Preparation of Sodalite and Faujasite Clay Composite Membranes and Their Utilization in the Decontamination of Dye Effluents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of Clay Supports
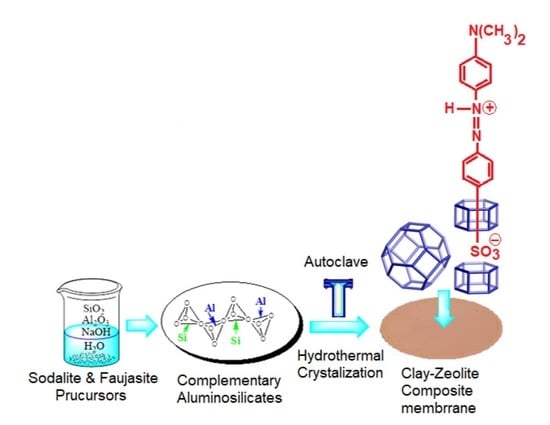
2.3. Preparation of the Clay/Zeolite Composite Membranes
2.3.1. Synthesis of Sodalite Zeolite
2.3.2. Synthesis of Faujasite Zeolite
2.3.3. Sodalite (SOM) and Faujasite (FAM) Composite Membranes’ Preparation
2.4. Characterization Techniques
2.5. Filtration Tests and MO Concentration Measurements
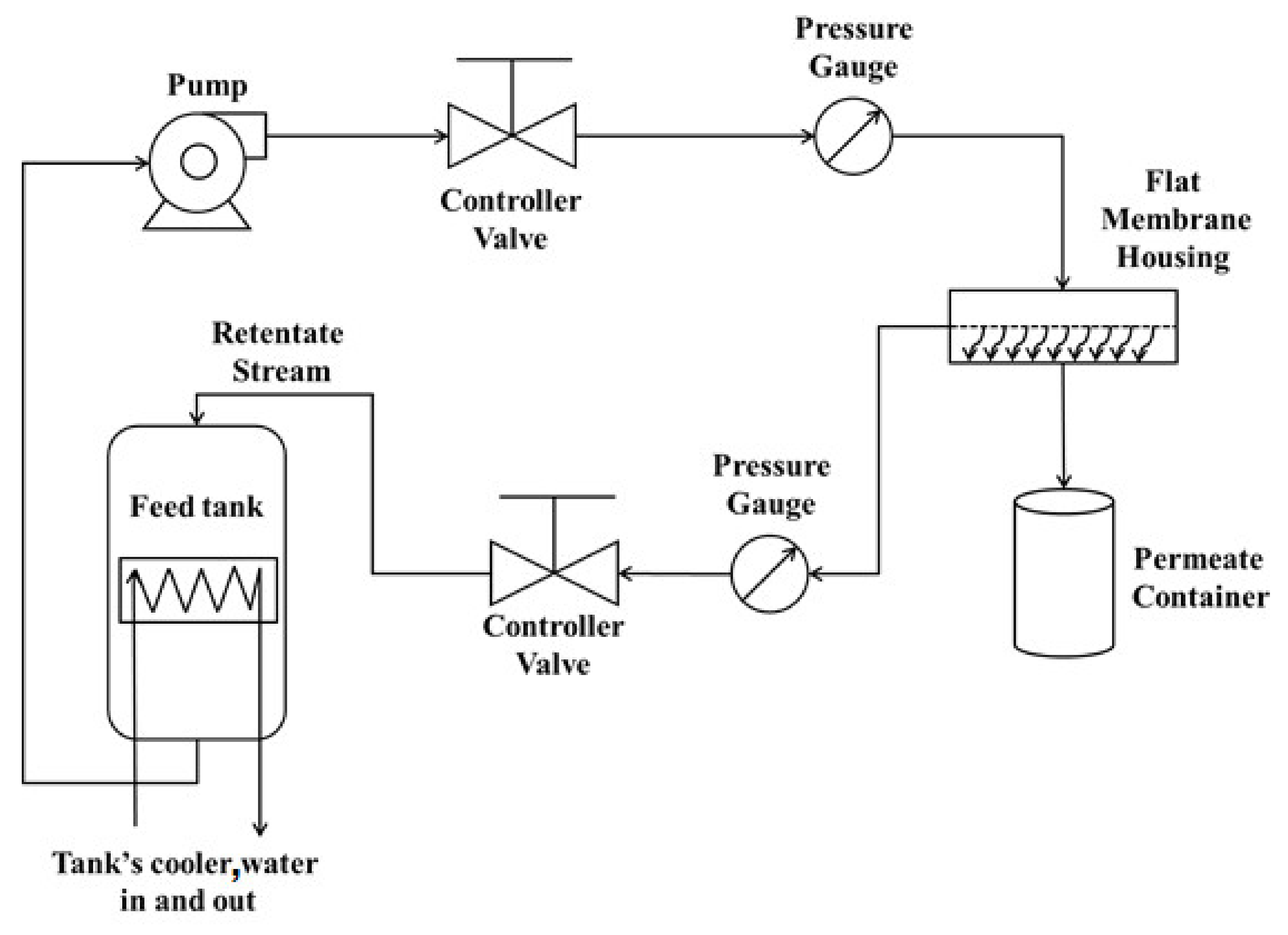
2.6. The Filtration Flow Loop
3. Results and Discussion
3.1. X-ray Fluorescence Analysis
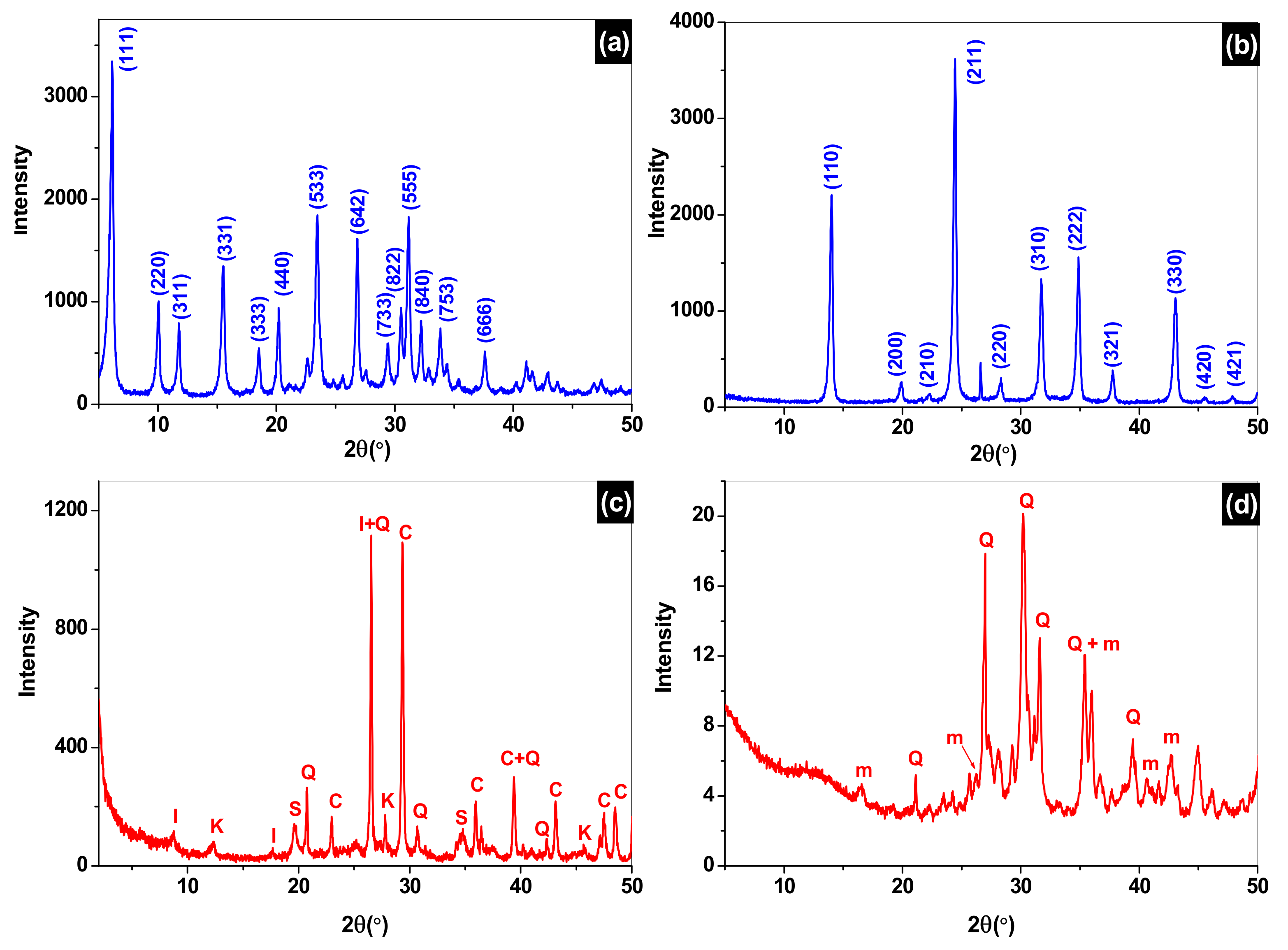
3.2. X-ray Diffraction Analysis
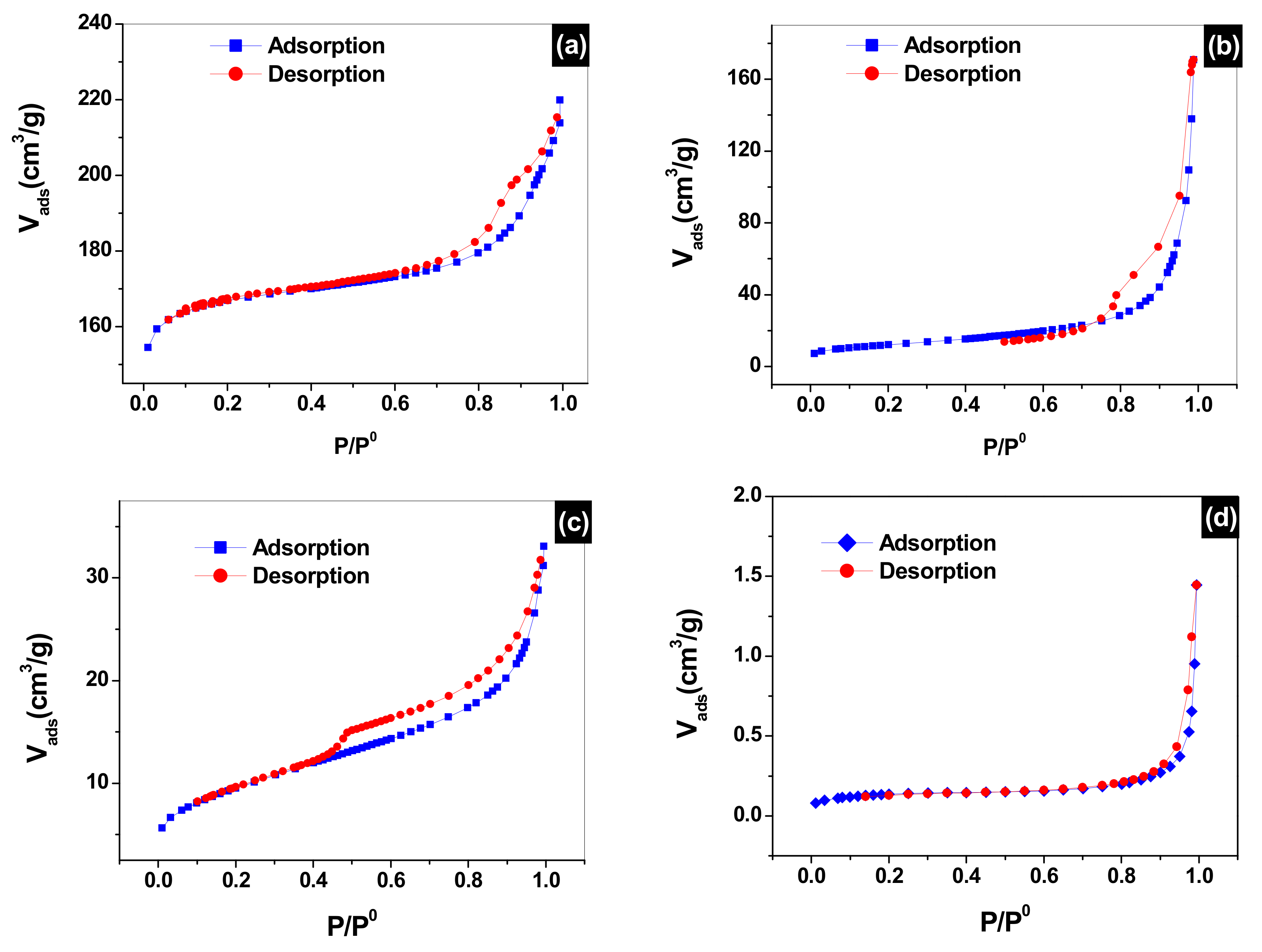
3.3. Adsorption/Desorption of Nitrogen (N2) Analysis
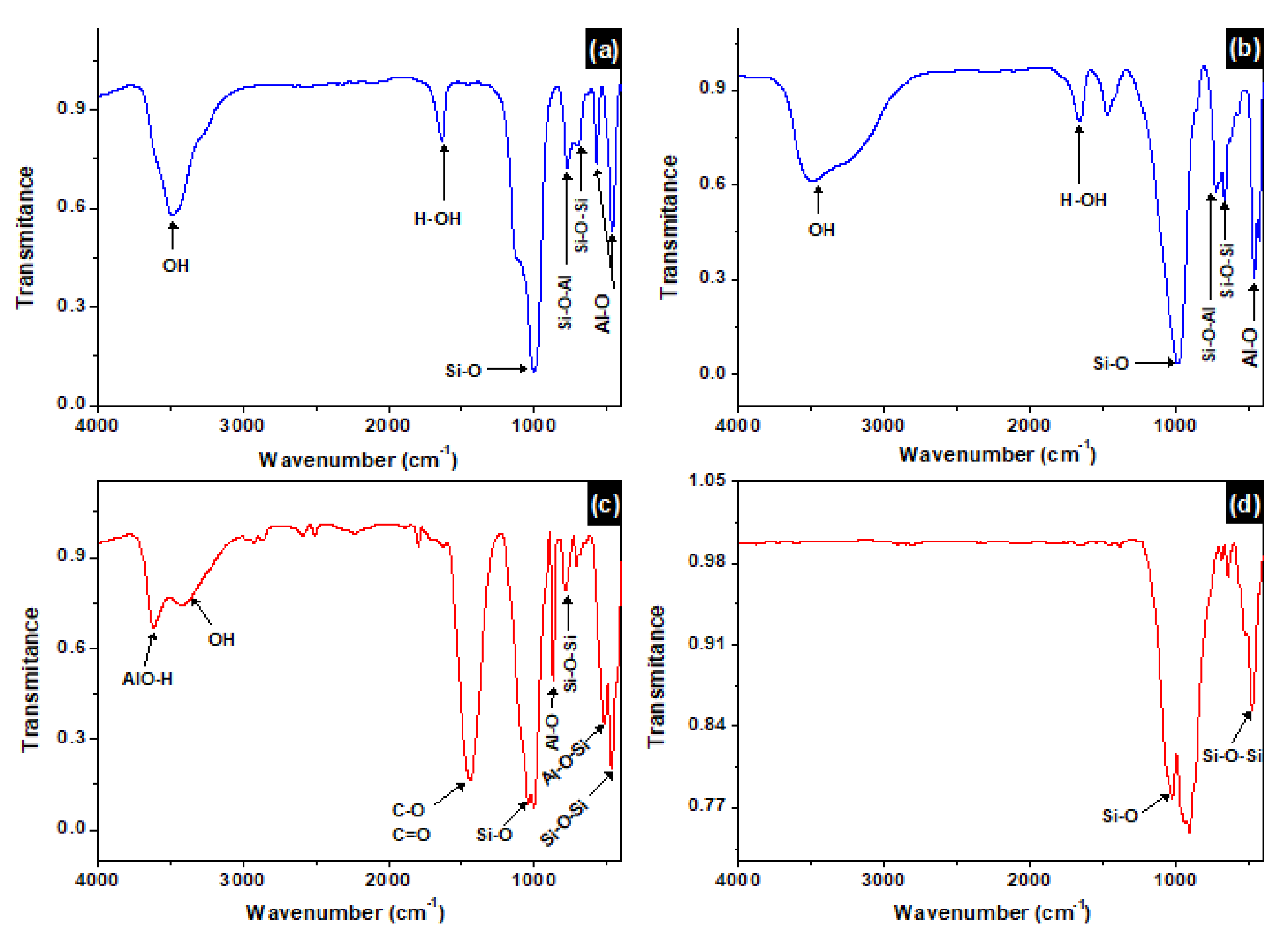
3.4. FTIR Analysis
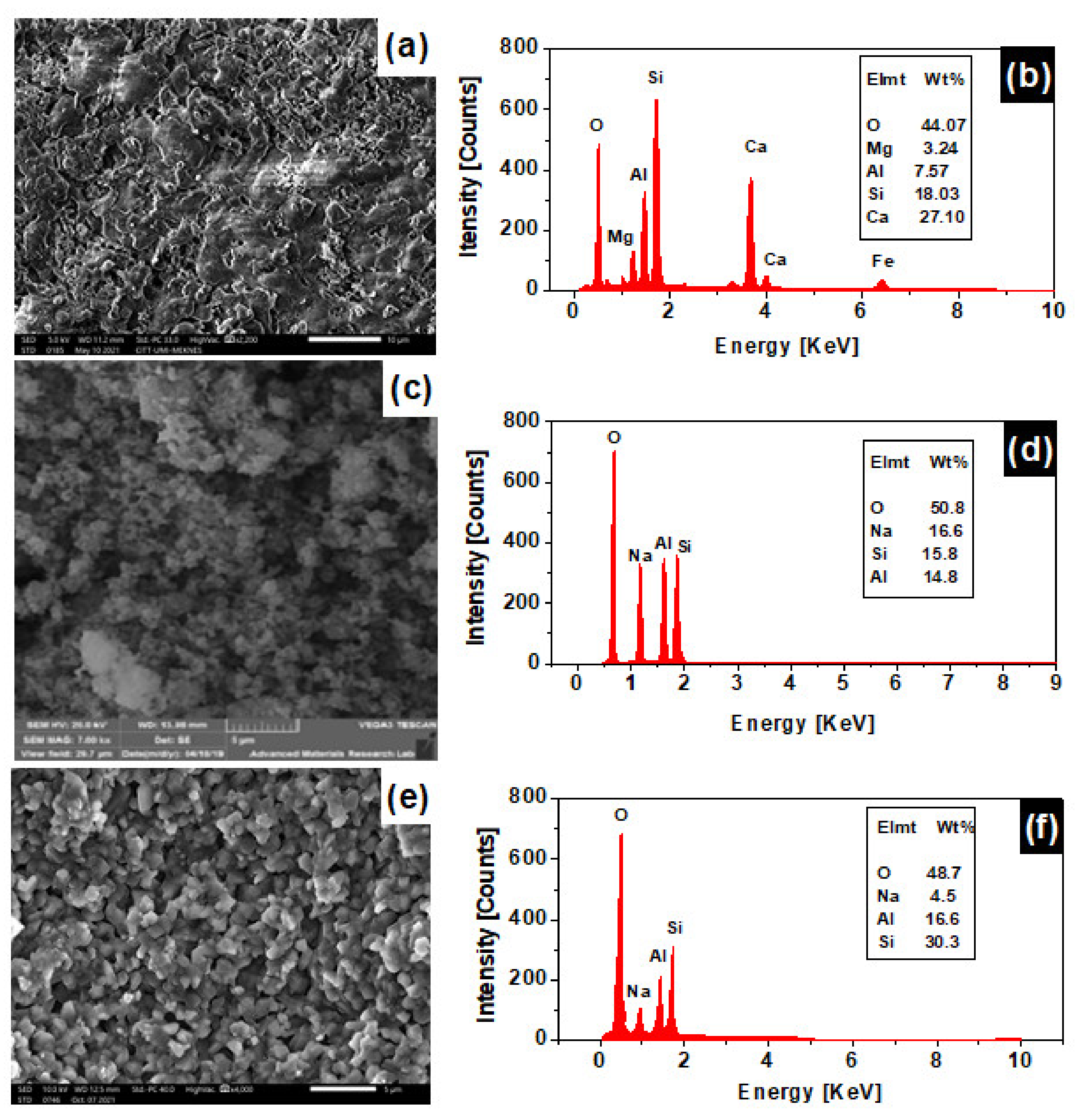
3.5. Scanning Electron Microscopy Analysis (SEM)
3.6. The Point of Zero Charge of the Clay Supports, SOM and FAM Membranes
3.7. Calibration of the Spectrophotometer for Methyl Orange Analysis
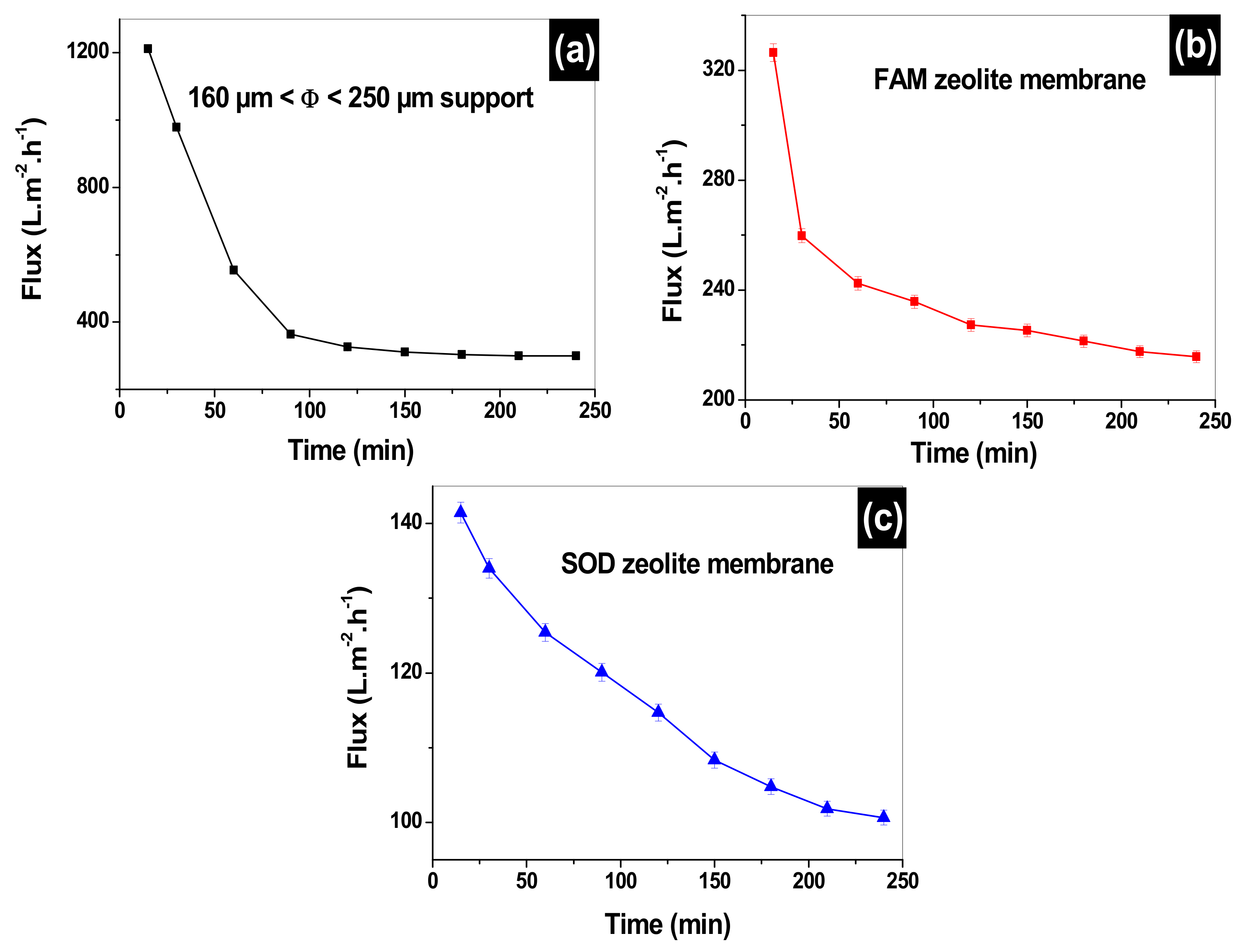
3.8. Water Flux Permeability for the Clay Supports, SOM, and FAM Membranes
3.9. Filtration through 160 µm ≤ Φ ≤ 250 µm Support, SOM, and FAM Composite Zeolite/Clay Membranes
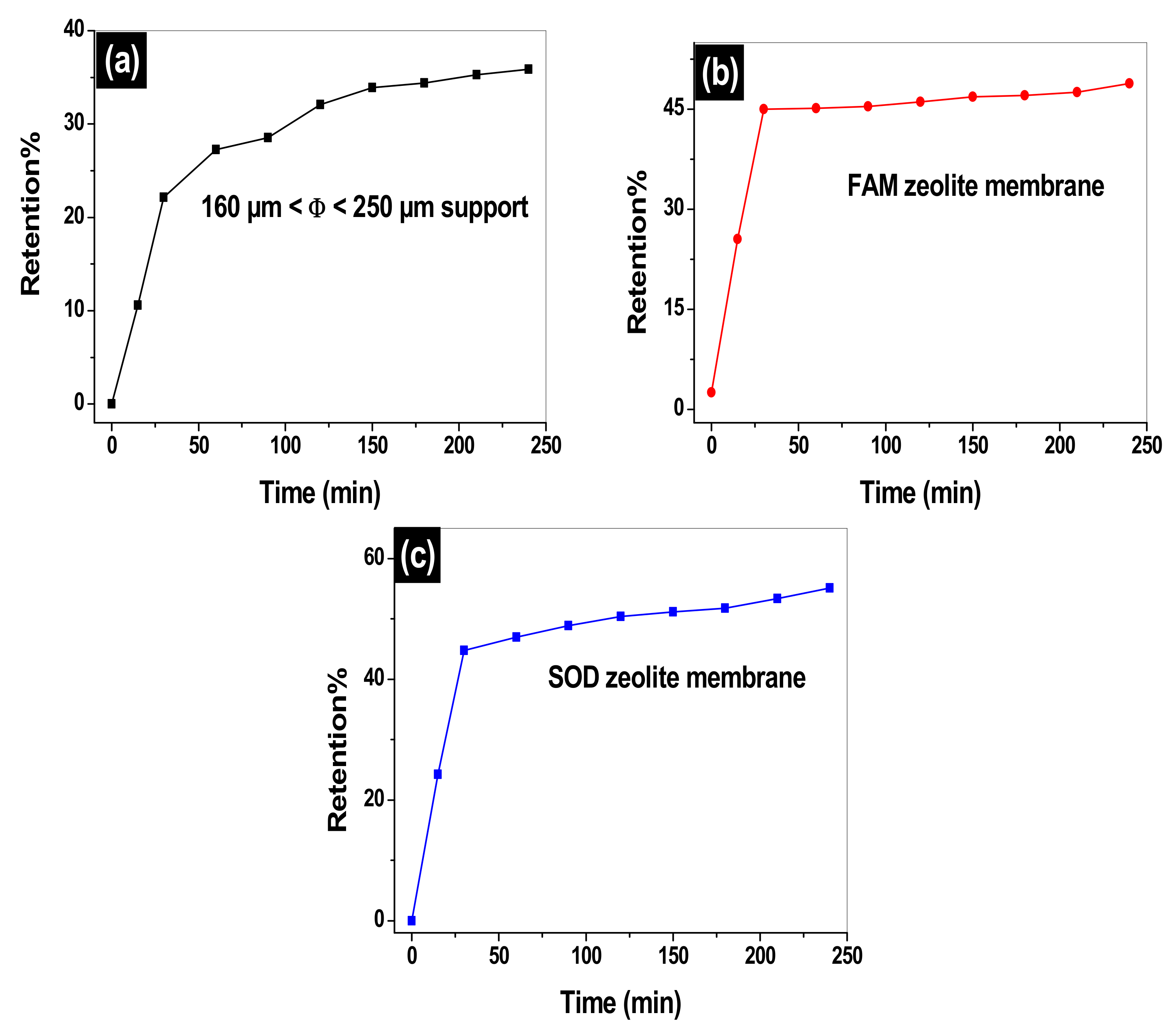
3.10. Retention of MO on the 160 µm ≤ Φ ≤ 250 µm Support, SOM, and FAM Composite Zeolite/Clay Membranes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hanafi, M.F.; Sapawe, N. A review on the water problem associate with organic pollutants derived from phenol, methyl orange, and remazol brilliant blue dyes. Mater. Today Proc. 2020, 31, A141–A150. [Google Scholar] [CrossRef]
- Jamee, R.; Siddique, R. Biodegradation of synthetic dyes of textile effluent by microorganisms: An environmentally and economically sustainable approach. Eur. J. Microbiol. Immunol. 2019, 9, 114–118. [Google Scholar] [CrossRef]
- Keshvardoostchokami, M.; Majidi, M.; Zamani, A.; Liu, B. Adsorption of phenol on environmentally friendly Fe3O4/chitosan/zeolitic imidazolate framework-8 nanocomposite: Optimization by experimental design methodology. J. Mol. Liq. 2021, 323, 115064. [Google Scholar] [CrossRef]
- Gadow, S.; Estrada, A.L.; YouLic, Y. Characterization and potential of two different anaerobic mixed microflora for bioenergy recovery and decolorization of textile wastewater: Effect of C/N ratio, dye concentration and Ph. J. Bioresour. Technol. Rep. 2021, 17, 100886. [Google Scholar] [CrossRef]
- Mohamad, D.F.; Osman, N.S.; Nazri, M.K.H.M.; Mazlan, A.A.; Hanafi, M.F.; Esa, Y.A.M.; Rafi, M.I.I.M.; Zailani, M.N.; Rahman, N.N.; Abd Rahman, A.H. Synthesis of mesoporous silica nanoparticle from banana peel ash for removal of phenol and methyl orange in aqueous solution. Mater. Today Proc. 2019, 19, 1119–1125. [Google Scholar] [CrossRef]
- Mansour, H.; Boughzala, D.; Barilier, D.; Dridi, D.; Cheekir-Ghedira, L.; Mosrati, R. Textiles dyes as a source of wastewater contamination: Screening of the toxicity and treatment methods. J. Water Sci. 2021, 24, 209–238. [Google Scholar]
- Dariani, R.S.; Nazari, M. Comparison of stress, strain, and elastic properties for porous silicon layers supported by substrate and corresponding membranes. J. Mol. Struct. 2016, 1119, 308–313. [Google Scholar] [CrossRef]
- Ouallal, H.; Azrour, M.; Messaoudi, M.; Moussout, H.; Messaoudi, L.; Tijani, N. Incorporation effect of olive pomace on the properties of tubular membranes. J. Environ. Chem. Eng. 2020, 8, 103668. [Google Scholar] [CrossRef]
- Phan, D.D.; Babick, F.; Trịnh, T.H.T.; Nguyen, M.T.; Samhaber, W.; Stintz, M. Investigation of fixed-bed photocatalytic membrane reactors based on submerged ceramic membranes. Chem. Eng. Sci. 2018, 191, 332–342. [Google Scholar] [CrossRef]
- Zhang, W.; Ding, L.; Luo, J.; Jaffrin, M.Y.; Tang, B. Membrane fouling in photocatalytic membrane reactors (PMRs) for water and wastewater treatment: A critical review. Chem. Eng. J. 2016, 302, 446–458. [Google Scholar] [CrossRef]
- Tahiri, A.; Messaoudi, L.; Tijani, N.; Zerrouk, M.H.; Messaoudi, M. Manufacture and characterization of flat membrane supports based on Moroccan Rif clay. Mater. Today Proc. 2021, 43, 209–215. [Google Scholar] [CrossRef]
- Elgamouz, A.; Tijani, N. From a naturally occurring material (clay mineral) to the production of porous ceramic membranes. Microporous Mesoporous Mater. 2018, 271, 52–58. [Google Scholar] [CrossRef]
- Elgamouz, A.; Tijani, N. Dataset in the production of composite clay-zeolite membranes made from naturally occurring clay minerals. Data Brief 2018, 19, 2267–2278. [Google Scholar] [CrossRef]
- Abdullah, N.; Yusof, N.; Lau, W.J.; Jaafar, J.; Ismail, A.F. Recent trends of heavy metal removal from water/wastewater by membrane technologies. J. Ind. Eng. Chem. 2019, 76, 17–38. [Google Scholar] [CrossRef]
- Wu, T.; Wang, N.; Li, J.; Wang, L.; Zhang, W.; Zhang, G.; Ji, S. Tubular thermal crosslinked-PEBA/ceramic membrane for aromatic/aliphatic pervaporation. J. Membr. Sci. 2015, 486, 1–9. [Google Scholar] [CrossRef]
- Chougui, A.; Zaiter, K.; Belouatek, A.; Asli, B. Heavy metals and color retention by a synthesized inorganic membrane. Arab. J. Chem. 2014, 7, 817–822. [Google Scholar] [CrossRef] [Green Version]
- Aguado, S.; Polo, A.C.; Bernal, M.P.; Coronas, J.; Santamaría, J. Removal of pollutants from indoor air using zeolite membranes. J. Membr. Sci. 2004, 240, 159–166. [Google Scholar] [CrossRef]
- Wang, C.; Leng, S.; Guo, H.; Cao, L.; Huang, J. Acid and alkali treatments for regulation of hydrophilicity/hydrophobicity of natural zeolite. Appl. Surf. Sci. 2019, 478, 319–326. [Google Scholar] [CrossRef]
- Mohammed, B.B.; Yamni, K.; Tijani, N.; Lee, H.S.; Dehmani, Y.; El Hamdani, H.; Alrashdi, A.A.; Ramola, S.; Belwal, T.; Lgaz, H. Enhanced removal efficiency of NaY zeolite toward phenol from aqueous solution by modification with nickel (Ni-NaY). J. Saudi Chem. Soc. 2021, 25, 101224. [Google Scholar] [CrossRef]
- Vilaseca, M.; Mateo, E.; Palacio, L.; Prádanos, P.; Hernández, A.; Paniagua, A.; Coronas, J.; Santamaría, J. AFM characterization of the growth of MFI-type zeolite films on alumina substrates. Microporous Mesoporous Mater. 2004, 71, 33–37. [Google Scholar] [CrossRef]
- Arivithamani, N.; Dev, V.R.G. Cationization of cotton for industrial scale salt-free reactive dyeing of garments. J. Clean Technol. Environ. Policy 2017, 19, 2317–2326. [Google Scholar] [CrossRef]
- Elgamouz, A.; Tijani, N.; Shehadi, I.; Hasan, K.; Al-Farooq Kawam, M. Characterization of the firing behaviour of an illite-kaolinite clay mineral and its potential use as membrane support. Heliyon 2019, 5, e02281. [Google Scholar] [CrossRef] [Green Version]
- Workneh, S.; Shukla, A. Synthesis of sodalite octahydrate zeolite-clay composite membrane and its use in separation of SDS. J. Membr. Sci. 2008, 309, 189–195. [Google Scholar] [CrossRef]
- Felsche, J.; Luger, S.; Baerlocher, C. Crystal structures of the hydro-sodalite Na6 [AlSiO4] 6 8H2O and of the anhydrous sodalite Na6 [AlSiO4]. Zeolites 1986, 6, 367–372. [Google Scholar] [CrossRef]
- Lahnafi, A.; Elgamouz, A.; Tijani, N.; Shehadi, I. Hydrothermal synthesis of zeolite A and Y membrane layers on clay flat disc support and their potential use in the decontamination of water polluted with toxic heavy metals. Desalin. Water Treat. 2020, 182, 175–186. [Google Scholar] [CrossRef]
- Dehmani, Y.; Ed-Dra, A.; Zennouhi, O.; Bouymajane, A.; Filali, F.R.; Nassiri, L.; Abouarnadasse, S. Chemical characterization and adsorption of oil mill wastewater on Moroccan clay in order to be used in the agricultural field. Heliyon 2020, 6, e03164. [Google Scholar] [CrossRef] [Green Version]
- Messaoudi, M.; Tijani, N.; Baya, S.; Lahnafi, A.; Ouallal, H.; Moussout, H.; Messaoudi, L. Characterization of ceramic pieces shaped from clay intended for the development of filtration membranes. S. Afr. J. Chem. Eng. 2021, 37, 1–11. [Google Scholar] [CrossRef]
- Oakes, J.; Gratton, P. Kinetic investigations of the oxidation of Methyl Orange and substituted arylazonaphthol dyes by peracids in aqueous solution. J. Chem. Soc. Perkin Trans. 2 1998, 12, 2563–2568. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Li, X.; Li, J.; Yin, J. Photocatalytic degradation of methyl orange by TiO2-coated activated carbon and kinetic study. Water Res. 2006, 40, 1119–1126. [Google Scholar] [CrossRef] [PubMed]
- Alves, M.E.; Mascarenhas, Y.P.; French, D.H.; Vaz, C. Rietveld-based mineralogical quantitation of deferrified oxisol clays. Soil Res. 2007, 45, 224–232. [Google Scholar] [CrossRef]
- Jiang, J.; Zhu, M.; Liu, Y.; Li, Y.; Gui, T.; Hu, N.; Zhang, F.; Chen, X.; Kita, H. Influences of synthesis conditions on preparation and characterization of Ti-MWW zeolite membrane by secondary hydrothermal synthesis. Microporous Mesoporous Mater. 2020, 297, 110004. [Google Scholar] [CrossRef]
- Vezzalini, G.; Quartieri, S.; Galli, E.; Alberti, A.; Cruciani, G.; Kvick, A. Crystal structure of the zeolite mutinaite, the natural analog of ZSM-5. Zeolites 1997, 19, 323–325. [Google Scholar] [CrossRef]
- Selim, M.M.; EL-Mekkawi, D.M.; Aboelenin, R.M.; Sayed Ahmed, S.A.; Mohamed, G.M. Preparation and characterization of Na-A zeolite from aluminum scrub and commercial sodium silicate for the removal of Cd2 from water. J. Assoc. Arab. Univ. Basic Appl. Sci. 2017, 24, 19–25. [Google Scholar] [CrossRef] [Green Version]
- Hailu, S.L.; Nair, B.U.; Redi-Abshiro, M.; Diaz, I.; Tessema, M. Preparation and characterization of cationic surfactant modified zeolite adsorbent material for adsorption of organic and inorganic industrial pollutants. J. Environ. Chem. Eng. 2017, 5, 3319–3329. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, G.; Wang, Y.; Chai, Y.; Liu, C. Preparation, mechanism and applications of oriented MFI zeolite membranes: A review. Microporous Mesoporous Mater. 2020, 312, 110790. [Google Scholar] [CrossRef]
- Senthil Kumar, R.; Rajkumar, P. Characterization of minerals in air dust particles in the state of Tamilnadu, India through ftir spectroscopy. Atmos. Chem. Phys. Discuss. 2013, 13, 22221–22248. [Google Scholar]
- Lynch, J.L.V.; Baykara, H.; Cornejo, M.; Soriano, G.; Ulloa, N.A. Preparation, characterization, and determination of mechanical and thermal stability of natural zeolite-based foamed geopolymers. Constr. Build Mater. 2018, 172, 448–456. [Google Scholar] [CrossRef]
- Liu, X.; Mäki-Arvela, P.; Aho, A.; Vajglova, Z.; Gun’ko, V.M.; Heinmaa, I.; Kumar, N.; Eränen, K.; Salmi, T.; Murzin, D.Y. Zeta potential of beta zeolites: Influence of structure, acidity, pH, temperature and concentration. Molecules 2018, 23, 946. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.; Liang, H.; Li, M.; Liu, B.; Xu, Z. Hierarchically porous carbon membranes derived from PAN and their selective adsorption of organic dyes. Chin. J. Polym. Sci. 2016, 34, 23–33. [Google Scholar] [CrossRef]
- Barakat, M.A.; Selim, A.Q.; Mobarak, M.; Kumar, R.; Anastopoulos, I.; Giannakoudakis, D.; Bonilla-Petriciolet, A.; Mohamed, E.A.; Seliem, M.K.; Komarneni, S. Experimental and Theoretical Studies of Methyl Orange Uptake by Mn–Rich Synthetic Mica: Insights into Manganese Role in Adsorption and Selectivity. Nanomaterials 2020, 10, 1464. [Google Scholar] [CrossRef]
- Filicea, S.; D’Angelo, D.; Libertino, S.; Nicotera, I.; Kosma, V.; Privitera, V.; Scales, S. Graphene oxide and titania hybrid Nafion membranes for efficient removal of methyl orange dye from water. Carbon 2015, 82, 489. [Google Scholar] [CrossRef]
- Li, T.; Zhang, Z.; Liu, L.; Gao, M.; Han, Z. A stable metal-organic framework nanofibrous membrane as photocatalyst for simultaneous removal of methyl orange and formaldehyde from aqueous solution. Colloids Surf. A Physicochem. Eng. 2021, 20, 126359. [Google Scholar] [CrossRef]
- Yadav, A.; Patel, R.V.; Singh, C.P.; Labhasetwar, P.K.; Shahi, V.K. Experimental study and numerical optimization for removal of methyl orange using polytetrafluoroethylene membranes in vacuum membrane distillation process. Colloids Surf. A Physicochem. Eng. 2022, 635, 128070. [Google Scholar] [CrossRef]



| %Oxide | SiO2 | Al2O3 | CaO | Fe2O3 | MgO | K2O | TiO2 | Na2O | P2O5 | Mn2O3 | SrO | ZnO | Cr2O3 | LOI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Raw Clay | 39.33 | 10.44 | 18.79 | 5.22 | 3.11 | 2.52 | 0.62 | 0.23 | 0.09 | 0.07 | 0.05 | 0.01 | 0.01 | 19.5 |
| Materials | Parameters | Crystalline System | Chemical Formula |
|---|---|---|---|
| Faujasite Zeolite | a = b = c = 24.79 A α = β = ϫ = 90° | cubic | (Na2)3.5[Al7Si17O48]·32 (H2O) |
| Sodalite Zeolite | a = b = c = 8.84 Å α = β = γ = 90° | Square bipyramidale | Na6(H20)8[Si6Al6O24] |
| Materials | SBET (m2/g) | Vp (cm3/g) | dp (Å) |
|---|---|---|---|
| Raw clay | 34.40 | 0.04108 | 47.77 |
| 160 µm ≤ Φ ≤ 250 Support at 1000 °C | 0.481 | 2.13 × 10−3 | 35.5 |
| Faujasite | 558.75 | 0.31837 | 22.80 |
| Sodalite | 43.34 | 0.31837 | 22.79 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-kordy, A.; Elgamouz, A.; Lemdek, E.M.; Tijani, N.; Alharthi, S.S.; Kawde, A.-N.; Shehadi, I. Preparation of Sodalite and Faujasite Clay Composite Membranes and Their Utilization in the Decontamination of Dye Effluents. Membranes 2022, 12, 12. https://doi.org/10.3390/membranes12010012
El-kordy A, Elgamouz A, Lemdek EM, Tijani N, Alharthi SS, Kawde A-N, Shehadi I. Preparation of Sodalite and Faujasite Clay Composite Membranes and Their Utilization in the Decontamination of Dye Effluents. Membranes. 2022; 12(1):12. https://doi.org/10.3390/membranes12010012
Chicago/Turabian StyleEl-kordy, Abderrazek, Abdelaziz Elgamouz, El Mokhtar Lemdek, Najib Tijani, Salman S. Alharthi, Abdel-Nasser Kawde, and Ihsan Shehadi. 2022. "Preparation of Sodalite and Faujasite Clay Composite Membranes and Their Utilization in the Decontamination of Dye Effluents" Membranes 12, no. 1: 12. https://doi.org/10.3390/membranes12010012
APA StyleEl-kordy, A., Elgamouz, A., Lemdek, E. M., Tijani, N., Alharthi, S. S., Kawde, A.-N., & Shehadi, I. (2022). Preparation of Sodalite and Faujasite Clay Composite Membranes and Their Utilization in the Decontamination of Dye Effluents. Membranes, 12(1), 12. https://doi.org/10.3390/membranes12010012