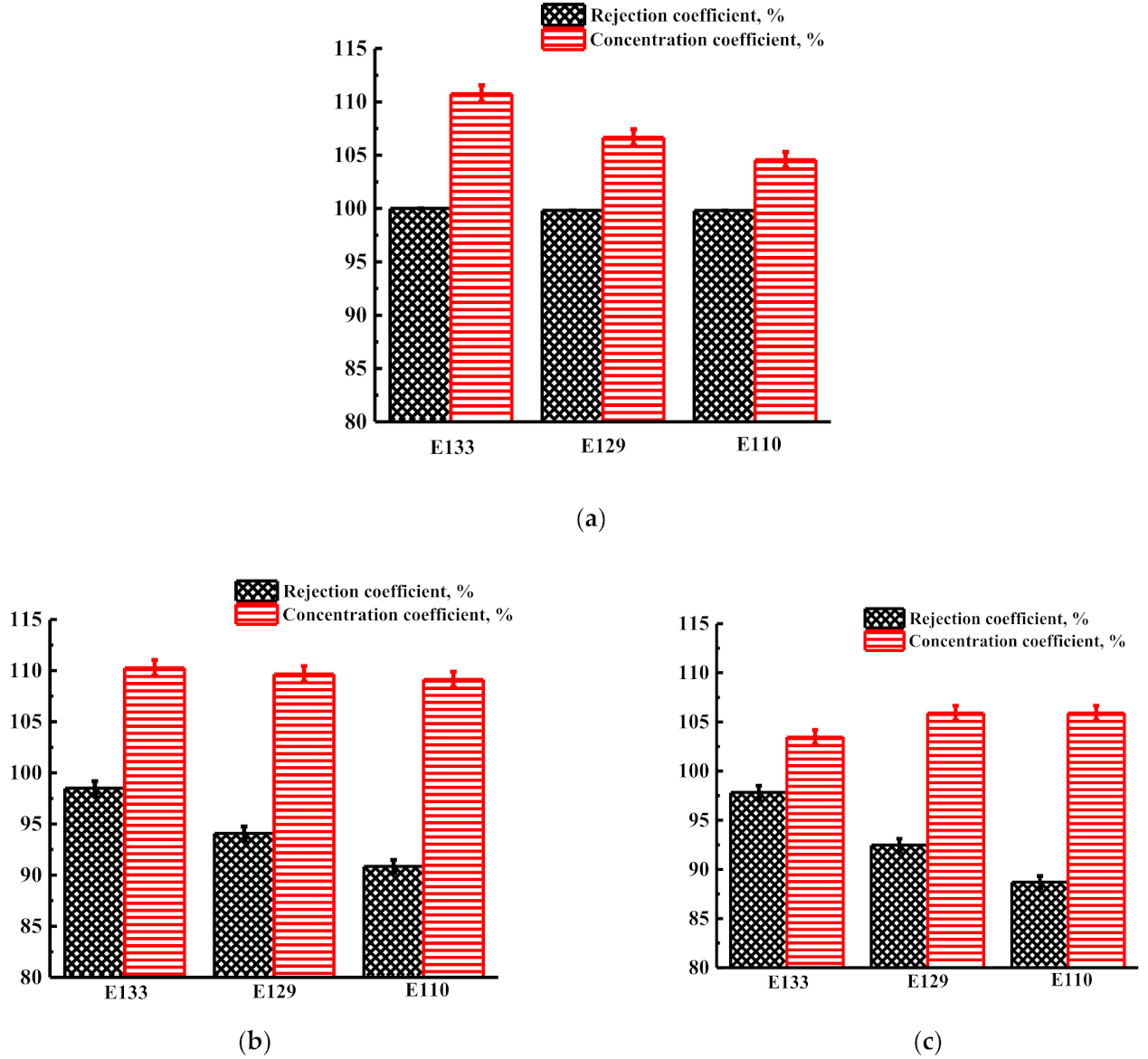
3.1.3. Concentration of Food Dyes
3.2. Structure and Physicochemical Properties Investigation of Nanofiltration Membranes
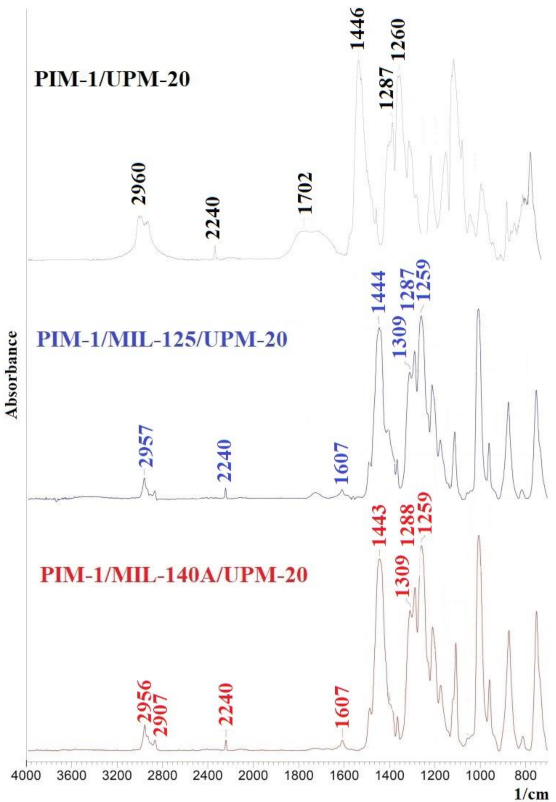
3.2.1. Study by Fourier Transform Infrared Spectroscopy (FTIR)
3.2.2. Study by Scanning Electron Microscopy (SEM)
3.2.3. Study by Atomic Force Microscopy (AFM)
3.2.4. Contact Angle Measurement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Atlaskin, A.A.; Trubyanov, M.M.; Yanbikov, N.R.; Vorotyntsev, A.V.; Drozdov, P.N.; Vorotyntsev, V.M.; Vorotyntsev, I.V. Comprehensive experimental study of membrane cascades type of “continuous membrane column” for gases high-purification. J. Membr. Sci. 2019, 572, 92–101. [Google Scholar] [CrossRef]
- Anokhina, T.S.; Yushkin, A.A.; Makarov, I.S.; Ignatenko, V.Y.; Kostyuk, A.V.; Antonov, S.V.; Volkov, A.V. Cellulose composite membranes for nanofiltration of aprotic solvents. Pet. Chem. 2016, 56, 1085–1092. [Google Scholar] [CrossRef]
- Ilyin, S.O.; Makarova, V.V.; Anokhina, T.S.; Ignatenko, V.Y.; Brantseva, T.V.; Volkov, A.V.; Antonov, S.V. Diffusion and phase separation at the morphology formation of cellulose membranes by regeneration from N-methylmorpholine N-oxide solutions. Cellulose 2018, 25, 2515–2530. [Google Scholar] [CrossRef]
- Zhu, C.-Y.; Liu, C.; Yang, J.; Guo, B.-B.; Li, H.-N.; Xu, Z.-K. Polyamide nanofilms with linearly-tunable thickness for high performance nanofiltration. J. Membr. Sci. 2021, 627, 119142. [Google Scholar] [CrossRef]
- Yushkin, A.A.; Anokhina, T.S.; Volkov, A.V. Application of cellophane films as nanofiltration membranes. Pet. Chem. 2015, 55, 746–752. [Google Scholar] [CrossRef]
- Upadhyaya, L.; Chiao, Y.-H.; Wickramasinghe, S.; Qian, X. Cu(I/II) Metal–Organic Frameworks Incorporated Nanofiltration Membranes for Organic Solvent Separation. Membranes 2020, 10, 313. [Google Scholar] [CrossRef] [PubMed]
- Kossov, A.A.; Yushkin, A.A.; Khotimskiy, V.S.; Volkov, A.V. Study of accessible free volume and transport properties of TFPS-co-TMSP copolymer. Pet. Chem. 2015, 55, 783–790. [Google Scholar] [CrossRef]
- Zhu, L.; Wu, M.; Van der Bruggen, B.; Lei, L.; Zhu, L. Effect of TiO2 content on the properties of polysulfone nanofiltration membranes modified with a layer of TiO2–graphene oxide. Sep. Purif. Technol. 2020, 242, 116770. [Google Scholar] [CrossRef]
- Zhang, Q.; Shi, B. Effect of Isomeric Propanols on the Performances of Polyethersulfone Nanofiltration Membranes. Sep. Sci. Technol. 2009, 44, 3876–3887. [Google Scholar] [CrossRef]
- Li, J.; Zhang, M.; Feng, W.; Zhu, L.; Zhang, L. PIM-1 pore-filled thin film composite membranes for tunable organic solvent nanofiltration. J. Membr. Sci. 2020, 601, 117951. [Google Scholar] [CrossRef]
- Tsarkov, S.; Khotimskiy, V.; Budd, P.M.; Volkov, V.; Kukushkina, J.; Volkov, A. Solvent nanofiltration through high permeability glassy polymers: Effect of polymer and solute nature. J. Membr. Sci. 2012, 423, 65–72. [Google Scholar] [CrossRef]
- Tsar’Kov, S.E.; Malakhov, A.O.; Litvinova, E.G.; Volkov, A.V. Nanofiltration of dye solutions through membranes based on poly(trimethylsilylpropyne). Pet. Chem. 2013, 53, 537–545. [Google Scholar] [CrossRef]
- Volkov, A.; Stamatialis, D.; Khotimsky, V.; Wessling, M.; Plate, N. Poly[1-(trimethylsilyl)-1-propyne] as a solvent resistance nanofiltration membrane material. J. Membr. Sci. 2006, 281, 351–357. [Google Scholar] [CrossRef]
- Volkov, A.V.; Parashchuk, V.V.; Stamatialis, D.F.; Khotimsky, V.S.; Volkov, V.V.; Wessling, M. High permeable PTMSP/PAN composite membranes for solvent nanofiltration. J. Membr. Sci. 2009, 333, 88–93. [Google Scholar] [CrossRef]
- Volkov, A.; Yushkin, A.; Kachula, Y.; Khotimsky, V.; Volkov, V. Application of negative retention in organic solvent nanofiltration for solutes fractionation. Sep. Purif. Technol. 2014, 124, 43–48. [Google Scholar] [CrossRef]
- Zheng, F.; Zhang, Z.; Li, C.; Yuan, Q. A comparative study of suitability on different molecular size descriptors with the consideration of molecular geometry in nanofiltration. J. Membr. Sci. 2009, 332, 13–23. [Google Scholar] [CrossRef]
- Darvishmanesh, S.; Degrève, J.; Van der Bruggen, B. Mechanisms of solute rejection in solvent resistant nanofiltration: The effect of solvent on solute rejection. Phys. Chem. Chem. Phys. 2010, 12, 13333–13342. [Google Scholar] [CrossRef] [PubMed]
- Darvishmanesh, S.; Vanneste, J.; Tocci, E.; Jansen, J.C.; Tasselli, F.; Degrève, J.; Drioli, E.; Van Der Bruggen, B. Physicochemical Characterization of Solute Retention in Solvent Resistant Nanofiltration: The Effect of Solute Size, Polarity, Dipole Moment, and Solubility Parameter. J. Phys. Chem. B 2011, 115, 14507–14517. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Feng, P.; Zhang, L.; Chen, Y. Mussel-inspired method to decorate commercial nanofiltration membrane for heavy metal ions removal. Polym. Adv. Technol. 2020, 31, 665–674. [Google Scholar] [CrossRef]
- Godiya, C.B.; Ruotolo, L.A.M.; Cai, W. Functional biobased hydrogels for the removal of aqueous hazardous pollutants: Current status, challenges, and future perspectives. J. Mater. Chem. A 2020, 8, 21585–21612. [Google Scholar] [CrossRef]
- Dmitrenko, M.; Penkova, A.; Kuzminova, A.; Missyul, A.; Ermakov, S.; Roizard, D. Development and Characterization of New Pervaporation PVA Membranes for the Dehydration Using Bulk and Surface Modifications. Polymers 2018, 10, 571. [Google Scholar] [CrossRef] [Green Version]
- Dmitrenko, M.; Zolotarev, A.; Liamin, V.; Kuzminova, A.; Mazur, A.; Semenov, K.; Ermakov, S.; Penkova, A. Novel Membranes Based on Hydroxyethyl Cellulose/Sodium Alginate for Pervaporation Dehydration of Isopropanol. Polymers 2021, 13, 674. [Google Scholar] [CrossRef]
- Penkova, A.; Acquah, S.; Piotrovskiy, L.B.; Markelov, D.; Semisalova, A.S.; Kroto, H.W. Fullerene derivatives as nano-additives in polymer composites. Russ. Chem. Rev. 2017, 86, 530–566. [Google Scholar] [CrossRef]
- Penkova, A.V.; Dmitrenko, M.E.; Ermakov, S.S.; Toikka, A.M.; Roizard, D. Novel green PVA-fullerenol mixed matrix supported membranes for separating water-THF mixtures by pervaporation. Environ. Sci. Pollut. Res. 2018, 25, 20354–20362. [Google Scholar] [CrossRef]
- Dudek, G.; Turczyn, R.; Gnus, M.; Konieczny, K. Pervaporative dehydration of ethanol/water mixture through hybrid alginate membranes with ferroferic oxide nanoparticles. Sep. Purif. Technol. 2018, 193, 398–407. [Google Scholar] [CrossRef]
- Dudek, G.; Krasowska, M.; Turczyn, R.; Strzelewicz, A.; Djurado, D.; Pouget, S. Clustering analysis for pervaporation performance assessment of alginate hybrid membranes in dehydration of ethanol. Chem. Eng. Res. Des. 2019, 144, 483–493. [Google Scholar] [CrossRef]
- Nigiz, F.U.; Hilmioglu, N.D. Pervaporation of ethanol/water mixtures by zeolite filled sodium alginate membrane. Desalin. Water Treat. 2012, 51, 637–643. [Google Scholar] [CrossRef]
- Hosseini, S.; Charkhi, A.; Minuchehr, A.; Ahmadi, S.J. Dehydration of acetonitrile using cross-linked sodium alginate membrane containing nano-sized NaA zeolite. Chem. Pap. 2017, 71, 1143–1153. [Google Scholar] [CrossRef]
- Rowsell, J.L.; Yaghi, O.M. Metal–organic frameworks: A new class of porous materials. Microporous Mesoporous Mater. 2004, 73, 3–14. [Google Scholar] [CrossRef]
- Jia, Z.; Wu, G. Metal-organic frameworks based mixed matrix membranes for pervaporation. Microporous Mesoporous Mater. 2016, 235, 151–159. [Google Scholar] [CrossRef]
- Gong, X.-Y.; Huang, Z.-H.; Zhang, H.; Liu, W.-L.; Ma, X.-H.; Xu, Z.-L.; Tang, C.Y. Novel high-flux positively charged composite membrane incorporating titanium-based MOFs for heavy metal removal. Chem. Eng. J. 2020, 398, 125706. [Google Scholar] [CrossRef]
- Meng, Y.; Shu, L.; Liu, L.; Wu, Y.; Xie, L.-H.; Zhao, M.-J.; Li, J.-R. A high-flux mixed matrix nanofiltration membrane with highly water-dispersible MOF crystallites as filler. J. Membr. Sci. 2019, 591, 117360. [Google Scholar] [CrossRef]
- Sharma, U.; Shalini, S.; Basu, S.; Saravanan, P.; Jang, M. Active layer modification of commercial nanofiltration membrane using CuBTC / PVA matrix for improved surface and separation characteristics. J. Appl. Polym. Sci. 2021, 138, 138. [Google Scholar] [CrossRef]
- Ren, Z.; Luo, J.; Wan, Y. Highly permeable biocatalytic membrane prepared by 3D modification: Metal-organic frameworks ameliorate its stability for micropollutants removal. Chem. Eng. J. 2018, 348, 389–398. [Google Scholar] [CrossRef]
- Ren, Y.; Li, T.; Zhang, W.; Wang, S.; Shi, M.; Shan, C.; Zhang, W.; Guan, X.; Lv, L.; Hua, M.; et al. MIL-PVDF blend ultrafiltration membranes with ultrahigh MOF loading for simultaneous adsorption and catalytic oxidation of methylene blue. J. Hazard. Mater. 2019, 365, 312–321. [Google Scholar] [CrossRef]
- Li, T.; Zhang, W.; Zhai, S.; Gao, G.; Ding, J.; Zhang, W.; Liu, Y.; Zhao, X.; Pan, B.; Lv, L. Efficient removal of nickel(II) from high salinity wastewater by a novel PAA/ZIF-8/PVDF hybrid ultrafiltration membrane. Water Res. 2018, 143, 87–98. [Google Scholar] [CrossRef]
- Kuzminova, A.I.; Dmitrenko, M.E.; Poloneeva, D.Y.; Selyutin, A.A.; Mazur, A.S.; Emeline, A.V.; Mikhailovskii, V.Y.; Solovyev, N.D.; Ermakov, S.S.; Penkova, A.V. Sustainable composite pervaporation membranes based on sodium alginate modified by metal organic frameworks for dehydration of isopropanol. J. Membr. Sci. 2021, 626, 119194. [Google Scholar] [CrossRef]
- Penkova, A.V.; Kuzminova, A.I.; Dmitrenko, M.E.; Surkova, V.A.; Liamin, V.P.; Markelov, D.A.; Komolkin, A.V.; Poloneeva, D.Y.; Selyutin, A.A.; Mazur, A.S.; et al. Novel pervaporation mixed matrix membranes based on polyphenylene isophtalamide modified by metal–organic framework UiO-66(NH2)-EDTA for highly efficient methanol isolation. Sep. Purif. Technol. 2021, 263, 118370. [Google Scholar] [CrossRef]
- Ashtiani, S.; Khoshnamvand, M.; Regmi, C.; Friess, K. Interfacial Design of Mixed Matrix Membranes via Grafting PVA on UiO-66-NH2 to Enhance the Gas Separation Performance. Membranes 2021, 11, 419. [Google Scholar] [CrossRef]
- Tang, P.-H.; So, P.; Li, W.-H.; Hui, Z.-Y.; Hu, C.-C.; Lin, C.-H. Carbon Dioxide Enrichment PEBAX/MOF Composite Membrane for CO2 Separation. Membranes 2021, 11, 404. [Google Scholar] [CrossRef]
- Liao, Z.; Fang, X.; Xie, J.; Li, Q.; Wang, D.; Sun, X.; Wang, L.; Li, J. Hydrophilic Hollow Nanocube-Functionalized Thin Film Nanocomposite Membrane with Enhanced Nanofiltration Performance. ACS Appl. Mater. Interfaces 2019, 11, 5344–5352. [Google Scholar] [CrossRef]
- Gnanasekaran, G.; Balaguru, S.; Arthanareeswaran, G.; Das, D.B. Removal of hazardous material from wastewater by using metal organic framework (MOF) embedded polymeric membranes. Sep. Sci. Technol. 2018, 54, 434–446. [Google Scholar] [CrossRef] [Green Version]
- Gnanaselvan, G.; Sasikumar, B.; Arthanareeswaran, G.; Mok, Y.S. Performance of composite PES/MOF-5 membranes for the treatment of textile wastewater. Desalin. Water Treat. 2019, 156, 220–228. [Google Scholar] [CrossRef]
- Campbell, J.; Burgal, J.D.S.; Szekely, G.; Davies, R.; Braddock, D.C.; Livingston, A. Hybrid polymer/MOF membranes for Organic Solvent Nanofiltration (OSN): Chemical modification and the quest for perfection. J. Membr. Sci. 2016, 503, 166–176. [Google Scholar] [CrossRef]
- Yang, L.; Wang, Z.; Zhang, J. Zeolite imidazolate framework hybrid nanofiltration (NF) membranes with enhanced permselectivity for dye removal. J. Membr. Sci. 2017, 532, 76–86. [Google Scholar] [CrossRef]
- Dai, J.; Li, S.; Liu, J.; He, J.; Li, J.; Wang, L.; Lei, J. Fabrication and characterization of a defect-free mixed matrix membrane by facile mixing PPSU with ZIF-8 core–shell microspheres for solvent-resistant nanofiltration. J. Membr. Sci. 2019, 589, 117261. [Google Scholar] [CrossRef]
- Friebe, S.; Mundstock, A.; Unruh, D.; Renz, F.; Caro, J. NH 2 -MIL-125 as membrane for carbon dioxide sequestration: Thin supported MOF layers contra Mixed-Matrix-Membranes. J. Membr. Sci. 2016, 516, 185–193. [Google Scholar] [CrossRef]
- Anjum, M.W.; Bueken, B.; De Vos, D.; Vankelecom, I.F. MIL-125(Ti) based mixed matrix membranes for CO2 separation from CH4 and N2. Membr. Sci. 2016, 502, 21–28. [Google Scholar] [CrossRef]
- Suhaimi, N.H.; Yeong, Y.F.; Jusoh, N.; Chew, T.L.; Bustam, M.A.; Suleman, S. Separation of CO2 from CH4 using mixed matrix membranes incorporated with amine functionalized MIL-125 (Ti) nanofiller. Chem. Eng. Res. Des. 2020, 159, 236–247. [Google Scholar] [CrossRef]
- Su, Z.; Chen, J.H.; Sun, X.; Huang, Y.; Dong, X. Amine-functionalized metal organic framework (NH2-MIL-125(Ti)) incorporated sodium alginate mixed matrix membranes for dehydration of acetic acid by pervaporation. RSC Adv. 2015, 5, 99008–99017. [Google Scholar] [CrossRef]
- Kadhom, M.; Hu, W.; Deng, B. Thin Film Nanocomposite Membrane Filled with Metal-Organic Frameworks UiO-66 and MIL-125 Nanoparticles for Water Desalination. Membranes 2017, 7, 31. [Google Scholar] [CrossRef]
- Anokhina, T.; Yushkin, A.; Budd, P.; Volkov, A. Application of PIM-1 for solvent swing adsorption and solvent recovery by nanofiltration. Sep. Purif. Technol. 2015, 156, 683–690. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, Z.; Hou, L.; Yang, C.; Shen, H.; Yang, K.; Wang, Z. Metal-organic framework MOF-801/PIM-1 mixed-matrix membranes for enhanced CO2/N2 separation performance. Sep. Purif. Technol. 2020, 250, 117198. [Google Scholar] [CrossRef]
- Mikami, H.; Higashi, S.; Muramoto, T.; Tanaka, M.; Yamato, M.; Kawakami, H. Gas Permeable Mixed Matrix Membranes Composed of a Polymer of Intrinsic Microporosity (PIM-1) and Surface-modified Pearl-necklace Silica Nanoparticles: Effect of Expansion of Nano-space on Gas Permeability. J. Photopolym. Sci. Technol. 2020, 33, 313–320. [Google Scholar] [CrossRef]
- Yan, X.; Ye, H.; Dong, C.; Wu, Y.; Shi, S. Preparation and characterization of PIM-1 and PIM-1/PU-blend membranes for pervaporation separation of phenol from water. Desalin. Water Treat. 2019, 138, 68–79. [Google Scholar] [CrossRef]
- Číhal, P.; Vopička, O.; Durďáková, T.-M.; Budd, P.M.; Harrison, W.; Friess, K. Pervaporation and vapour permeation of methanol—dimethyl carbonate mixtures through PIM-1 membranes. Sep. Purif. Technol. 2019, 217, 206–214. [Google Scholar] [CrossRef]
- Yushkin, A.A.; Anokhina, T.S.; Bazhenov, S.D.; Borisov, I.L.; Budd, P.M.; Volkov, A.V. Sorption and Nanofiltration Characteristics of PIM-1 Material in Polar and Non-Polar Solvents. Pet. Chem. 2018, 58, 1154–1158. [Google Scholar] [CrossRef]
- Shen, Q.; Cong, S.; He, R.; Wang, Z.; Jin, Y.; Li, H.; Cao, X.; Wang, J.; Van der Bruggen, B.; Zhang, Y. SIFSIX-3-Zn/PIM-1 mixed matrix membranes with enhanced permeability for propylene/propane separation. J. Membr. Sci. 2019, 588, 117201. [Google Scholar] [CrossRef]
- Khdhayyer, M.R.; Esposito, E.; Fuoco, A.; Monteleone, M.; Giorno, L.; Jansen, J.C.; Attfield, M.; Budd, P.M. Mixed matrix membranes based on UiO-66 MOFs in the polymer of intrinsic microporosity PIM-1. Sep. Purif. Technol. 2017, 173, 304–313. [Google Scholar] [CrossRef]
- Khdhayyer, M.; Bushell, A.F.; Budd, P.; Attfield, M.; Jiang, D.; Burrows, A.; Esposito, E.; Bernardo, P.; Monteleone, M.; Fuoco, A.; et al. Mixed matrix membranes based on MIL-101 metal–organic frameworks in polymer of intrinsic microporosity PIM. Sep. Purif. Technol. 2019, 212, 545–554. [Google Scholar] [CrossRef] [Green Version]
- Mason, C.R.; Buonomenna, M.G.; Golemme, G.; Budd, P.M.; Galiano, F.; Figoli, A.; Friess, K.; Hynek, V. New organophilic mixed matrix membranes derived from a polymer of intrinsic microporosity and silicalite-1. Polymer 2013, 54, 2222–2230. [Google Scholar] [CrossRef]
- Luque-Alled, J.M.; Ameen, A.W.; Alberto, M.; Tamaddondar, M.; Foster, A.B.; Budd, P.M.; Vijayaraghavan, A.; Gorgojo, P. Gas separation performance of MMMs containing (PIM-1)-functionalized GO derivatives. J. Membr. Sci. 2021, 623, 118902. [Google Scholar] [CrossRef]
- Du, N.; Robertson, G.P.; Song, J.; Pinnau, I.; Thomas, S.; Guiver, M. Polymers of Intrinsic Microporosity Containing Trifluoromethyl and Phenylsulfone Groups as Materials for Membrane Gas Separation. Macromolecules 2008, 41, 9656–9662. [Google Scholar] [CrossRef] [Green Version]
- Halder, K.; Khan, M.M.; Grünauer, J.; Shishatskiy, S.; Abetz, C.; Filiz, V.; Abetz, V. Blend membranes of ionic liquid and polymers of intrinsic microporosity with improved gas separation characteristics. J. Membr. Sci. 2017, 539, 368–382. [Google Scholar] [CrossRef]
- Baker, R.W. Membrane Technology and Applications; McGraw-Hill: New York, NY, USA, 2000. [Google Scholar]
- Meuleman, E.; Willemsen, J.; Mulder, M.; Strathmann, H. EPDM as a selective membrane material in pervaporation. J. Membr. Sci. 2001, 188, 235–249. [Google Scholar] [CrossRef]
- Brdar, M.; Sciban, M.; Kukic, D.; Dosenovic, T. Kinetic model for the sorption of copper ions onto sugar beet shreds. Chem. Ind. 2014, 68, 793–799. [Google Scholar] [CrossRef] [Green Version]
- Ho, Y.; McKay, G. Application of Kinetic Models to the Sorption of Copper(II) on to Peat. Adsorpt. Sci. Technol. 2002, 20, 797–815. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Sorption of Copper(II) from Aqueous Solution by Peat. Water Air Soil Pollut. 2004, 158, 77–97. [Google Scholar] [CrossRef]
- Li, X.; Hill, M.R.; Wang, H.; Zhang, H. Metal–Organic Framework-Based Ion-Selective Membranes. Adv. Mater. Technol. 2021, 6, 2000790. [Google Scholar] [CrossRef]
- Jian, M.; Qiu, R.; Xia, Y.; Lu, J.; Chen, Y.; Gu, Q.; Liu, R.; Hu, C.; Qu, J.; Wang, H.; et al. Ultrathin water-stable metal-organic framework membranes for ion separation. Sci. Adv. 2020, 6, 3998. [Google Scholar] [CrossRef]
- Yahia, M.; Le, Q.N.P.; Ismail, N.; Essalhi, M.; Sundman, O.; Rahimpour, A.; Dal-Cin, M.M.; Tavajohi, N. Effect of incorporating different ZIF-8 crystal sizes in the polymer of intrinsic microporosity, PIM-1, for CO2/CH4 separation. Microporous Mesoporous Mater. 2021, 312, 110761. [Google Scholar] [CrossRef]
- Satilmis, B. Amidoxime Modified Polymers of Intrinsic Microporosity (PIM-1); A Versatile Adsorbent for Efficient Removal of Charged Dyes; Equilibrium, Kinetic and Thermodynamic Studies. J. Polym. Environ. 2020, 28, 995–1009. [Google Scholar] [CrossRef]
- Lasseuguette, E.; McClements, J.; Koutsos, V.; Schäfer, T.; Ferrari, M.-C. Ionic liquid mediated surface micropatterning of polymer blends. J. Appl. Polym. Sci. 2017, 135, 46109. [Google Scholar] [CrossRef]

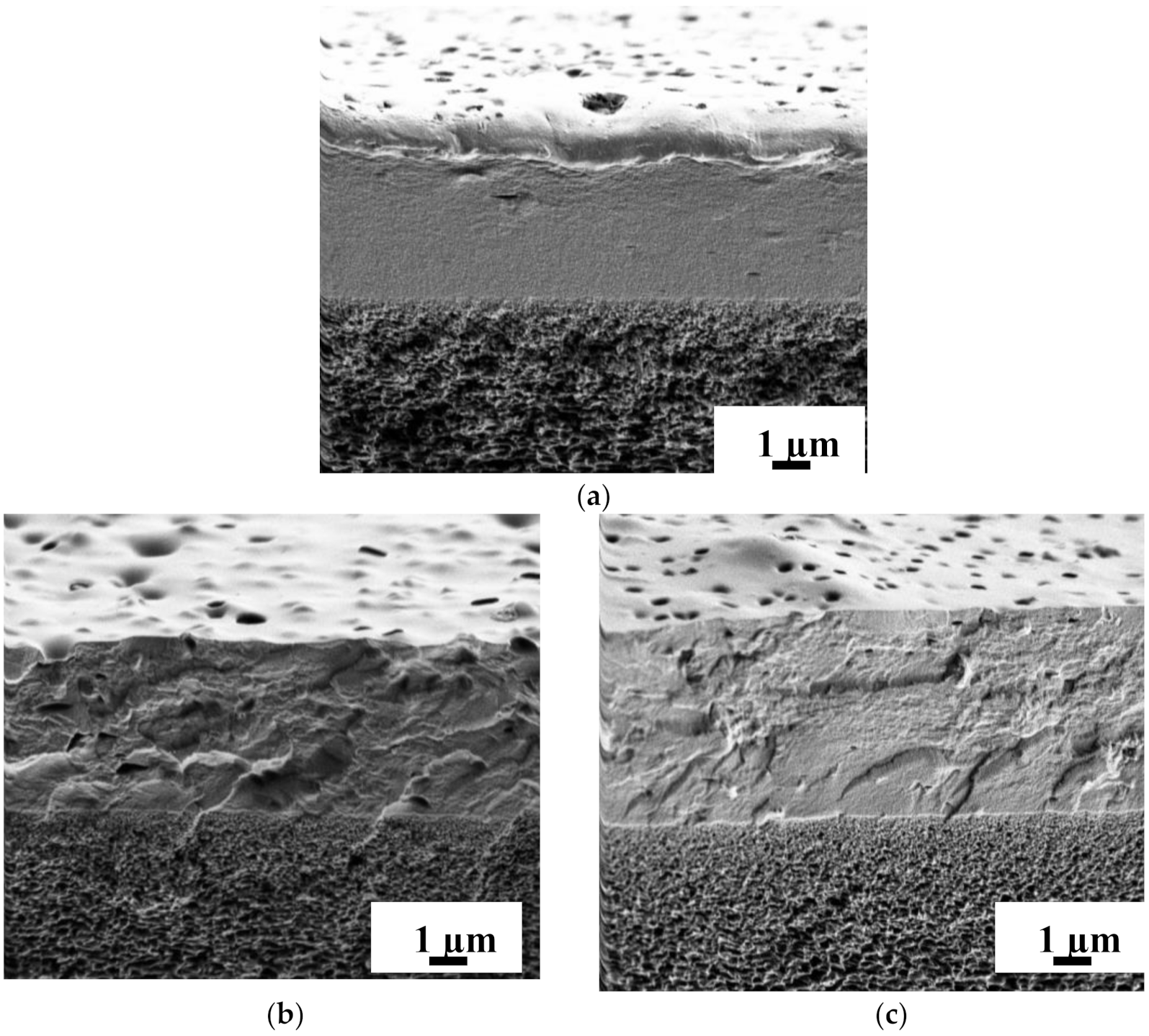
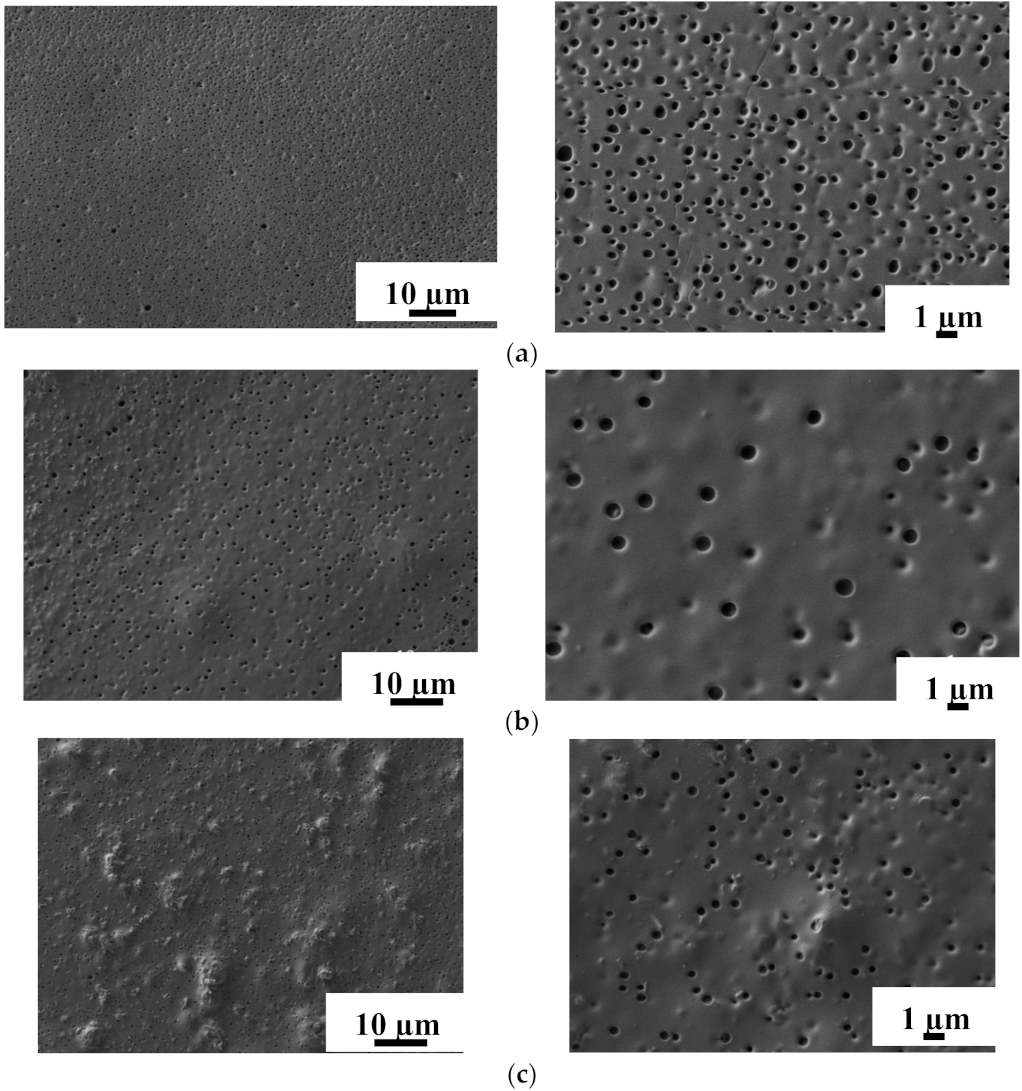
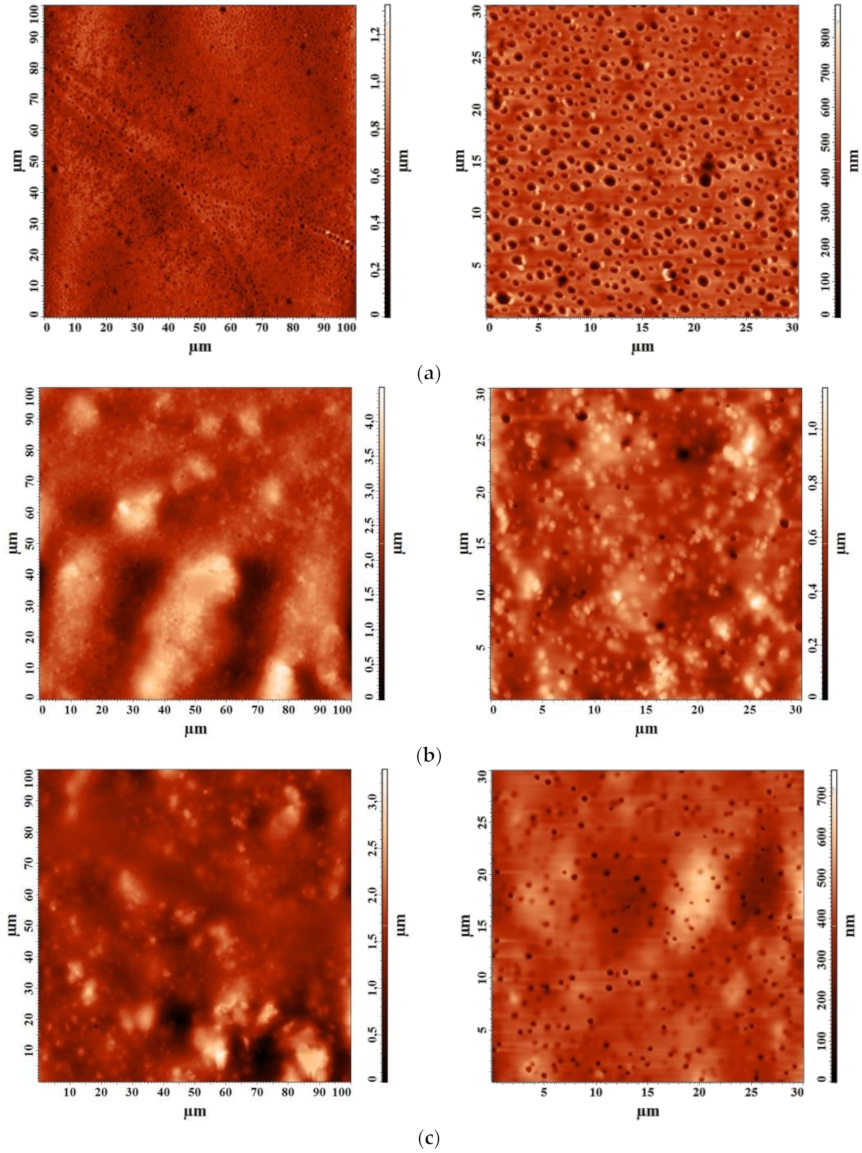
| Membrane | Ra, nm | Rq, nm |
|---|---|---|
| PIM-1 | 74.0 | 97.1 |
| PIM-1/MIL-125 | 87.5 | 114.2 |
| PIM-1/MIL-140A | 139.1 | 193.2 |
| Membrane | Contact Angle of Water, ° |
|---|---|
| PIM-1 | 72 |
| PIM-1/MIL-125 | 73 |
| PIM-1/MIL-140A | 68 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuzminova, A.; Dmitrenko, M.; Zolotarev, A.; Korniak, A.; Poloneeva, D.; Selyutin, A.; Emeline, A.; Yushkin, A.; Foster, A.; Budd, P.; et al. Novel Mixed Matrix Membranes Based on Polymer of Intrinsic Microporosity PIM-1 Modified with Metal-Organic Frameworks for Removal of Heavy Metal Ions and Food Dyes by Nanofiltration. Membranes 2022, 12, 14. https://doi.org/10.3390/membranes12010014
Kuzminova A, Dmitrenko M, Zolotarev A, Korniak A, Poloneeva D, Selyutin A, Emeline A, Yushkin A, Foster A, Budd P, et al. Novel Mixed Matrix Membranes Based on Polymer of Intrinsic Microporosity PIM-1 Modified with Metal-Organic Frameworks for Removal of Heavy Metal Ions and Food Dyes by Nanofiltration. Membranes. 2022; 12(1):14. https://doi.org/10.3390/membranes12010014
Chicago/Turabian StyleKuzminova, Anna, Mariia Dmitrenko, Andrey Zolotarev, Aleksandra Korniak, Daria Poloneeva, Artem Selyutin, Alexei Emeline, Alexey Yushkin, Andrew Foster, Peter Budd, and et al. 2022. "Novel Mixed Matrix Membranes Based on Polymer of Intrinsic Microporosity PIM-1 Modified with Metal-Organic Frameworks for Removal of Heavy Metal Ions and Food Dyes by Nanofiltration" Membranes 12, no. 1: 14. https://doi.org/10.3390/membranes12010014
APA StyleKuzminova, A., Dmitrenko, M., Zolotarev, A., Korniak, A., Poloneeva, D., Selyutin, A., Emeline, A., Yushkin, A., Foster, A., Budd, P., & Ermakov, S. (2022). Novel Mixed Matrix Membranes Based on Polymer of Intrinsic Microporosity PIM-1 Modified with Metal-Organic Frameworks for Removal of Heavy Metal Ions and Food Dyes by Nanofiltration. Membranes, 12(1), 14. https://doi.org/10.3390/membranes12010014










