Correlations between Properties of Pore-Filling Ion Exchange Membranes and Performance of a Reverse Electrodialysis Stack for High Power Density
Abstract
:1. Introduction
2. Materials and Methods
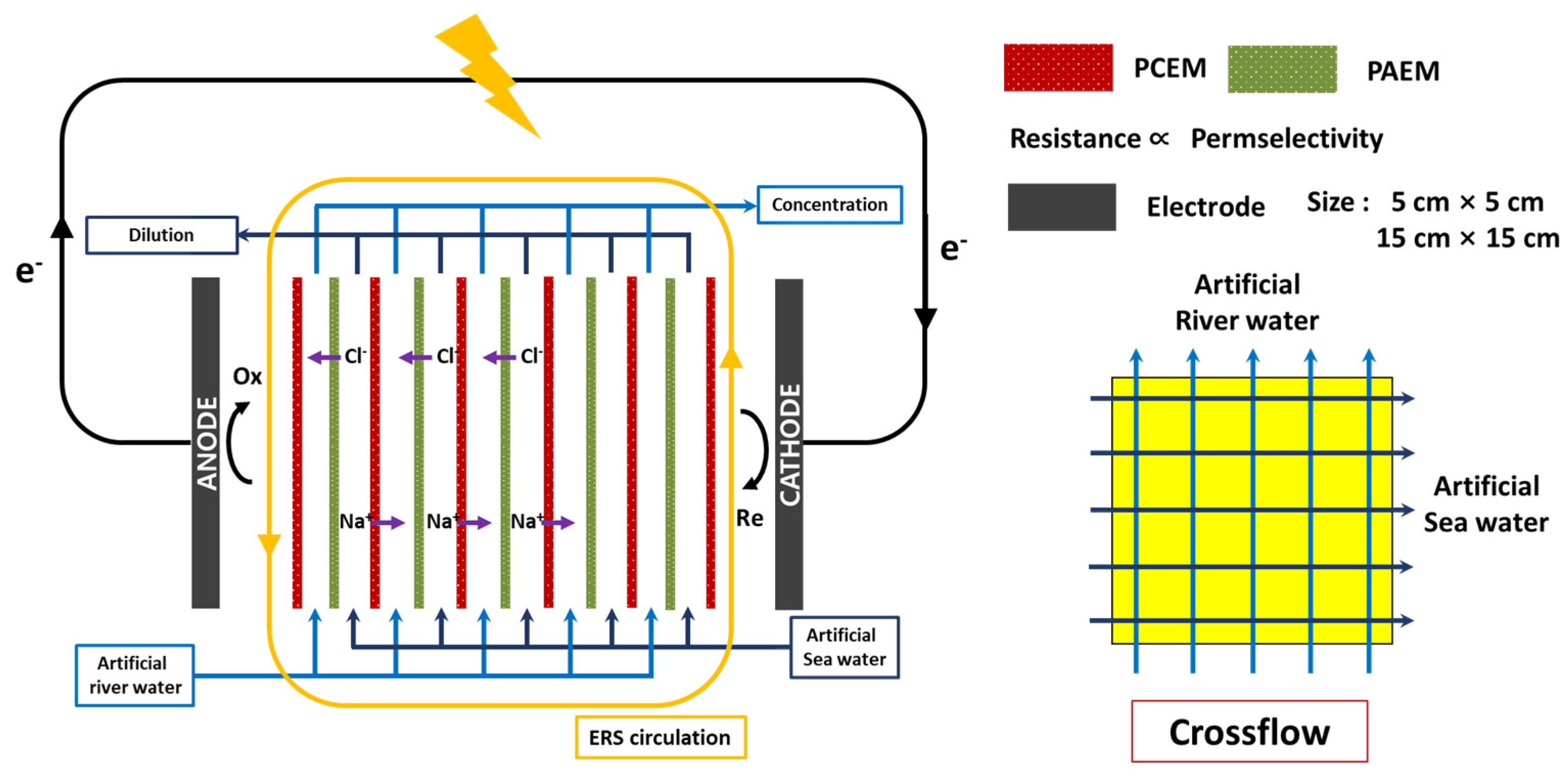
2.1. Reverse Electrodialysis (RED) System
2.2. Evaluation of the RED Stack Performance
2.3. Fabrication and Characterization of Pore-Filling Ion Exchange Membranes (PIEMs)
3. Results and Discussion
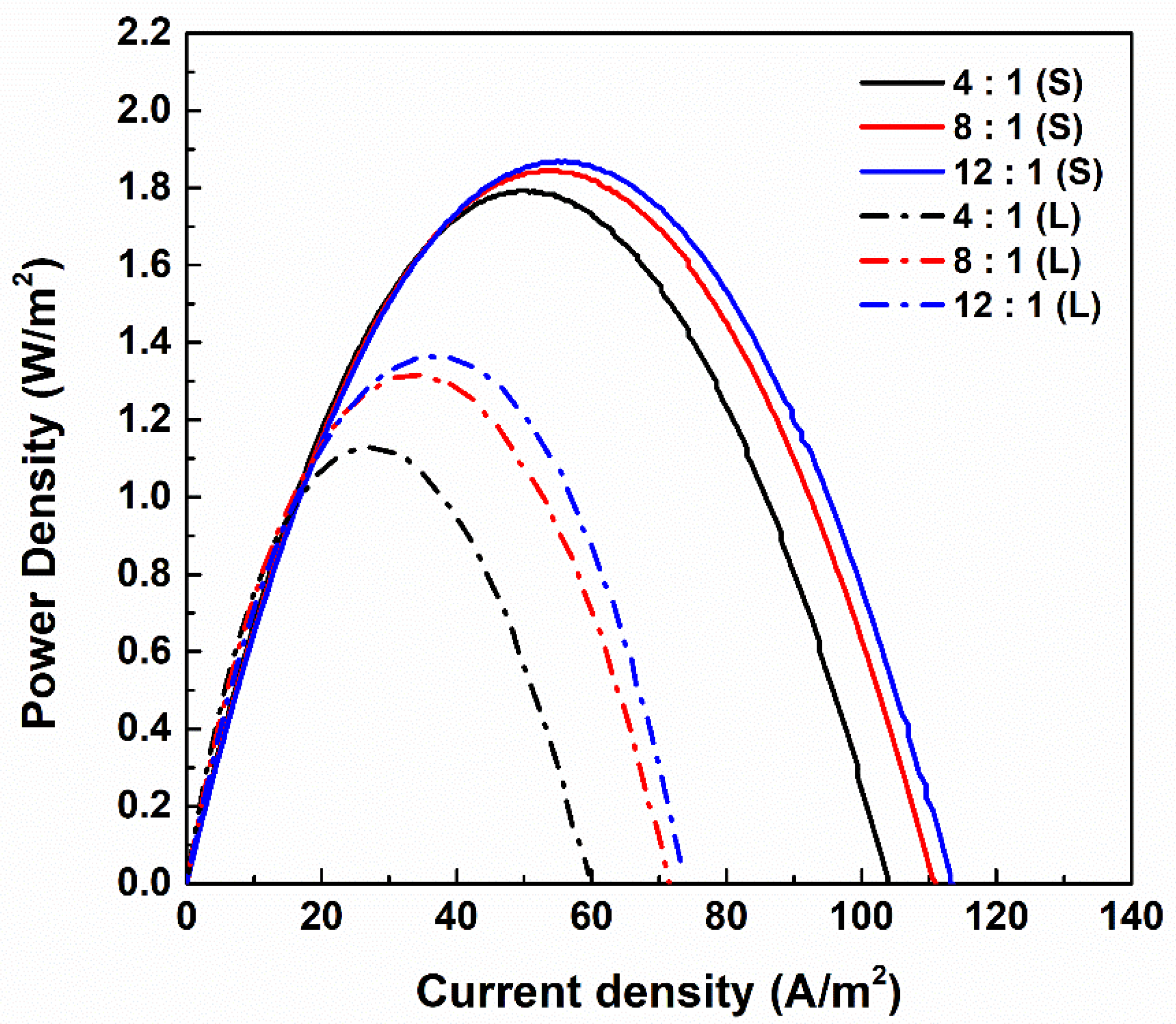
3.1. Effects of Composition of PIEMs on RED Performance
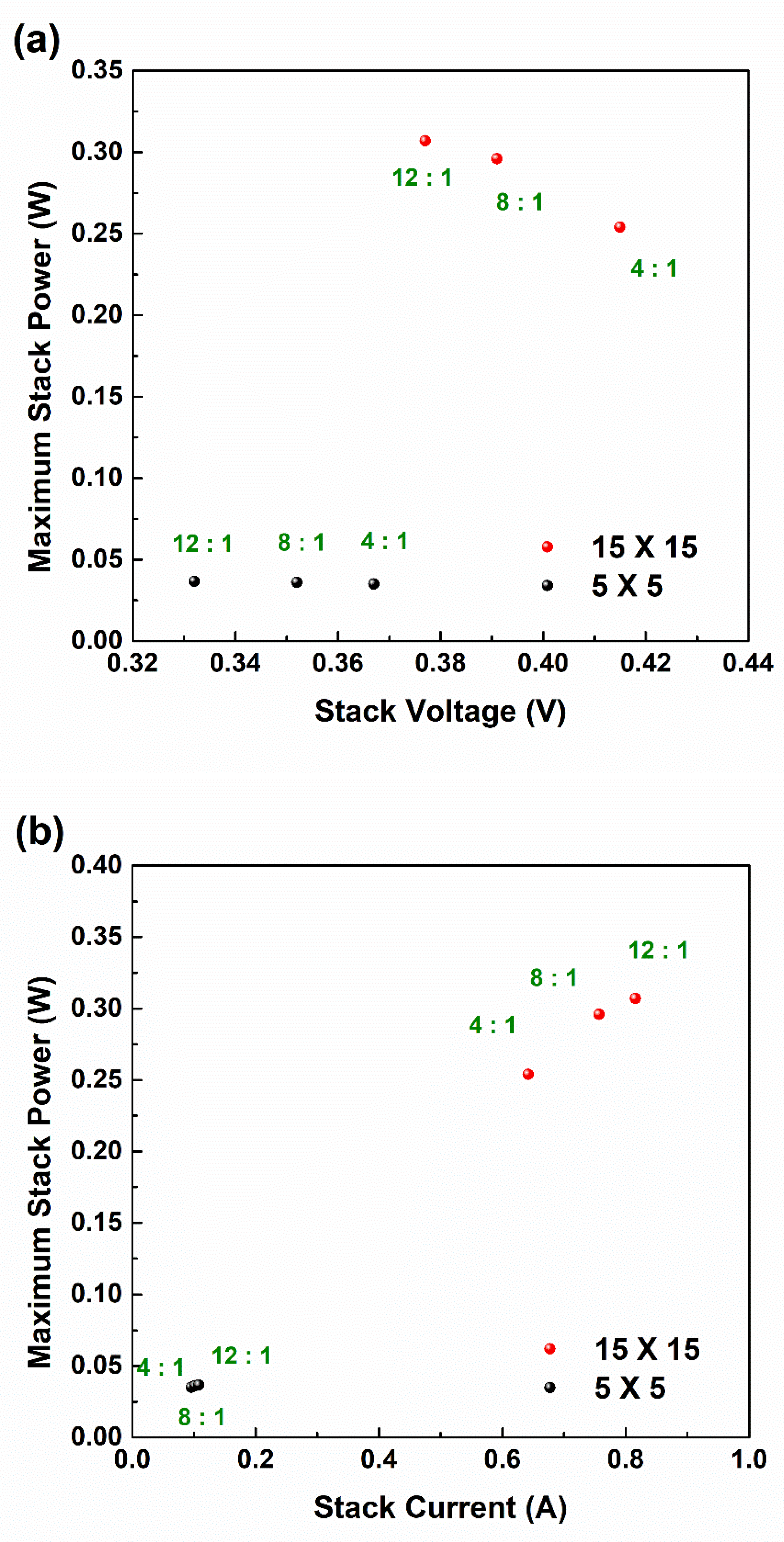
3.2. Correlation between Compositions of Ion Exchange Membranes (IEMs) and RED Performance
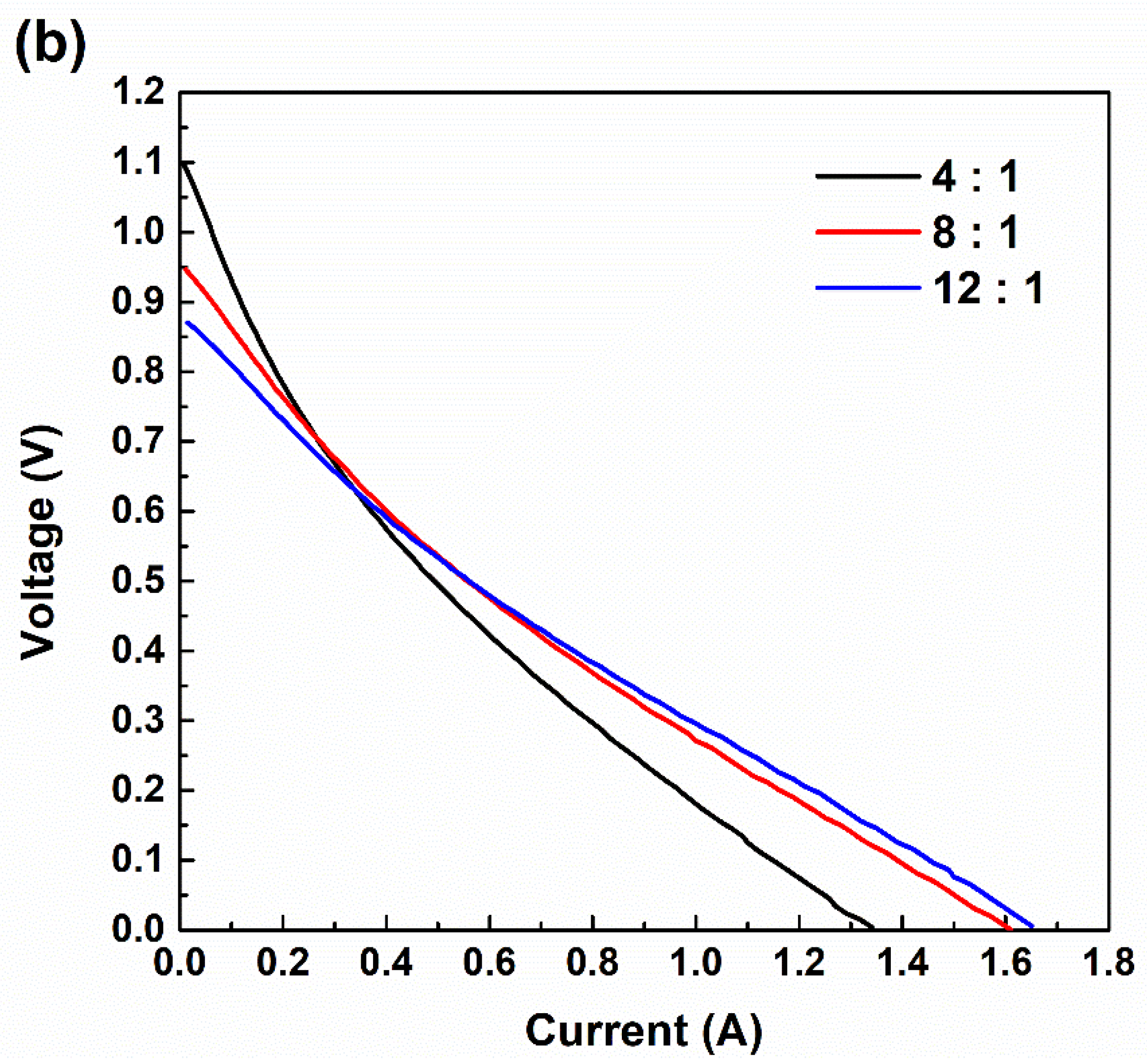
3.3. Current–Voltage (I–V) Curves According to RED Stack Size
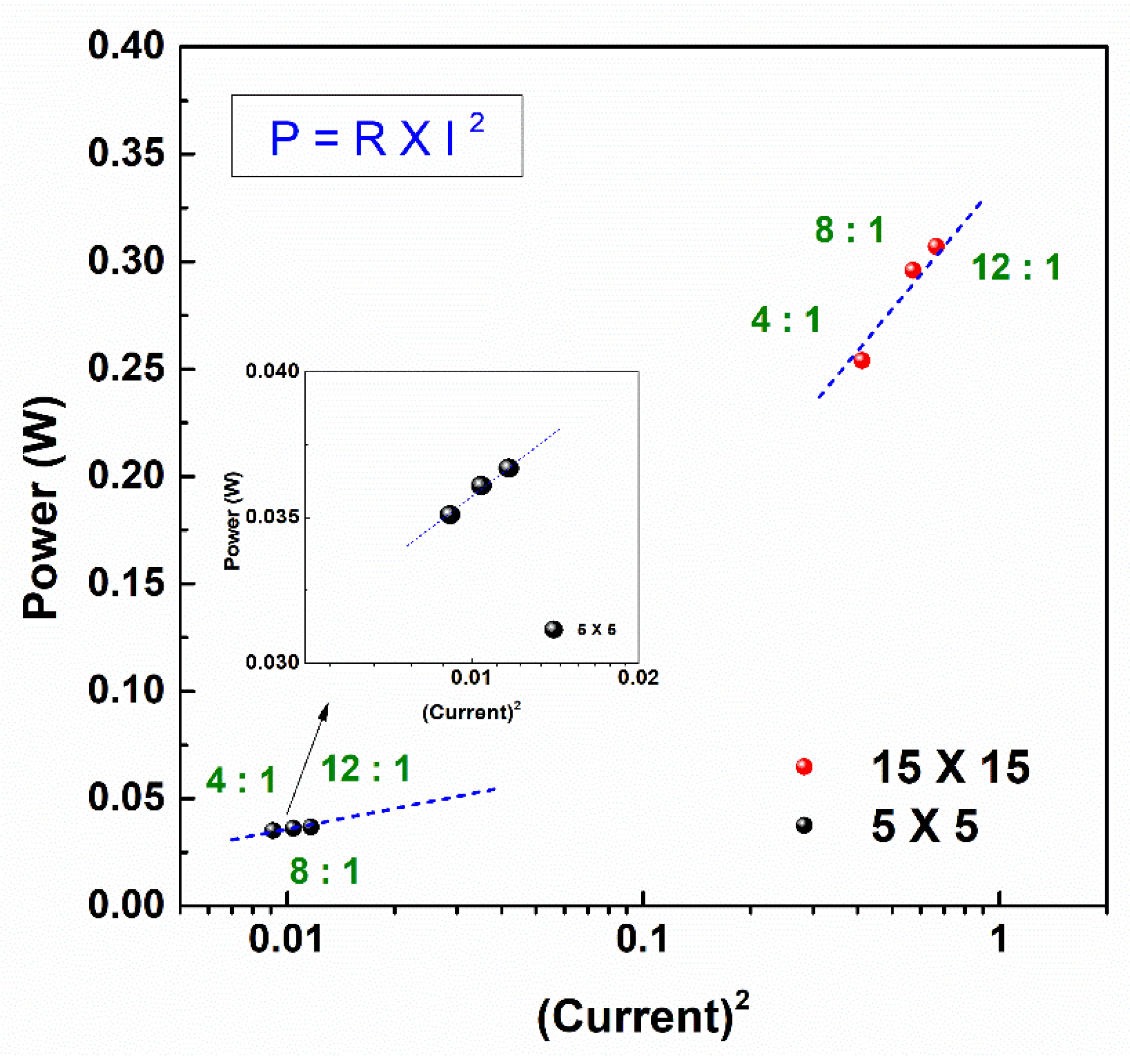
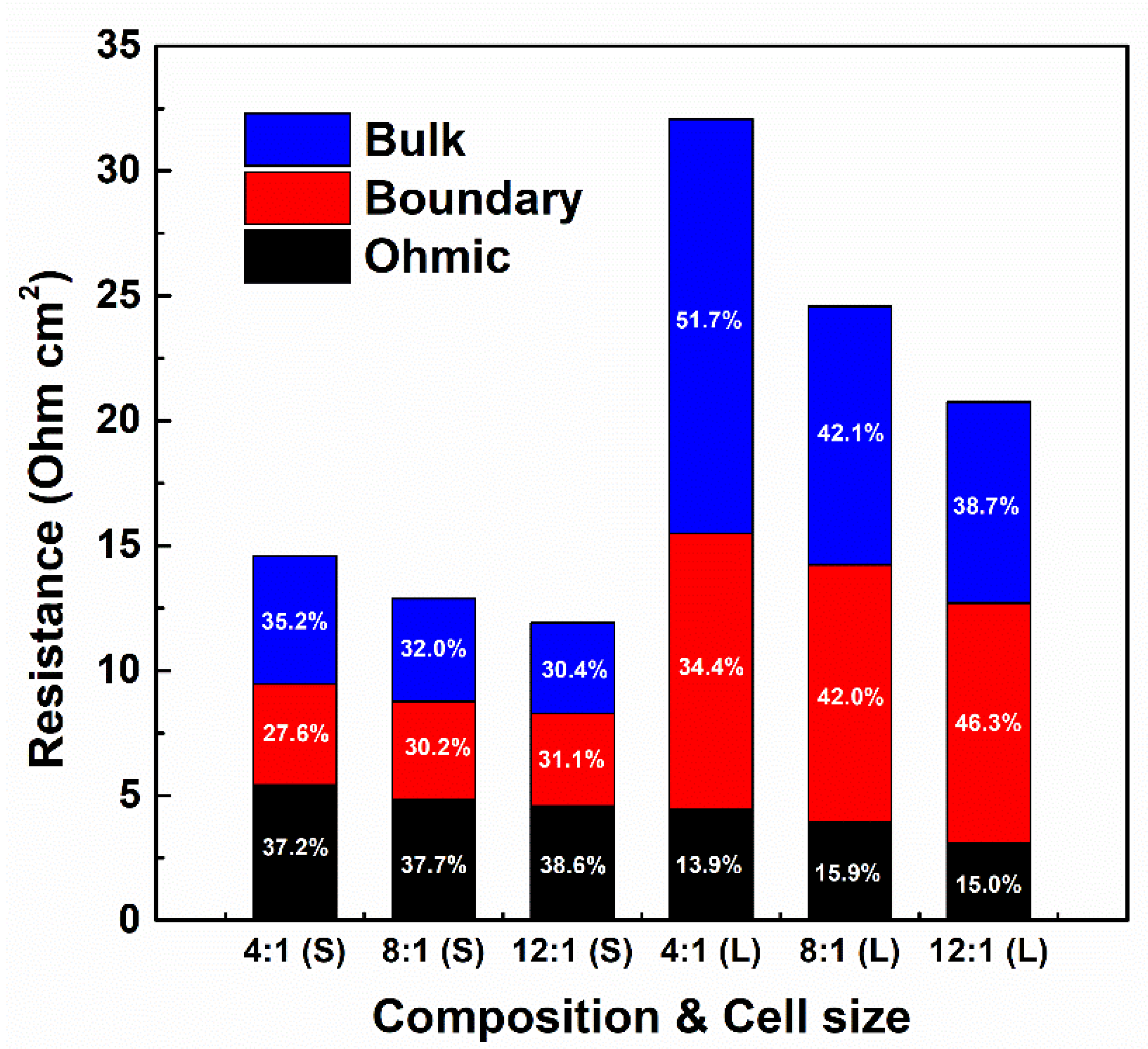
3.4. Ohmic and Non-Ohmic Resistances According to RED Stack Size
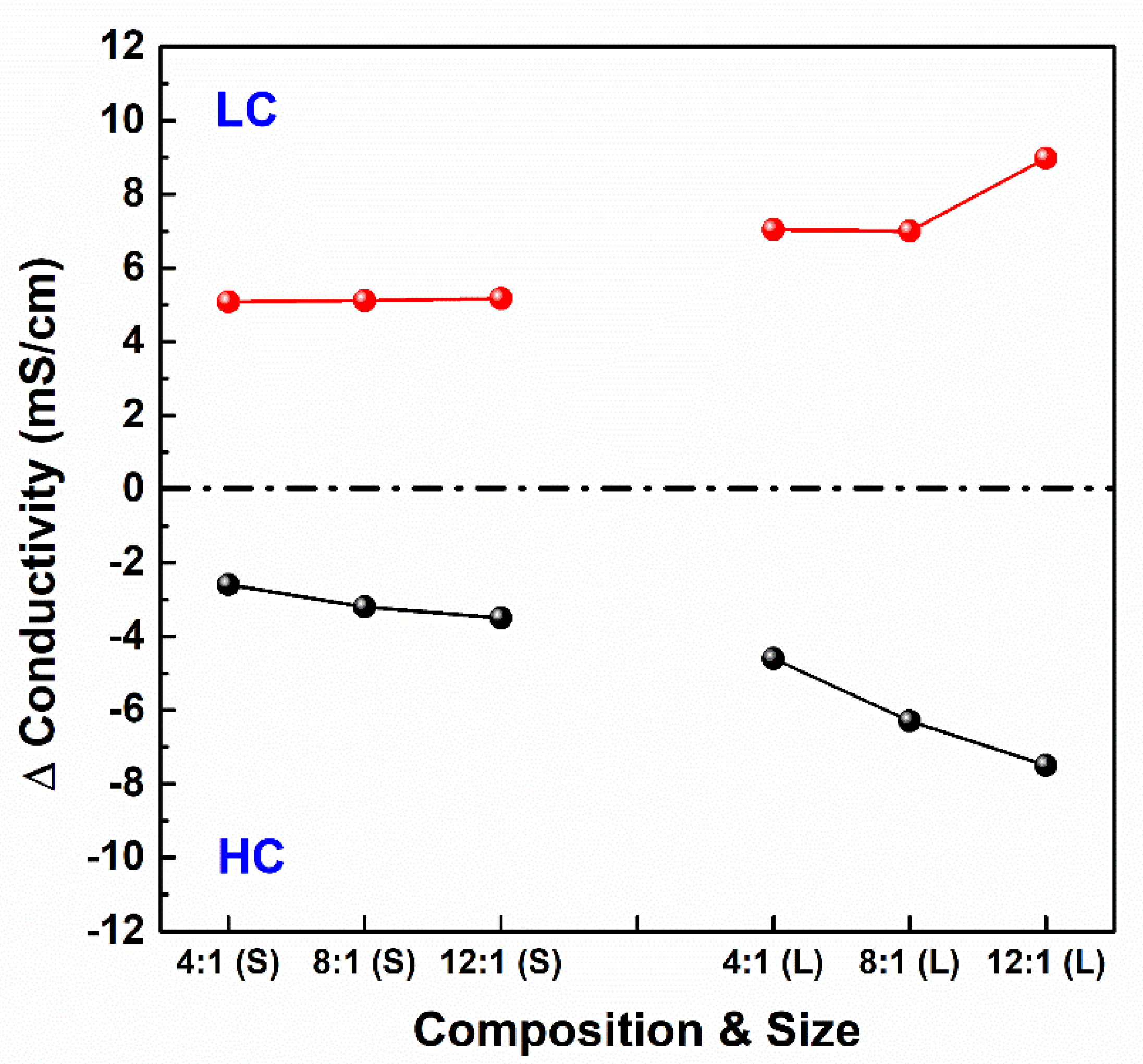
3.5. Conductivities of Influents and Effluents from the RED Stacks
4. Conclusions
- Increasing the electrolyte content in the composition of PIEMs reduced the electrical resistance and significantly affected the power density of the RED stack. The maximum power densities of 1.870 W/m2 and 1.364 W/m2 were achieved for the small and large RED stacks with a 12:1 PIEM composition, respectively.
- Enlarging the stack size significantly affected the power generation with a significant contribution of non-ohmic resistance to the internal resistance of the RED stack.
- The PIEM composition significantly affected the non-ohmic resistance of the RED stack. In the large RED stack, the bulk layer resistance contributed to 51.7% of the internal resistance of the RED stack with a 4:1 PIEM composition.
- The variations in non-ohmic resistances were attributed to the ion transport rate across the PIEMs with a salinity gradient reduction by enlarging stack size and ion transport enhancement by lowering electrical resistance according to PIEMs as major factors.
- Permselectivity was less sensitive to RED performance than electrical resistance when exceeding 90%.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Logan, B.E.; Elimelech, M. Membrane-based processes for sustainable power generation using water. Nature 2012, 488, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Yip, N.Y.; Brogioli, D.; Hamelers, H.V.; Nijmeijer, K. Salinity Gradients for Sustainable Energy: Primer, Progress, and Prospects. Environ. Sci. Technol. 2016, 50, 12072–12094. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Wang, B.; Song, S.; Fan, Y. Blue energy: Current technologies for sustainable power generation from water salinity gradient. Renew. Sustain. Energy Rev. 2014, 31, 91–100. [Google Scholar] [CrossRef]
- Mei, Y.; Tang, C.Y. Recent developments and future perspectives of reverse electrodialysis technology: A review. Desalination 2018, 425, 156–174. [Google Scholar] [CrossRef]
- Hong, J.G.; Zhang, B.; Glabman, S.; Uzal, N.; Dou, X.; Zhang, H.; Wei, X.; Chen, Y. Potential ion exchange membranes and system performance in reverse electrodialysis for power generation: A review. J. Membr. Sci. 2015, 486, 71–88. [Google Scholar] [CrossRef]
- Ortiz-Imedio, R.; Gomez-Coma, L.; Fallanza, M.; Ortiz, A.; Ibañez, R.; Ortiz, I. Comparative performance of Salinity Gradient Power-Reverse Electrodialysis under different operating conditions. Desalination 2019, 457, 8–21. [Google Scholar] [CrossRef]
- Vermaas, D.A.; Kunteng, D.; Veerman, J.; Saakes, M.; Nijmeijer, K. Periodic feedwater reversal and air sparging as antifouling strategies in reverse electrodialysis. Environ. Sci. Technol. 2014, 48, 3065–3073. [Google Scholar] [CrossRef]
- Nam, J.Y.; Hwang, K.S.; Kim, H.C.; Jeong, H.; Kim, H.; Jwa, E.; Yang, S.; Choi, J.; Kim, C.S.; Han, J.H.; et al. Assessing the behavior of the feed-water constituents of a pilot-scale 1000-cell-pair reverse electrodialysis with seawater and municipal wastewater effluent. Water Res. 2019, 148, 261–271. [Google Scholar] [CrossRef]
- Post, J.W.; Veerman, J.; Hamelers, H.V.M.; Euverink, G.J.W.; Metz, S.J.; Nymeijer, K.; Buisman, C.J.N. Salinity-gradient power: Evaluation of pressure-retardedosmosis and reverse electrodialysis. J. Membr. Sci. 2007, 288, 218–230. [Google Scholar] [CrossRef] [Green Version]
- Jwa, E.; Yun, Y.-M.; Kim, H.; Jeong, N.; Hwang, K.S.; Yang, S.C.; Nam, J.-Y. Energy-efficient seawater softening and power generation using a microbial electrolysis cell-reverse electrodialysis hybrid system. Chem. Eng. J. 2020, 391, 123480. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, C. Cost-effective iron-based aqueous redox flow batteries for large-scale energy storage application: A review. J. Power Sources 2021, 493, 229445. [Google Scholar] [CrossRef]
- Jang, J.; Kang, Y.; Han, J.-H.; Jang, K.; Kim, C.-M.; Kim, I.S. Developments and future prospects of reverse electrodialysis for salinity gradient power generation: Influence of ion exchange membranes and electrodes. Desalination 2020, 491, 114540. [Google Scholar] [CrossRef]
- Kim, H.-K.; Lee, M.-S.; Lee, S.-Y.; Choi, Y.-W.; Jeong, N.-J.; Kim, C.-S. High power density of reverse electrodialysis with pore-filling ion exchange membranes and a high-open-area spacer. J. Mater. Chem. A 2015, 3, 16302–16306. [Google Scholar] [CrossRef] [Green Version]
- Geise, G.M.; Hickner, M.A.; Logan, B.E. Ionic resistance and permselectivity tradeoffs in anion exchange membranes. ACS Appl. Mater. Interfaces 2013, 5, 10294–10301. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Yip, N.Y. Elucidating conductivity-permselectivity tradeoffs in electrodialysis and reverse electrodialysis by structure-property analysis of ion-exchange membranes. J. Membr. Sci. 2019, 573, 668–681. [Google Scholar] [CrossRef]
- Merino-Garcia, I.; Kotoka, F.; Portugal, C.A.; Crespo, J.G.; Velizarov, S. Characterization of poly (Acrylic) acid-modified heterogenous anion exchange membranes with improved monovalent permselectivity for RED. Membranes 2020, 10, 134. [Google Scholar] [CrossRef]
- Guler, E.; Zhang, Y.; Saakes, M.; Nijmeijer, K. Tailor-made anion-exchange membranes for salinity gradient power generation using reverse electrodialysis. ChemSusChem 2012, 5, 2262–2270. [Google Scholar] [CrossRef]
- Hong, J.G.; Chen, Y. Nanocomposite reverse electrodialysis (RED) ion-exchange membranes for salinity gradient power generation. J. Membr. Sci. 2014, 460, 139–147. [Google Scholar] [CrossRef]
- Tufa, R.A.; Piallat, T.; Hnát, J.; Fontananova, E.; Paidar, M.; Chanda, D.; Curcio, E.; di Profio, G.; Bouzek, K. Salinity gradient power reverse electrodialysis: Cation exchange membrane design based on polypyrrole-chitosan composites for enhanced monovalent selectivity. Chem. Eng. J. 2020, 380, 122461. [Google Scholar] [CrossRef]
- Güler, E.; Elizen, R.; Vermaas, D.A.; Saakes, M.; Nijmeijer, K. Performance-determining membrane properties in reverse electrodialysis. J. Membr. Sci. 2013, 446, 266–276. [Google Scholar] [CrossRef]
- Güler, E.; van Baak, W.; Saakes, M.; Nijmeijer, K. Monovalent-ion-selective membranes for reverse electrodialysis. J. Membr. Sci. 2014, 455, 254–270. [Google Scholar] [CrossRef]
- Hong, J.G.; Chen, Y. Evaluation of electrochemical properties and reverse electrodialysis performance for porous cation exchange membranes with sulfate-functionalized iron oxide. J. Membr. Sci. 2015, 473, 210–217. [Google Scholar] [CrossRef]
- Lee, Y.J.; Cha, M.S.; Oh, S.-G.; So, S.; Kim, T.-H.; Ryoo, W.S.; Hong, Y.T.; Lee, J.Y. Reinforced anion exchange membrane based on thermal cross-linking method with outstanding cell performance for reverse electrodialysis. RSC Adv. 2019, 9, 27500–27509. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Choi, Y.-W.; Choi, J.; Jeong, N.; Kim, H.; Jeong, H.; Byeon, S.Y.; Yoon, H.; Kim, Y.H. Green fabrication of pore-filling anion exchange membranes using R2R processing. J. Membr. Sci. 2019, 584, 181–190. [Google Scholar] [CrossRef]
- Yang, S.; Choi, Y.-W.; Choi, J.; Jeong, N.; Kim, H.; Nam, J.-Y.; Jeong, H. R2R Fabrication of pore-filling cation-exchange membranes via one-time impregnation and their application in reverse electrodialysis. ACS Sustain. Chem. Eng. 2019, 7, 12200–12213. [Google Scholar] [CrossRef]
- Moreno, J.; Grasman, S.; van Engelen, R.; Nijmeijer, K. Upscaling Reverse Electrodialysis. Environ. Sci. Technol. 2018, 52, 10856–10863. [Google Scholar] [CrossRef] [Green Version]
- Tedesco, M.; Scalici, C.; Vaccari, D.; Cipollina, A.; Tamburini, A.; Micale, G. Performance of the first reverse electrodialysis pilot plant for power production from saline waters and concentrated brines. J. Membr. Sci. 2016, 500, 33–45. [Google Scholar] [CrossRef] [Green Version]
- Han, J.-H.; Jeong, H.; Hwang, K.S.; Kim, C.-S.; Jeong, N.; Yang, S. Asymmetrical electrode system for stable operation of a large-scale reverse electrodialysis (RED) system. Environ. Sci. Wat. Res. Technol. 2020, 6, 1597–1605. [Google Scholar] [CrossRef]
- Veerman, J.; Saakes, M.; Metz, S.J.; Harmsen, G.J. Electrical power from sea and river water by reverse electrodialysis: A first step from the laboratory to a real power plant. Environ. Sci. Technol. 2010, 44, 9207–9212. [Google Scholar] [CrossRef]
- Tedesco, M.; Cipollina, A.; Tamburini, A.; Micale, G. Towards 1 kW power production in a reverse electrodialysis pilot plant with saline waters and concentrated brines. J. Membr. Sci. 2017, 522, 226–236. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.; Yang, S.; Jeong, N.-J.; Kim, H.; Kim, W.-S. Fabrication of an Anion-Exchange Membrane by Pore-Filling Using Catechol–1,4-Diazabicyclo-[2,2,2]octane Coating and Its Application to Reverse Electrodialysis. Langmuir 2018, 34, 10837–10846. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Kim, W.-S.; Choi, J.; Choi, Y.-W.; Jeong, N.; Kim, H.; Nam, J.-Y.; Jeong, H.; Kim, Y.H. Fabrication of photocured anion-exchange membranes using water-soluble siloxane resins as cross-linking agents and their application in reverse electrodialysis. J. Membr. Sci. 2019, 573, 544–553. [Google Scholar] [CrossRef]
- Lee, M.-S.; Kim, H.-K.; Kim, C.-S.; Suh, H.-Y.; Nahm, K.-S.; Choi, Y.-W. Thin Pore-Filled Ion Exchange Membranes for High Power Density in Reverse Electrodialysis: Effects of Structure on Resistance, Stability, and Ion Selectivity. ChemistrySelect 2017, 2, 1974–1978. [Google Scholar] [CrossRef]
- Vermaas, D.A.; Saakes, M.; Nijmeijer, K. Doubled power density from salinity gradients at reduced intermembrane distance. Environ. Sci. Technol. 2011, 45, 7089–7095. [Google Scholar] [CrossRef]
- Vermaas, D.A.; Veerman, J.; Yip, N.Y.; Elimelech, M.; Saakes, M.; Nijmeijer, K. High Efficiency in Energy Generation from Salinity Gradients with Reverse Electrodialysis. ACS Sustain. Chem. Eng. 2013, 1, 1295–1302. [Google Scholar] [CrossRef]
- Pawlowski, S.; Crespo, J.G.; Velizarov, S. Pressure drop in reverse electrodialysis: Experimental and modeling studies for stacks with variable number of cell pairs. J. Membr. Sci. 2014, 462, 96–111. [Google Scholar] [CrossRef]
- Long, R.; Li, B.; Liu, Z.; Liu, W. Reverse electrodialysis: Modelling and performance analysis based on multi-objective optimization. Energy 2018, 151, 1–10. [Google Scholar] [CrossRef]
- Moya, A. A Nernst-Planck analysis on the contributions of the ionic transport in permeable ion-exchange membranes to the open circuit voltage and the membrane resistance in reverse electrodialysis stacks. Electrochim. Acta 2017, 238, 134–141. [Google Scholar] [CrossRef]
- Tedesco, M.; Hamelers, H.; Biesheuvel, P. Nernst-Planck transport theory for (reverse) electrodialysis: I. Effect of co-ion transport through the membranes. J. Membr. Sci. 2016, 510, 370–381. [Google Scholar] [CrossRef]
- Tedesco, M.; Hamelers, H.; Biesheuvel, P. Nernst-Planck transport theory for (reverse) electrodialysis: III. Optimal membrane thickness for enhanced process performance. J. Membr. Sci. 2018, 565, 480–487. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.-S.; Kim, T.; Park, S.-H.; Kim, C.-S.; Choi, Y.-W. A highly durable cross-linked hydroxide ion conducting pore-filling membrane. J. Mater. Chem. 2012, 22, 13928–13931. [Google Scholar] [CrossRef]
- Kang, H.-G.; Lee, M.-S.; Sim, W.-J.; Yang, T.-H.; Shin, K.-H.; Shul, Y.-G.; Choi, Y.-W. Effect of number of cross-linkable sites on proton conducting, pore-filling membranes. J. Membr. Sci. 2014, 460, 178–184. [Google Scholar] [CrossRef]
- Kim, H.; Jeong, N.; Yang, S.; Choi, J.; Lee, M.-S.; Nam, J.-Y.; Jwa, E.; Kim, B.; Ryu, K.-S.; Choi, Y.-W. Nernst–Planck analysis of reverse-electrodialysis with the thin-composite pore-filling membranes and its upscaling potential. Water Res. 2019, 165, 114970. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Martínez, V.M.; Gómez-Coma, L.; Tristán, C.; Pérez, G.; Fallanza, M.; Ortiz, A.; Ibáñez, R.; Ortiz, I. A comprehensive study on the effects of operation variables on reverse electrodialysis performance. Desalination 2020, 482, 114389. [Google Scholar] [CrossRef]

| Notation | Composition (Weight Ratio) | Thickness (μm) | IEC (meq/g) | Permselectivity (%) | Resistance (Ω·cm2) | |||
|---|---|---|---|---|---|---|---|---|
| AMPS–Na + AMPS | ATAC | PDA | ||||||
| Fujifilm Type-1 CEM [25] | - | - | - | 135 | 1.83 | 97.4 | 2.10 | |
| PCEM | 4:1 | 4 | - | 1 | 16 | 1.54 | 99.9 | 0.75 |
| 8:1 | 8 | - | 1 | 1.67 | 98.7 | 0.49 | ||
| 12:1 | 12 | - | 1 | 1.85 | 93.7 | 0.37 | ||
| Fujifilm Type-1 AEM [25] | - | - | - | 125 | 1.84 | 93.8 | 1.22 | |
| PAEM | 4:1 | - | 4 | 1 | 16 | 1.50 | 99.5 | 0.77 |
| 8:1 | - | 8 | 1 | 1.64 | 97.1 | 0.48 | ||
| 12:1 | - | 12 | 1 | 1.81 | 93.4 | 0.37 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.; Choi, J.; Jeong, N.; Jung, Y.-G.; Kim, H.; Kim, D.; Yang, S. Correlations between Properties of Pore-Filling Ion Exchange Membranes and Performance of a Reverse Electrodialysis Stack for High Power Density. Membranes 2021, 11, 609. https://doi.org/10.3390/membranes11080609
Kim H, Choi J, Jeong N, Jung Y-G, Kim H, Kim D, Yang S. Correlations between Properties of Pore-Filling Ion Exchange Membranes and Performance of a Reverse Electrodialysis Stack for High Power Density. Membranes. 2021; 11(8):609. https://doi.org/10.3390/membranes11080609
Chicago/Turabian StyleKim, Hanki, Jiyeon Choi, Namjo Jeong, Yeon-Gil Jung, Haeun Kim, Donghyun Kim, and SeungCheol Yang. 2021. "Correlations between Properties of Pore-Filling Ion Exchange Membranes and Performance of a Reverse Electrodialysis Stack for High Power Density" Membranes 11, no. 8: 609. https://doi.org/10.3390/membranes11080609
APA StyleKim, H., Choi, J., Jeong, N., Jung, Y.-G., Kim, H., Kim, D., & Yang, S. (2021). Correlations between Properties of Pore-Filling Ion Exchange Membranes and Performance of a Reverse Electrodialysis Stack for High Power Density. Membranes, 11(8), 609. https://doi.org/10.3390/membranes11080609








