A Brief Review of the Status of Low-Pressure Membrane Technology Implementation for Petroleum Industry Effluent Treatment
Abstract
:1. Introduction
- (a).
- the legislated limits for discharge to the environment (i.e., to sea),
- (b).
- the contaminant level limits demanded by reuse of the treated effluent for reinjection into the reservoir (known as produced water reinjection, or PWRI), and/or
- (c).
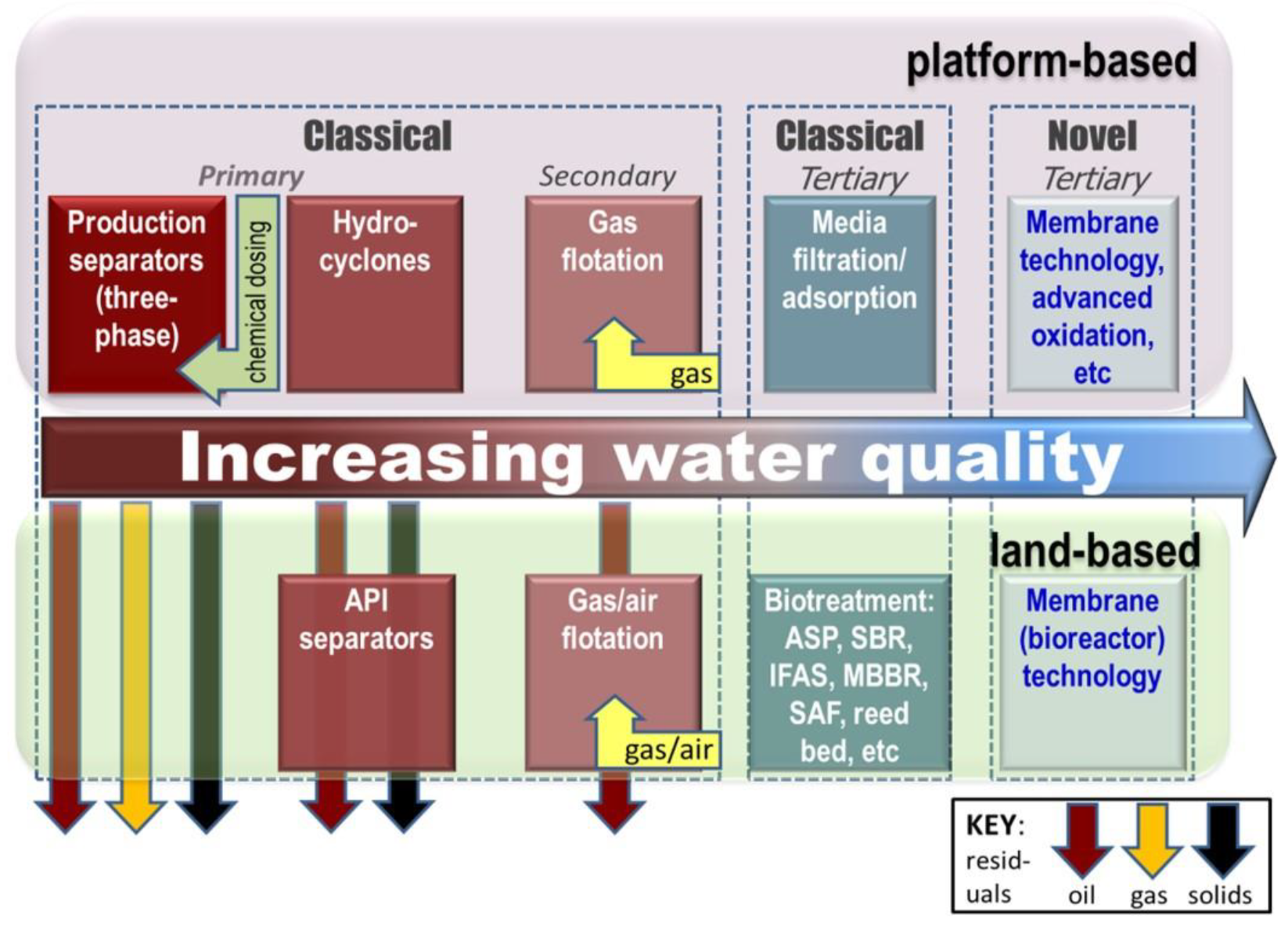
- the overall wastewater management strategy and treatment technologies selected, determined largely by whether the installation is based onshore or offshore (Figure 2).
- the influent water quality,
- system hydraulics, and specifically sustainability of flux and permeability (the flux per unit transmembrane pressure, TMP),
- organic carbon removal, as represented by the oil and/or chemical oxygen demand (COD), and
- requirement for supplementary system components, and specifically pretreatment and post-treatment.
2. Water Quality
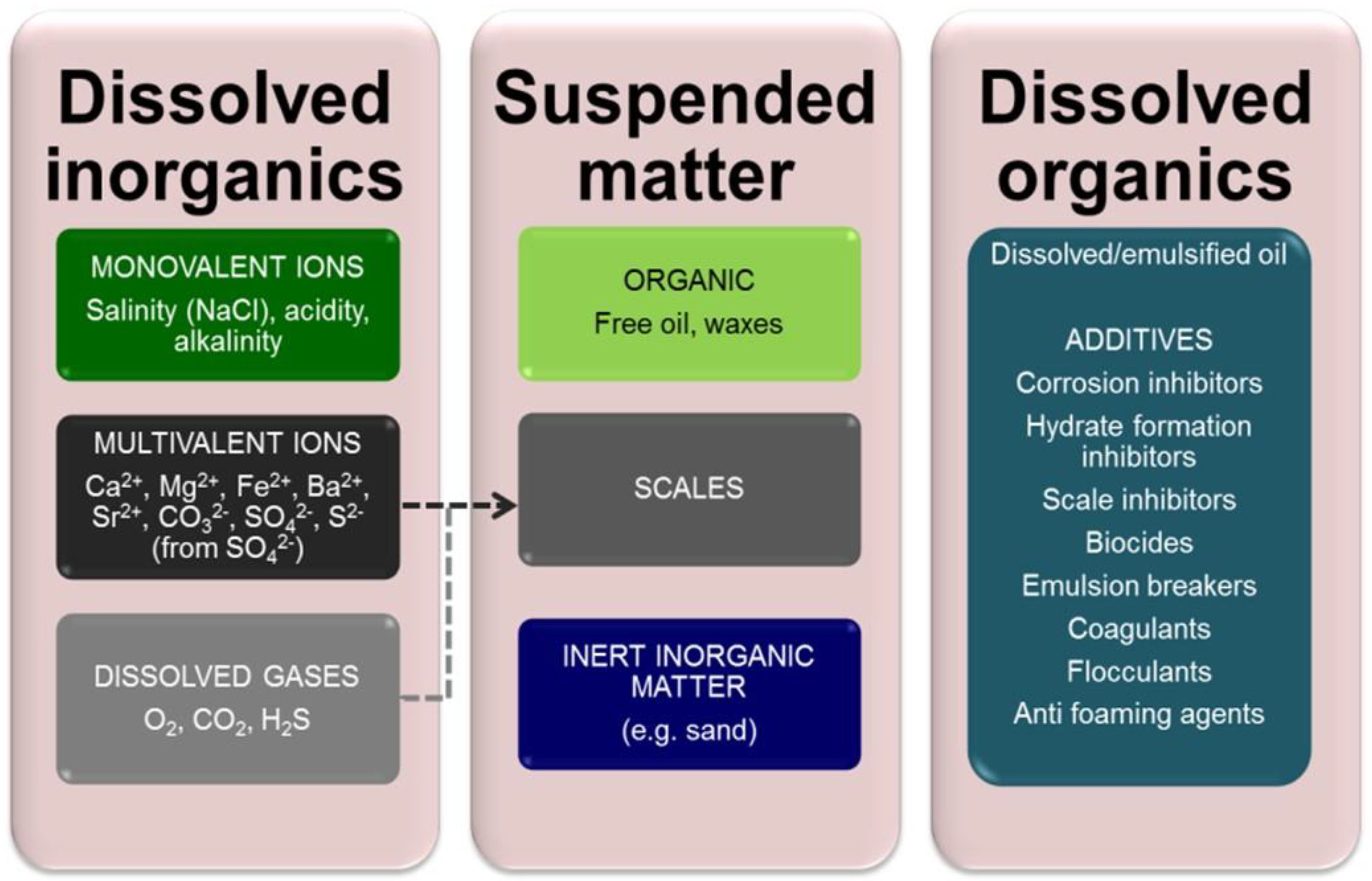
2.1. Produced Water (PW)
2.2. Refinery Effluent (RE)
- tank bottom draws
- desalter effluent
- stripped sour water, and
- cooling tower blowdown.
3. Abiotic Low-Pressure (UF/MF) Membrane Separation
- bench-scale studies,
- crossflow operation of tubular or square-channel membrane elements to sustain high shears and thus suppress membrane surface fouling,
- analogue (or synthetic) effluent feeds,
- refinery wastewaters, and
- limited duration (<6 h) trials conducted under constant transmembrane pressure (TMP) conditions.
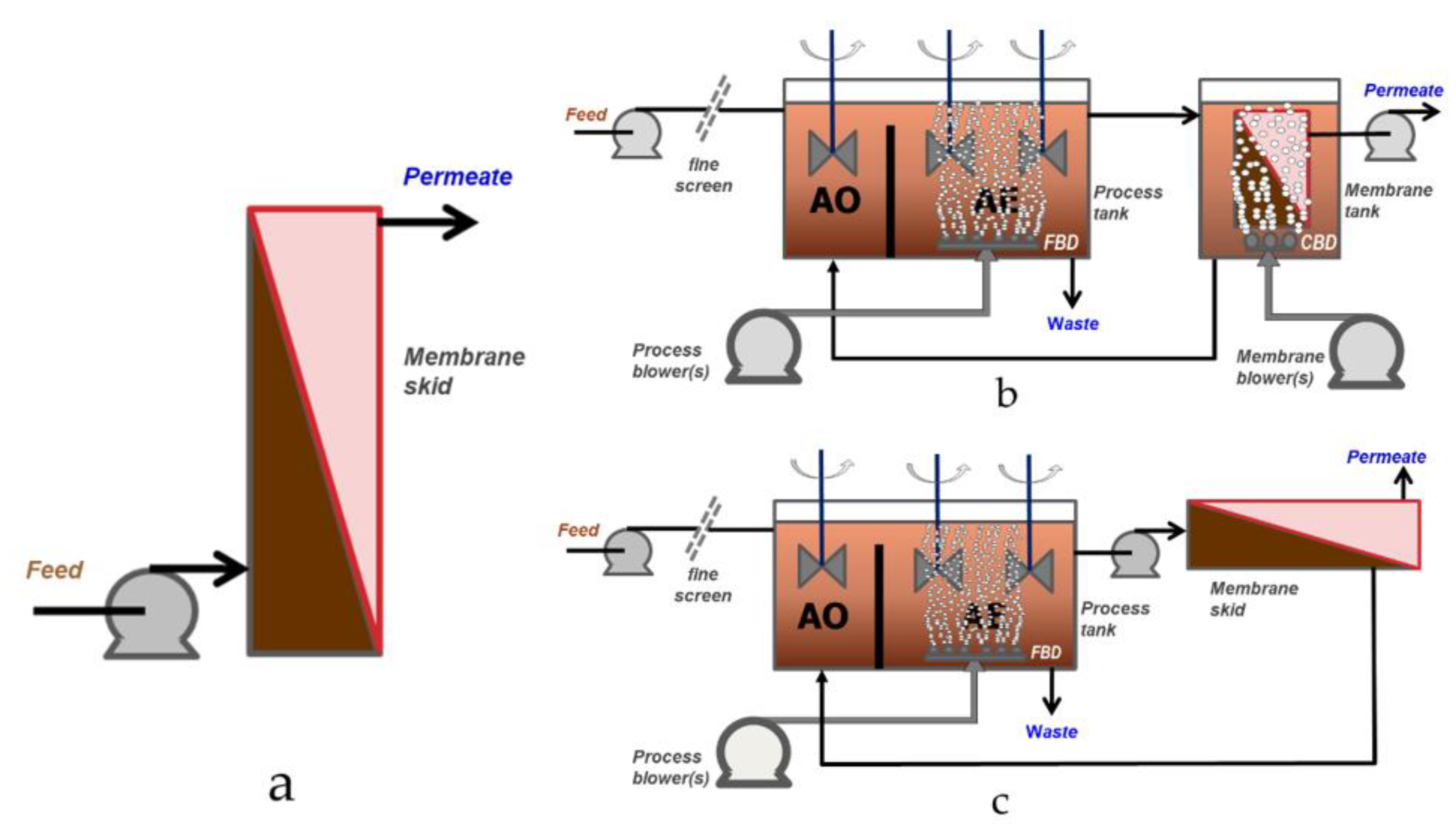
4. Membrane Biological Treatment (MBRs)
5. Conclusions
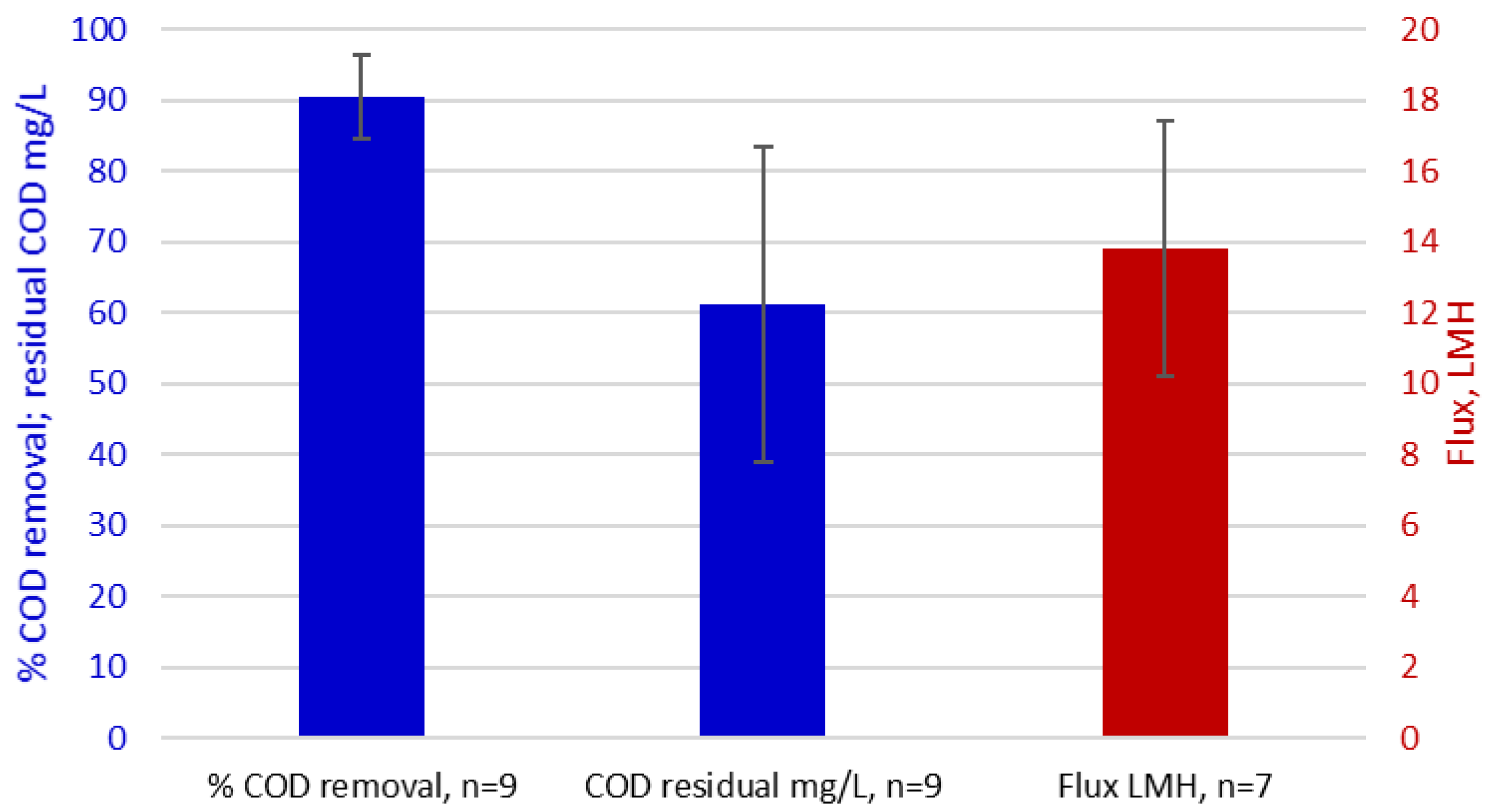
- PW UF/MF membrane filtration studies have been limited in scale and duration, and largely based on synthetic/analogue feedwaters, which are not necessarily representative of real effluents. A wide range of final fluxes (4–700 LMH) and permeabilities (5–1240 LMH/bar) have consequently been reported, which may not be representative of full-scale operation.
- The most economical method of enhanced oil recovery (EOR) is through dosing with polymer. Whilst this improves the yield of oil from reservoirs, it also causes significant membrane fouling [23,45]. This decreases the flux, commensurately increasing the required membrane area and associated footprint to beyond the threshold where implementation on offshore oil platforms can be considered feasible [20,22].
- Ceramic membranes have been successfully implemented for onshore applications, with examples from 1997 onwards of abiotic potable water installations in Japan, [53] and, from 2007 onwards, MBR technologies for wastewater treatment [54]. Despite the apparent viability of ceramic membranes for these onshore duties, there has been no significant implementation for the key offshore application of PW filtration using either ceramic or polymeric membrane materials and no successful demonstration-scale trials reported.
- Onshore treatment of refinery and petrochemical effluents specifically using MBR technology, providing advanced biological treatment, has been established since the early noughties. Such treatment is appropriate since removal of both the suspended and dissolved organic matter (manifested as the COD) for this duty rather than just suspended oil removal as for PW treatment.
- The main contributors to MBR operational costs have been shown to be labor effort, sustainable flux, energy, and membrane replacement [48]. The comparative economic viability of ceramic membrane-based MBR technology for effluent treatment compared with the polymeric materials is thus dependent on the cost benefit offered by the reduced labor effort, longer membrane life and higher fluxes weighed against the cost penalty of the membrane material, as demonstrated by a recent review of potable water applications [55].
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADF | Average daily flow |
| AE | Aerobic |
| AlO | Aluminum oxide |
| AO | Anoxic |
| API | American Petroleum Institute |
| ASF | Advance sand filtration |
| BOD | Biological oxygen demand |
| BS and W | Bottom sediment and water |
| BTEX | Benzene, toluene, ethylbenzene, xylene |
| CBD | Course bubble diffuser |
| CEOR | Chemically-enhanced oil recovery |
| CFV | Crossflow velocity |
| COD | Chemical oxygen demand |
| CW | Cooling water |
| DAF | Dissolved air flotation |
| DN | Denitrification |
| EQ | Equalization |
| FBD | Fine bubble diffuser |
| HDPE | High density polyethylene |
| HRT | Hydraulic retention time |
| IFAS | Integrated fixed-film activated sludge |
| IGF | Induced gas flotation |
| iHF | Immersed hollow fiber |
| iMBR | Immersed membrane bioreactor |
| LMH | Liter/m2/h |
| MBBR | Moving bed bioreactor |
| MBR | Membrane bioreactor |
| MLD | Millions of liters per day |
| MLSS | Mixed liquor suspended solids |
| MT | Multitube |
| MF | Microfiltration |
| OFPW | Oilfield produced water |
| PAHs | Polycyclic aromatic hydrocarbons |
| Peak daily flow | |
| PES | Polyethylsulphone |
| PS | Polysulphone |
| PVDF | Polyvinylidene fluoride |
| PW | Produced water |
| PWRI | Produced water reinjection |
| RE | Refinery effluent |
| SAGD | Steam-assisted gravity drainage |
| SBR | Sequence batch reactor |
| SEDm | Specific energy consumption of membrane permeation |
| SiC | Silicon carbide |
| sMBR | Sidestream membrane bioreactor |
| sMT | Sidestream multitube |
| SRT | Solid retention time |
| SWOT | Strengths, weaknesses, opportunities and threats |
| TDS | Total dissolved solids |
| TiO | Titanium oxide |
| TMP | Transmembrane pressure |
| TOC | Total organic carbon |
| TSS | Total suspended solids |
| UF | Ultrafiltration |
| WQ | Water quality |
| WwTP | Wastewater treatment plant |
| ZrO | Zirconium oxide |
References and Note
- Lin, H.; Gao, W.; Meng, F.; Liao, B.-Q.; Leung, K.-T.; Zhao, L.; Chen, J.; Hong, H. Membrane Bioreactors for Industrial Wastewater Treatment: A Critical Review. Crit. Rev. Environ. Sci. Technol. 2012, 42, 677–740. [Google Scholar] [CrossRef]
- Munirasu, S.; Abu Haija, M.; Banat, F. Use of membrane technology for oil field and refinery produced water treatment—A review. Process Saf. Environ. Prot. 2016, 100, 183–202. [Google Scholar] [CrossRef]
- Al Jabri, F.; Muruganandam, L.; Aljuboury, D.A.D.A. Treatment of the oilfield-produced water and oil refinery wastewater by using inverse fluidization—A review. Global Nest Rev. 2019, 21, 204–210. [Google Scholar] [CrossRef]
- Amakiri, K.T.; Canon, A.R.; Molinari, M.; Angelis-Dimakis, A. Review of oilfield produced water treatment technologies. Chemosphere 2022, 298. [Google Scholar] [CrossRef] [PubMed]
- Judd, S.; Qiblawey, H.; Al-Marri, M.J.; Clarkin, C.; Watson, S.; Ahmed, A.; Bach, S. The size and performance of offshore produced water oil-removal technologies for reinjection. Sep. Purif. Technol. 2014, 134, 241–246. [Google Scholar] [CrossRef]
- Stewart, M.; Arnold, K. Produced Water Treatment Field Manual; Gulf Professional Publishing: New York, NY, USA, 2011. [Google Scholar] [CrossRef]
- Bayat, M.; Mehrnia, M.R.; Hosseinzadeh, M.; Sheikh-Sofla, R. Petrochemical wastewater treatment and reuse by MBR: A pilot study for ethylene oxide/ethylene glycol and olefin units. J. Ind. Eng. Chem. 2015, 25, 265–271. [Google Scholar] [CrossRef]
- Amaral, M.C.S.; De Andrade, L.H.; Neta, L.S.F.; Moravia, W.G. Long-term use of the critical flux for fouling control in membrane bioreactors treating different industrial effluents: Bench and pilot scale. Desalination Water Treat. 2015, 55, 859–869. [Google Scholar] [CrossRef]
- Zhong, J.; Sun, X.; Wang, C. Treatment of oily wastewater produced from refinery processes using flocculation and ceramic membrane filtration. Sep. Purif. Technol. 2003, 32, 93–98. [Google Scholar] [CrossRef]
- Chakrabarty, B.; Ghoshal, A.K.; Purkait, M.K. Cross-flow ultrafiltration of stable oil-in-water emulsion using polysulfone membranes. Chem. Eng. J. 2010, 165, 447–456. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Willershausen, D.; Ashaghi, K.S.; Engel, L.; Placido, L.; Mund, P.; Bolduan, P.; Czermak, P. Investigations on the use of different ceramic membranes for efficient oil-field produced water treatment. Desalination 2010, 250, 991–996. [Google Scholar] [CrossRef] [Green Version]
- Silalahi, S.H.; Leiknes, T. Cleaning strategies in ceramic microfiltration membranes fouled by oil and particulate matter in produced water. Desalination 2009, 236, 160–169. [Google Scholar] [CrossRef]
- Subramani, A.; Schlicher, R.; Long, J.; Yu, J.; Lehman, S.; Jacangelo, J.G. Recovery optimization of membrane processes for treatment of produced water with high silica content. Desalination Water Treat. 2011, 36, 297–309. [Google Scholar] [CrossRef]
- CoMeTas. Commissioning of the SemCoMem unit, Company report, 2011, Project no. 110–11.
- Pedenaud, P.; Heng, S.; Evans, W.; Bieonneau, D. Ceramic Membrane and Core Pilot Results for Produced Water, OTC-22371-PP. In Proceedings of the Offshore Technology Conference, Rio de Janeiro, Brazil, 4–6 October 2011. [Google Scholar]
- Prado-Rubio, O.A.; Cardona, D.; Svendsen, T.; Yuan, L. SiC Membrane Pilot Plant Ultrafiltration Test for Produced Water Treatment, Ocelote Field—Hocol (Colombia); Company Report; Liqtech: Ballerup, Denmark, 2012. [Google Scholar]
- Madaeni, S.S.; Gheshlaghi, A.; Rekabdar, F. Membrane treatment of oily wastewater from refinery processes: Membrane Treatment of Oily Wastewater. Asia-Pac. J. Chem. Eng. 2013, 8, 45–53. [Google Scholar] [CrossRef]
- Guirgis, A.; Gay-De-Montella, R.; Faiz, R. Treatment of produced water streams in SAGD processes using tubular ceramic membranes. Desalination 2015, 358, 27–32. [Google Scholar] [CrossRef]
- Weschenfelder, S.E.; Louvisse, A.M.; Borges, C.; Meabe, E.; Izquierdo, J.; Campos, J. Evaluation of ceramic membranes for oilfield produced water treatment aiming reinjection in offshore units. J. Pet. Sci. Eng. 2015, 131, 51–57. [Google Scholar] [CrossRef]
- Weschenfelder, S.E.; Fonseca, M.J.d.C.; Borges, C.; Campos, J. Application of ceramic membranes for water management in offshore oil production platforms: Process design and economics. Sep. Purif. Technol. 2016, 171, 214–220. [Google Scholar] [CrossRef]
- Reyhani, A.; Meighani, H.M. Optimal operating conditions of micro- and ultra-filtration systems for produced-water purification: Taguchi method and economic investigation. Desalination Water Treat. 2016, 57, 19642–19654. [Google Scholar] [CrossRef]
- Zsirai, T.; Qiblawey, H.; Buzatu, P.; Al-Marri, M.; Judd, S. Cleaning of ceramic membranes for produced water filtration. J. Pet. Sci. Eng. 2018, 166, 283–289. [Google Scholar] [CrossRef] [Green Version]
- Weschenfelder, S.; Fonseca, M.; Borges, C. Treatment of produced water from polymer flooding in oil production by ceramic membranes. J. Pet. Sci. Eng. 2020, 196, 108021. [Google Scholar] [CrossRef]
- Judd, S.J. Industrial MBRs; Judd & Judd: Cranfield, UK, 2014. [Google Scholar]
- The MBR Site. Available online: www.thembrsite.com (accessed on 17 March 2022).
- Cerqueira, A.C.; Lopes, T.; Santiago, V.; Vallero, M.; Trovati, J.; Arntsen, B.; Syed, W. Design and Performance of the First Full Scale Membrane Bioreactor Plant Treating Oil Refinery Effluent in Brazil. Proc. Water Environ. Fed. 2013, 2013, 3573–3584. [Google Scholar] [CrossRef]
- Ahmadi, M.; Benis, K.Z.; Faraji, M.; Shakerkhatibi, M.; Aliashrafi, A. Process performance and multi-kinetic modeling of a membrane bioreactor treating actual oil refinery wastewater. J. Water Process Eng. 2019, 28, 115–122. [Google Scholar] [CrossRef]
- Sambusiti, C.; Saadouni, M.; Gauchou, V.; Segues, B.; Leca, M.A.; Baldoni-Andrey, P.; Jacob, M. Influence of HRT reduction on pilot scale flat sheet submerged membrane bioreactor (sMBR) performances for Oil&Gas wastewater treatment. J. Membr. Sci. 2020, 594, 117459. [Google Scholar] [CrossRef]
- Asante-Sackey, D.; Rathilal, S.; Tetteh, E.K.; Armah, E.K. Membrane Bioreactors for Produced Water Treatment: A Mini-Review. Membranes 2022, 12, 275. [Google Scholar] [CrossRef] [PubMed]
- Samaei, S.M.; Gato-Trinidad, S.; Altaee, A. The application of pressure-driven ceramic membrane technology for the treatment of industrial wastewaters—A review. Sep. Purif. Technol. 2018, 200, 198–220. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, H.; Li, Y.; Xu, H.; Pan, Z.; Dai, P.; Wang, H.; Yang, Q. A review of treatment technologies for produced water in offshore oil and gas fields. Sci. Total Environ. 2021, 775, 145485. [Google Scholar] [CrossRef]
- Fakhru’L-Razi, A.; Pendashteh, A.; Abdullah, L.C.; Biak, D.R.A.; Madaeni, S.S.; Abidin, Z.Z. Review of technologies for oil and gas produced water treatment. J. Hazard. Mater. 2009, 170, 530–551. [Google Scholar] [CrossRef]
- Pal, S.; Banat, F.; Almansoori, A.; Haija, M.A. Review of technologies for biotreatment of refinery wastewaters: Progress, challenges and future opportunities. Environ. Technol. Rev. 2016, 5, 12–38. [Google Scholar] [CrossRef]
- IPIECA. Petroleum Refining Water/Wastewater Use and Management 2010, Operations Best Practice Series, IPIECA, London. Available online: https://www.ipieca.org/resources/good-practice/petroleum-refining-water-wastewater-use-and-management/ (accessed on 17 March 2022).
- El-Naas, M.; Acio, J.A.; El Telib, A.E. Aerobic biodegradation of BTEX: Progresses and Prospects. J. Environ. Chem. Eng. 2014, 2, 1104–1122. [Google Scholar] [CrossRef]
- Tong, K.; Zhang, Y.; Liu, G.; Ye, Z.; Chu, P. Treatment of heavy oil wastewater by a conventional activated sludge process coupled with an immobilized biological filter. Int. Biodeterior. Biodegradation 2013, 84, 65–71. [Google Scholar] [CrossRef]
- Gargouri, B.; Karray, F.; Mhiri, N.; Aloui, F.; Sayadi, S. Application of a continuously stirred tank bioreactor (CSTR) for bioremediation of hydrocarbon-rich industrial wastewater effluents. J. Hazard. Mater. 2011, 189, 427–434. [Google Scholar] [CrossRef]
- Khaing, T.-H.; Li, J.; Li, Y.; Wai, N.; Wong, F.-S. Feasibility study on petrochemical wastewater treatment and reuse using a novel submerged membrane distillation bioreactor. Sep. Purif. Technol. 2010, 74, 138–143. [Google Scholar] [CrossRef]
- Wei, L.; Guo, S.; Yan, G.; Chen, C.; Jiang, X. Electrochemical pretreatment of heavy oil refinery wastewater using a three-dimensional electrode reactor. Electrochim. Acta 2010, 55, 8615–8620. [Google Scholar] [CrossRef]
- Ma, F.; Guo, J.-B.; Zhao, L.-J.; Chang, C.-C.; Cui, D. Application of bioaugmentation to improve the activated sludge system into the contact oxidation system treating petrochemical wastewater. Bioresour. Technol. 2009, 100, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Zhang, Z.; Yu, W.; Zhu, W. Biological treatment of oilfield-produced water: A field pilot study. Int. Biodeterior. Biodegrad. 2009, 63, 316–321. [Google Scholar] [CrossRef]
- Coelho, A.; Castro, A.V.; Dezotti, M.; Sant’Anna, G. Treatment of petroleum refinery sourwater by advanced oxidation processes. J. Hazard. Mater. 2006, 137, 178–184. [Google Scholar] [CrossRef]
- Dold, P. Current Practice for Treatment of Petroleum Refinery Wastewater and Toxics Removal. Water Pollut. Res. J. 1989, 24, 363–390. [Google Scholar] [CrossRef]
- Goldsmith, R.L.; Roberts, D.A.; Burre, D.L. Ultrafiltration of soluble oil wastes. J. Water Poll. Control Fed. 1974, 46, 2183–2192. [Google Scholar]
- Li, C.; Li, J.; Wang, N.; Zhao, Q.; Wang, P. Status of the treatment of produced water containing polymer in oilfields: A review. J. Environ. Chem. Eng. 2021, 9, 105303. [Google Scholar] [CrossRef]
- Zhang, J.; Xiao, K.; Liu, Z.; Gao, T.; Liang, S.; Huang, X. Large-Scale Membrane Bioreactors for Industrial Wastewater Treatment in China: Technical and Economic Features, Driving Forces, and Perspectives. Engineering 2021, 7, 868–880. [Google Scholar] [CrossRef]
- Li, P.; Liu, L.; Wu, J.; Cheng, R.; Shi, L.; Zheng, X.; Zhang, Z. Identify driving forces of MBR applications in China. Sci. Total Environ. 2018, 647, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Qiblawey, H.; Judd, S. Industrial effluent treatment with immersed MBRs: Treatability and cost. Water Sci. Technol. 2019, 80, 762–772. [Google Scholar] [CrossRef] [PubMed]
- Alsalhy, Q.F.; Almukhtar, R.S.; Alani, H.A. Oil Refinery Wastewater Treatment by Using Membrane Bioreactor (MBR). Arab. J. Sci. Eng. 2015, 41, 2439–2452. [Google Scholar] [CrossRef]
- Razavi, S.M.R.; Miri, T. A real petroleum refinery wastewater treatment using hollow fiber membrane bioreactor (HF-MBR). J. Water Process Eng. 2015, 8, 136–141. [Google Scholar] [CrossRef]
- Alkmim, A.R.; da Costa, P.R.; Moser, P.B.; Neta, L.S.F.; Santiago, V.M.J.; Cerqueira, A.C.; Amaral, M.C.S. Long-term evaluation of different strategies of cationic polyelectrolyte dosage to control fouling in a membrane bioreactor treating refinery effluent. Environ. Technol. 2016, 37, 1026–1035. [Google Scholar] [CrossRef]
- Jelic, A.; Di Fabio, S.; Vecchiato, G.; Cecchi, F.; Fatone, F. Nano-occurrence and removal of PCBs within the Europe’s largest petrochemical MBR system. Water Res. 2015, 83, 329–336. [Google Scholar] [CrossRef]
- Hattori, K. Operation with ceramic membrane filtration system for SWTP in Japan. In Sborník Konference Pitn´a Voda; W&ET Team, Co.: Budejovice, Czech Republic, 2010; pp. 101–106. ISBN 978-80-254-6854-8. [Google Scholar]
- Binkle, O.; Gabriel, K.; Braun, G.; Nonninger, R. Small-scale sewage plant tests ceramic flat membranes. Water Wastewater Int. 2005, 20, 42–43. Available online: https://www.waterworld.com/technologies/article/16201625/smallscale-sewage-plant-tests-ceramic-flat-membranes (accessed on 17 March 2022).
- Jarvis, P.; Carra, I.; Jafari, M.; Judd, S. Ceramic vs polymeric membrane implementation for potable water treatment. Water Res. 2022, 215, 118269. [Google Scholar] [CrossRef]
- Kurth, C.J.; Wise, B.L.; Smith, S. Design considerations for implementing ceramics in new and existing polymeric UF systems. Water Pr. Technol. 2018, 13, 725–737. [Google Scholar] [CrossRef]

| Parameter | Units | Min | Max |
|---|---|---|---|
| Density | kg/m3 | 1014 | 1140 |
| Conductivity | μS/cm | 4200 | 58,600 |
| Salinity | mg/L | 1000 | >300,000 |
| Total organic carbon, TOC | mg/L | - | 1500 |
| Chemical oxygen demand, COD | mg/L | 1220 | 2600 |
| BTEX a | mg/L | 0.7 | 24 |
| Oil and grease | mg/L | 2 | 565 |
| Total suspended solids, TSS | mg/L | 1.2 | 1000 |
| Total dissolved solids, TDS | mg/L | 100 | 400,000 |
| Parameter | BS and W a | Desalter | Stripped Sour Water | Cooling Tower Blowdown |
|---|---|---|---|---|
| COD | 400–1000 | 400–1000 | 600–1200 | 150 |
| Free hydrocarbons | Up to 1000 | Up to 1000 | <10 | <5 |
| SS | Up to 500 | Up to 500 | <10 | Up to 200 |
| Phenol | - | 10–100 | Up to 200 | - |
| Benzene | - | 5–15 | negligible | - |
| Sulphides | Up to 100 | Up to 100 | - | - |
| Ammonia | - | Up to 100 | - | - |
| TDS | High | High | Low | Intermediate |
| Parameter | [35] | [36] | [37] | [38] | [39] | [40] | [41] | [42] | [43] |
|---|---|---|---|---|---|---|---|---|---|
| pH | 8.3–8.9 | 6.3 | 8 | 7.5–10.3 | 6.7–8.2 | 7–9 | 5.6–6.0 | 8.0–8.2 | - |
| BOD | - | 61.4 | 950 | - | 300–630 | 150–350 | 65–80 | 570 | 150–350 |
| COD | 3600–5300 | 209 | 4800 | 330–556 | 2500–4100 | 300–600 | 228–481 | 850–1020 | 300–800 |
| Phenol | 11–14 | - | - | - | - | - | - | 98–128 | 20–200 |
| Oil | 160–185 | 11.3 | - | 40–91 | 50–100 | 50 | 76–105 | 12.7 | 3000 |
| TPH | 1.8–1.85 | - | 320 | - | - | - | - | - | - |
| TOC | - | - | - | 57–126 | 1290–2360 | - | 76–105 | - | - |
| TSS | 0.03–0.04 | 33.1 | - | 130–250 | - | 150 | 90–180 | nd | 100 |
| BTEX | - | - | - | 57–126 | - | - | - | 23.9 | 1–100 |
| Sulphides | - | - | - | - | - | - | - | 15–23 | - |
| NH3 | - | 11.9 | - | 33–41 | - | 10–30 | 82 | 2.1–5.1 | - |
| Oil Concn mg/L, Water Source | Scale | Material | Pore Size μm | Init Flux, LMH | Fin Flux, LMH | TMP, bar | Fin Perm, LMH/bar | Ref |
|---|---|---|---|---|---|---|---|---|
| 6000, RE | b | ZrO | 0.2 | 240 | 120–175 | 1.1 | 109–159 | [9] |
| 366, PW | b | PS | 0.007 | 225 | 128 | 1–1.7 | 95 | [10] |
| b | PS | 0.006 | 100 | 70 | 1–1.7 | 74 | ||
| 200–1000, tank dewatering effl. | b | AlO | 0.2 | 128 | 28 | 1 | 28 | [11] |
| b | TiO | 0.05 | 80 | 4 | 1 | <5 | ||
| b | TiO | 0.05 | 120 | 30 | 1 | 120–30 | ||
| 50–350, synth | p | AlO | 0.1–0.5 | - | 80–175 | 0.06–0.25 | 400–800 | [12] |
| Gas field PW | p | ZrO | 0.05 | - | 170–255 | - | 190–250 | [13] |
| 3–25 (24–74), PW | p | SiC | 0.1–0.5 | 25–120 | 50 | 0.3–1.5 | 150 | [14] |
| PW | p | TiO | 0.01–0.1 | 200 | - | 0.5–3.5 | 60 | [15] |
| SiC | 0.25–1.5 | 135 | ||||||
| 221–722, PW | p | SiC | 0.04–0.1 | - | 135–590 | 0.35–0.95 | 450–1020 | [16] |
| 20, PW | p | 0.6 | 520 | |||||
| 3000, RE | b | PS | 0.1–0.2 | 145 | 65 | 1.5 | 50–15 | [17] |
| SAGD effl. | p | AlO | 0.05 | 200 | - | 1.52 | 132 | [18] |
| p | ZrO | 202 | - | 1.52 | 45 | |||
| 100, synth | b, p | ZrO | 0.1 | 910 | 194–240 | 2 | 97–120 | [19] |
| ~60, RE | b, p | ZrO | 0.1 | 910 | 175 | 2 | 88 | |
| ~250, RE | b | ZrO | 0.1 | 1000 | 290 | 1.5 | 193 | [20] |
| 9–43, PW | p | AlO | 0.2 | - | 295–312 | 2.5 | 118–125 | [21] |
| b | PAN | 202 | - | 104–280 | 5 | 20–36 | ||
| 24–95, PW | p | SiC | 0.04 | - | ~350 | 0.55–0.6 | 370–380 | [22] |
| 0.5 | - | ~700 | 1190–1240 | |||||
| 100, synth + poly | b | ZrO | 0.1 | 430 | 69 | 1–2 | 35 | [23] |
| Porta Maghera | Sinopec Guangzhou | Sinopec Jinling | Yunlin/Formosa | Dalsung | Petrobras [24] | Yanan Fengfuchuan | |
|---|---|---|---|---|---|---|---|
| Country | Italy | China | China | Taiwan | Korea | Brazil | China |
| Application | Petrochemical | Refinery | Petroleum | Petroleum | Industrial park | Refinery | Oilfield reinjection |
| Configuration | iHF | iHF | iHF | iHF | iHF | iHF | sMT |
| Material | PVDF | PVDF | PVDF | PVDF | HDPE | PVDF | PES |
| Selection reason(s) | Required discharge WQ | Re-use (cooling towers) | Re-use (cooling towers) | Restricted footprint and water re-use. | Required discharge WQ and restricted footprint. | Required discharge WQ | Limit risk of reservoir pore plugging |
| Capacity (MLD) | 47.5 PDF | 4.8 ADF | 6 ADF | 25 PDF | 25 PDF | 7.2 PDF | 1.5 PDF |
| HRT, h | 20 | 18 | 16 | - | 8 | - | - |
| SRT, d | - | 49 | 26 | - | 45 | 36 | - |
| Feed COD, mg/L | 280 | ~150 | ~300 | 990 | 50 | 850 | 45 (oil) |
| % COD rem | >96% | >80% | >80% | 95% | >80% | 93% | >98% (oil) |
| MLSS, g/L | 8.4 (design) | 3 (3.5 MT) | 3 (4.5, MT) | 3.5 | 8 | 10 | - |
| Flux (LMH) | 9 | 9 | 10 | 17 | 12.5 | 16.4 | 44 |
| SECm (kWh/m3) | - | 0.60 | 0.60 | - | 0.70 | - | - |
| Notes | Pre and post DN employed | MBR downstream of DAF + oxidation ditch. 12 h EQ | MBR downstream of primary sed. 40 h EQ | Probably downstream of primary sedimentation | 30% sewage feed. MBR downstream of primary sedimentation | 75% sewage feed. 25% industrial stream pre-clarified and w. 8 h EQ | MBR downstream of skimmer and DAF. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dizayee, K.K.H.; Judd, S.J. A Brief Review of the Status of Low-Pressure Membrane Technology Implementation for Petroleum Industry Effluent Treatment. Membranes 2022, 12, 391. https://doi.org/10.3390/membranes12040391
Dizayee KKH, Judd SJ. A Brief Review of the Status of Low-Pressure Membrane Technology Implementation for Petroleum Industry Effluent Treatment. Membranes. 2022; 12(4):391. https://doi.org/10.3390/membranes12040391
Chicago/Turabian StyleDizayee, Kasro Kakil Hassan, and Simon J. Judd. 2022. "A Brief Review of the Status of Low-Pressure Membrane Technology Implementation for Petroleum Industry Effluent Treatment" Membranes 12, no. 4: 391. https://doi.org/10.3390/membranes12040391
APA StyleDizayee, K. K. H., & Judd, S. J. (2022). A Brief Review of the Status of Low-Pressure Membrane Technology Implementation for Petroleum Industry Effluent Treatment. Membranes, 12(4), 391. https://doi.org/10.3390/membranes12040391





