Membrane-Based Harvesting Processes for Microalgae and Their Valuable-Related Molecules: A Review
Abstract
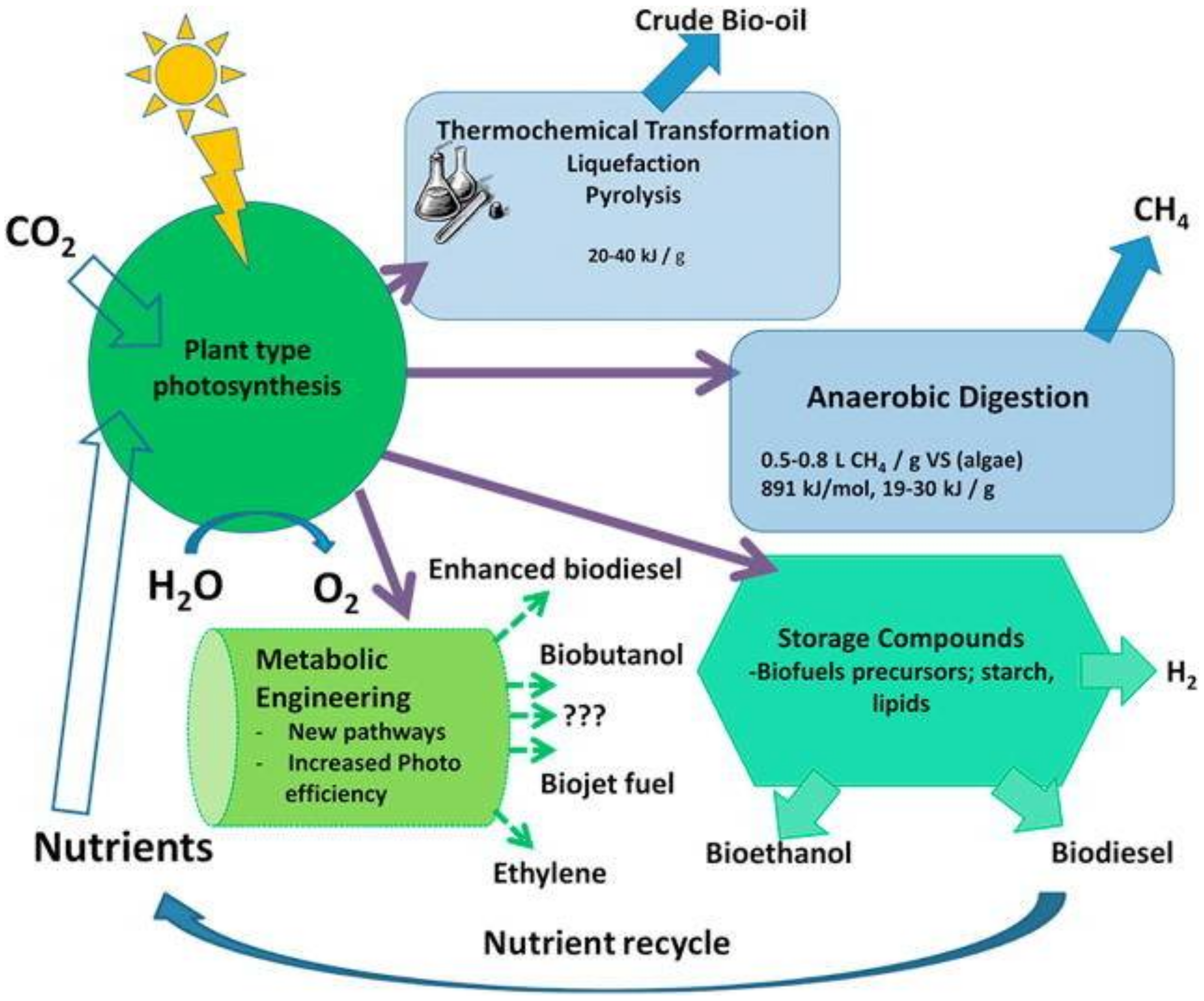
:1. Introduction
2. Principles of Membrane-Based Processes and Factors Influencing Their Performance
- Asymmetry properties of a membrane: In the case of polymer membranes, phase inversion technique is commonly used for the preparation of porous membranes [20]. Such a fabrication protocol often, depending on the type of method and conditions, generates asymmetric properties on the resulting membranes, which means that the membrane does not present a uniform pore size over the membrane structure. Such an asymmetry is a result of handling different parameters, such as exposure time, humidity, polymer concentration, in the preparation protocol. Importantly, an asymmetric structure is the most preferred since it combines high selectivity of small pores in a thin selective layer and high permeability due to low resistance of the support layer.
- Intrinsic properties of a membrane: As it is well known, the membranes, depending on the polymer or inorganic material, may either present hydrophilic or hydrophobic properties. In MF and UF processes, hydrophobic materials are preferred (polyethersulfone, polysulfone, etc.) since they repel water molecules, along with all those water-soluble compounds. In addition to this, the surface morphology influences the separation performance, but more importantly, contributes to some specific issues on the operation; for example, the membrane roughness, especially a rougher membrane surface, contributes to fouling. To some extent, the protuberances on a surface allow the capture of organic matter. Here, if there is an accumulation of organic material that may represent a source of microorganism proliferation, the membranes will be susceptible to biofouling formation as well [21].
- Membrane–molecule interactions: In general, electrostatic interactions may occur between membrane surfaces and specific solutes that present any charge. Of course, the membrane should also reveal any type of charge, which is often associated with the availability of functional groups on the membrane surface. Eventually, specific solute–membrane interactions, such as the hydrophobic interaction, Coulombic intermolecular attraction and repulsion, are among the most identified forces in membrane processes [22].
- Membrane fouling: This factor acts as the main bottleneck of membrane processes since it can lower the flux by pore blocking. The membrane fouling depends crucially on the physicochemical composition of the feed solution to be treated. Here, the possible interactions among the solutes and the membrane can introduce the degree and type of membrane fouling [23]. However, it is worth mentioning that the parameters of the operation may also foster such a phenomenon.
- Operating parameters: The permeate flux is usually increased as a function of the driving force; this is possible until the limiting transmembrane pressure is reached [24], in which after such limiting pressure the permeation becomes governed by the fouling and concentration polarization phenomenon. Similarly, the permeate flux can be raised as a function of temperature increase, which is a result of decreasing the viscosity of the fluid and the increasing diffusion of the components. When dealing with fouling issues, the feed flow rate, temperature and transmembrane pressure are important parameters in the membrane fouling. For instance, the feed flow speed influences the shear forces on the membrane surface; as mentioned previously, this generates the partial removal of solutes from the surfaces and, thus, reducing the fouling formation [25]. On the other hand, the retained molecules on the membrane provoke the pressure increment since the fouling layer acts as an additional barrier.
3. Development Works on Membrane-Aided Harvesting Process for Microalgae
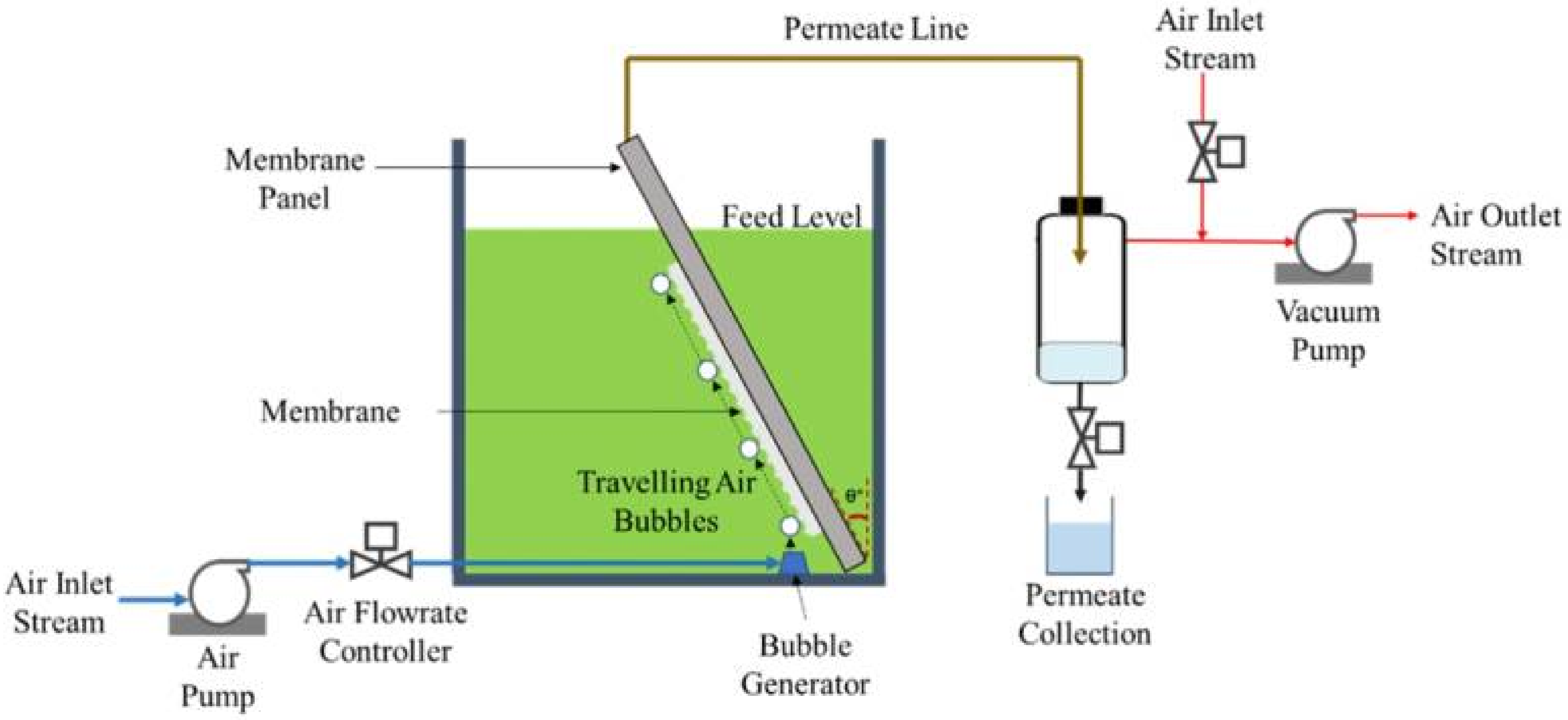
3.1. Air-Assisted Backwashing Technology
3.2. Dynamic Filtration Systems
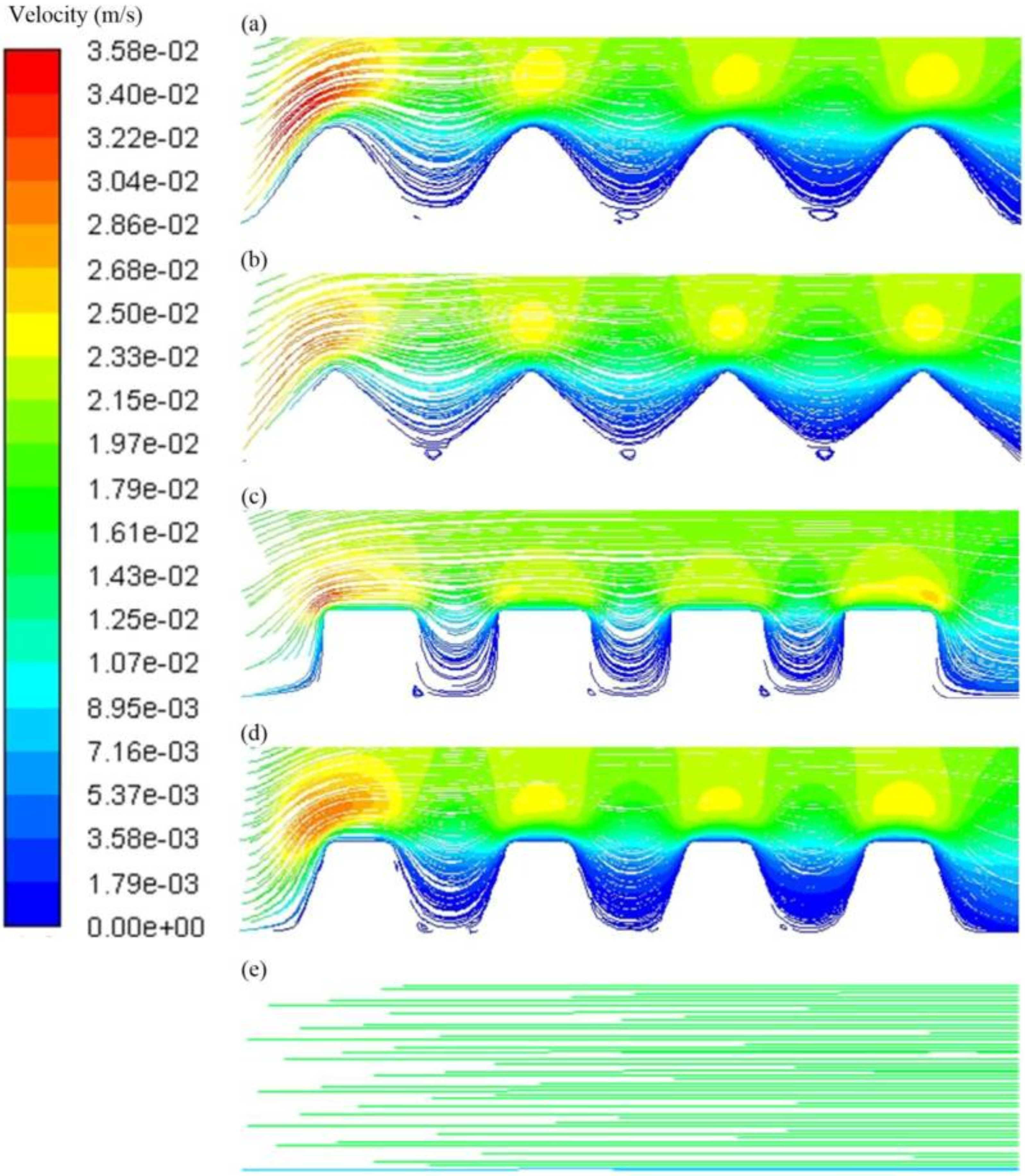
3.3. Membrane Manufacture
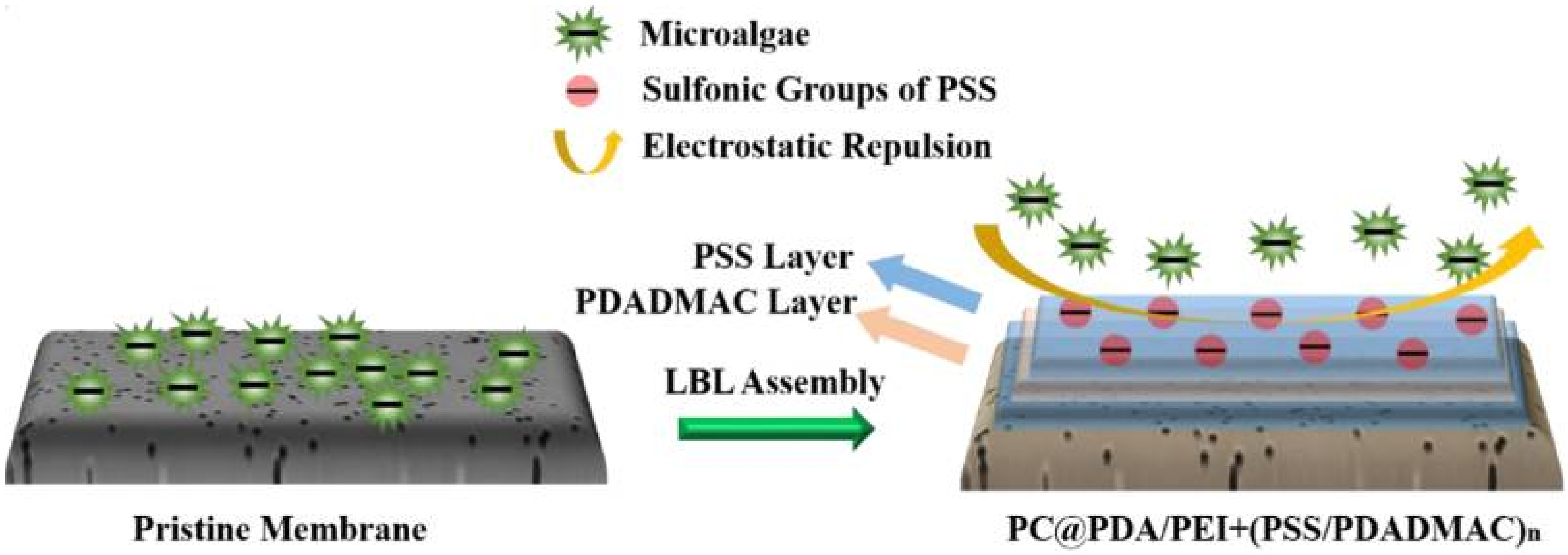
3.4. Emerging Membrane-Based Microalgae Harvesting Technologies
3.5. Pilot-Scale Studies
4. Membrane Technology for the Downstream Processing of Valuable Products Derived from Microalgal Biomass
4.1. Algal Protein Recovery by Membrane Technology
4.2. Application of Membrane Filtration to Recover Algal Exopolysaccharides
4.3. Recovery of Lipids
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhushan, S.; Kalra, A.; Simsek, H.; Kumar, G.; Prajapati, S.K. Current trends and prospects in microalgae-based bioenergy production. J. Environ. Chem. Eng. 2020, 8, 104025. [Google Scholar] [CrossRef]
- Leam, J.J.; Bilad, M.R.; Wibisono, Y.; Wirzal, M.D.H.; Ahmed, I. Membrane Technology for Microalgae Harvesting; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128175361. [Google Scholar]
- Ahmad, A.L.; Yasin, N.H.M.; Derek, C.J.C.; Lim, J.K. Microalgae as a sustainable energy source for biodiesel production: A review. Renew. Sustain. Energy Rev. 2011, 15, 584–593. [Google Scholar] [CrossRef]
- Kang, A.; Lee, T.S. Converting sugars to biofuels: Ethanol and beyond. Bioengineering 2015, 2, 184–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hallenbeck, P.C.; Grogger, M.; Mraz, M.; Veverka, D. Solar biofuels production with microalgae. Appl. Energy 2016, 179, 136–145. [Google Scholar] [CrossRef] [Green Version]
- Morales-Jiménez, M.; Gouveia, L.; Yañez-Fernandez, J.; Castro-Muñoz, J.; Barragan-Huerta, B.E. Production, preparation and characterization of microalgae-based biopolymer as a potential bioactive film. Coatings 2020, 10, 120. [Google Scholar] [CrossRef] [Green Version]
- Borowitzka, M.A. Commercial production of microalgae: Ponds, tanks, and fermenters. Prog. Ind. Microbiol. 1999, 35, 313–321. [Google Scholar] [CrossRef]
- Miao, X.; Wu, Q. Biodiesel production from heterotrophic microalgal oil. Bioresour. Technol. 2006, 97, 841–846. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, Y.; Guo, S. Growth and phycocyanin formation of Spirulina platensis in photoheterotrophic culture. Biotechnol. Lett. 1996, 18, 603–608. [Google Scholar] [CrossRef]
- Brennan, L.; Owende, P. Biofuels from microalgae-A review of technologies for production, processing, and extractions of biofuels and co-products. Renew. Sustain. Energy Rev. 2010, 14, 557–577. [Google Scholar] [CrossRef]
- Dębowski, M.; Zieliński, M.; Kazimierowicz, J.; Kujawska, N.; Talbierz, S. Microalgae cultivation technologies as an opportunity for bioenergetic system development—advantages and limitations. Sustainability 2020, 12, 9980. [Google Scholar] [CrossRef]
- Sharma, K.K.; Garg, S.; Li, Y.; Malekizadeh, A.; Schenk, P.M. Critical analysis of current microalgae dewatering techniques. Biofuels 2013, 4, 397–407. [Google Scholar] [CrossRef]
- Danquah, M.K.; Gladman, B.; Moheimani, N.; Forde, G.M. Microalgal growth characteristics and subsequent influence on dewatering efficiency. Chem. Eng. J. 2009, 151, 73–78. [Google Scholar] [CrossRef]
- Chen, C.Y.; Yeh, K.L.; Aisyah, R.; Lee, D.J.; Chang, J.S. Cultivation, photobioreactor design and harvesting of microalgae for biodiesel production: A critical review. Bioresour. Technol. 2011, 102, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Castro-Muñoz, R.; Yáñez-Fernández, J.; Fíla, V. Phenolic compounds recovered from agro-food by-products using membrane technologies: An overview. Food Chem. 2016, 213, 753–762. [Google Scholar] [CrossRef]
- Cassano, A.; Conidi, C.; Figueroa, R.R.; Muñoz, R.C. A two-step nanofiltration process for the production of phenolic-rich fractions from artichoke aqueous extracts. Int. J. Mol. Sci. 2015, 16, 8968–8987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castro-Muñoz, R.; Fíla, V. Membrane-based technologies as an emerging tool for separating high- added-value compounds from natural products. Trends Food Sci. Technol. 2018, 82, 8–20. [Google Scholar] [CrossRef]
- Bilad, M.R.; Arafat, H.A.; Vankelecom, I.F.J. Membrane technology in microalgae cultivation and harvesting: A review. Biotechnol. Adv. 2014, 32, 1283–1300. [Google Scholar] [CrossRef]
- Díaz-Montes, E.; Castro-Muñoz, R. Metabolites recovery from fermentation broths via pressure-driven membrane processes. Asia-Pacific J. Chem. Eng. 2019, e2332. [Google Scholar] [CrossRef]
- Russo, F.; Castro-Muñoz, R.; Galiano, F.; Figoli, A. Unprecedented preparation of porous Matrimid® 5218 membranes. J. Memb. Sci. 2019, 585, 166–174. [Google Scholar] [CrossRef]
- Pichardo-Romero, D.; Garcia-Arce, Z.P.; Zavala-Ramirez, A.; Castro-Muñoz, R. Current advances in biofouling mitigation in membranes for water treatment: An overview. Processes 2020, 8, 182. [Google Scholar] [CrossRef] [Green Version]
- Ali, J.; Wang, L.; Waseem, H.; Sharif, H.M.A.; Djellabi, R.; Zhang, C.; Pan, G. Bioelectrochemical recovery of silver from wastewater with sustainable power generation and its reuse for biofouling mitigation. J. Clean. Prod. 2019, 235, 1425–1437. [Google Scholar] [CrossRef]
- Susanto, H.; Feng, Y.; Ulbricht, M. Fouling behavior of aqueous solutions of polyphenolic compounds during ultrafiltration. J. Food Eng. 2009, 91, 333–340. [Google Scholar] [CrossRef]
- Cassano, A.; Conidi, C.; Timpone, R.; D’Avella, M.; Drioli, E. A membrane-based process for the clarification and the concentration of the cactus pear juice. J. Food Eng. 2007, 80, 914–921. [Google Scholar] [CrossRef]
- Ergön-Can, T.; Köse-Mutlu, B.; Koyuncu, İ.; Lee, C.H. Biofouling control based on bacterial quorum quenching with a new application: Rotary microbial carrier frame. J. Memb. Sci. 2017, 525, 116–124. [Google Scholar] [CrossRef]
- Unay, E.; Ozkaya, B.; Yoruklu, H.C. A multicriteria decision analysis for the evaluation of microalgal growth and harvesting. Chemosphere 2021, 279, 130561. [Google Scholar] [CrossRef]
- Singh, G.; Patidar, S.K. Microalgae harvesting techniques: A review. J. Environ. Manag. 2018, 217, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Pan, B.; Gao, Y.; Li, C.; Ye, J.; Yang, L.; Chen, Y.; Hu, Q.; Zhang, X. Efficient membrane microalgal harvesting: Pilot-scale performance and techno-economic analysis. J. Clean. Prod. 2019, 218, 83–95. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, W.; Zhang, X.; Amendola, P.; Hu, Q.; Chen, Y. Characterization of dissolved organic matters responsible for ultrafiltration membrane fouling in algal harvesting. Algal Res. 2013, 2, 223–229. [Google Scholar] [CrossRef]
- Zhao, F.; Chu, H.; Yu, Z.; Jiang, S.; Zhao, X.; Zhou, X.; Zhang, Y. The filtration and fouling performance of membranes with different pore sizes in algae harvesting. Sci. Total Environ. 2017, 587–588, 87–93. [Google Scholar] [CrossRef]
- Zhao, Z.; Mertens, M.; Li, Y.; Muylaert, K.; Vankelecom, I.F.J. A highly efficient and energy-saving magnetically induced membrane vibration system for harvesting microalgae. Bioresour. Technol. 2020, 300, 122688. [Google Scholar] [CrossRef]
- Khairuddin, N.F.M.; Idris, A.; Hock, L.W. Harvesting Nannochloropsis sp. using PES/MWCNT/LiBr membrane with good antifouling properties. Sep. Purif. Technol. 2019, 212, 1–11. [Google Scholar] [CrossRef]
- Park, K.; Kim, P.; Kim, H.G.; Kim, J.H. Membrane fouling mechanisms in combined microfiltration-coagulation of algal rich water applying ceramic membranes. Membranes 2019, 9, 33. [Google Scholar] [CrossRef] [Green Version]
- Monte, J.; Sá, M.; Galinha, C.F.; Costa, L.; Hoekstra, H.; Brazinha, C.; Crespo, J.G. Harvesting of Dunaliella salina by membrane filtration at pilot scale. Sep. Purif. Technol. 2018, 190, 252–260. [Google Scholar] [CrossRef]
- De Baerdemaeker, T.; Lemmens, B.; Dotremont, C.; Fret, J.; Roef, L.; Goiris, K.; Diels, L. Benchmark study on algae harvesting with backwashable submerged flat panel membranes. Bioresour. Technol. 2013, 129, 582–591. [Google Scholar] [CrossRef] [Green Version]
- Roy, M.; Mohanty, K. A comprehensive review on microalgal harvesting strategies: Current status and future prospects. Algal Res. 2019, 44, 101683. [Google Scholar] [CrossRef]
- Fasaei, F.; Bitter, J.H.; Slegers, P.M.; van Boxtel, A.J.B. Techno-economic evaluation of microalgae harvesting and dewatering systems. Algal Res. 2018, 31, 347–362. [Google Scholar] [CrossRef]
- Xu, M.; Wang, X.; Zhou, B.; Zhou, L. Pre-coagulation with cationic flocculant-composited titanium xerogel coagulant for alleviating subsequent ultrafiltration membrane fouling by algae-related pollutants. J. Hazard. Mater. 2021, 407, 124838. [Google Scholar] [CrossRef] [PubMed]
- Marbelia, L.; Mulier, M.; Vandamme, D.; Muylaert, K.; Szymczyk, A.; Vankelecom, I.F.J. Polyacrylonitrile membranes for microalgae filtration: Influence of porosity, surface charge and microalgae species on membrane fouling. Algal Res. 2016, 19, 128–137. [Google Scholar] [CrossRef]
- Shekhar, M.; Shriwastav, A.; Bose, P.; Hameed, S. Microfiltration of algae: Impact of algal species, backwashing mode and duration of filtration cycle. Algal Res. 2017, 23, 104–112. [Google Scholar] [CrossRef]
- Chu, H.; Zhao, F.; Tan, X.; Yang, L.; Zhou, X.; Zhao, J.; Zhang, Y. The impact of temperature on membrane fouling in algae harvesting. Algal Res. 2016, 16, 458–464. [Google Scholar] [CrossRef]
- Laksono, S.; ElSherbiny, I.M.A.; Huber, S.A.; Panglisch, S. Fouling scenarios in hollow fiber membranes during mini-plant filtration tests and correlation to microalgae-loaded feed characteristics. Chem. Eng. J. 2020, 127723. [Google Scholar] [CrossRef]
- Das, P.; Thaher, M.; Khan, S.; AbdulQuadir, M.; Al-Jabri, H. The effect of culture salinity on the harvesting of microalgae biomass using pilot-scale tangential-flow-filter membrane. Bioresour. Technol. 2019, 293, 122057. [Google Scholar] [CrossRef]
- Eliseus, A.; Bilad, M.R.; Nordin, N.A.H.M.; Putra, Z.A.; Wirzal, M.D.H. Tilted membrane panel: A new module concept to maximize the impact of air bubbles for membrane fouling control in microalgae harvesting. Bioresour. Technol. 2017, 241, 661–668. [Google Scholar] [CrossRef]
- Kim, K.; Jung, J.Y.; Kwon, J.H.; Yang, J.W. Dynamic microfiltration with a perforated disk for effective harvesting of microalgae. J. Memb. Sci. 2015, 475, 252–258. [Google Scholar] [CrossRef]
- Zhao, F.; Chu, H.; Su, Y.; Tan, X.; Zhang, Y.; Yang, L.; Zhou, X. Microalgae harvesting by an axial vibration membrane: The mechanism of mitigating membrane fouling. J. Memb. Sci. 2016, 508, 127–135. [Google Scholar] [CrossRef]
- Zhao, F.; Zhang, Y.; Chu, H.; Jiang, S.; Yu, Z.; Wang, M.; Zhou, X.; Zhao, J. A uniform shearing vibration membrane system reducing membrane fouling in algae harvesting. J. Clean. Prod. 2018, 196, 1026–1033. [Google Scholar] [CrossRef]
- Discart, V.; Bilad, M.R.; Moorkens, R.; Arafat, H.; Vankelecom, I.F.J. Decreasing membrane fouling during Chlorella vulgaris broth filtration via membrane development and coagulant assisted filtration. Algal Res. 2015, 9, 55–64. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, Y.; Muylaert, K.; Vankelecom, I.F.J. Synergy between membrane filtration and flocculation for harvesting microalgae. Sep. Purif. Technol. 2020, 240, 116603. [Google Scholar] [CrossRef]
- Zhao, Z.; Cuellar Bermudez, S.; Ilyas, A.; Muylaert, K.; Vankelecom, I.F.J. Optimization of negatively charged polysulfone membranes for concentration and purification of extracellular polysaccharides from Arthrospira platensis using the response surface methodology. Sep. Purif. Technol. 2020, 252, 117385. [Google Scholar] [CrossRef]
- Nawi, N.I.M.; Halim, N.S.A.; Lee, L.C.; Wirzal, M.D.H.; Bilad, M.R.; Nordin, N.A.H.; Putra, Z.A. Improved nylon 6,6 nanofiber membrane in a tilted panel filtration system for fouling control in microalgae harvesting. Polymers 2020, 12, 252. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Ilyas, A.; Muylaert, K.; Vankelecom, I.F.J. Optimization of patterned polysulfone membranes for microalgae harvesting. Bioresour. Technol. 2020, 309, 123367. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, H.; Gao, T.; Teng, J. Impacts of applied voltage on forward osmosis process harvesting microalgae: Filtration behaviors and lipid extraction efficiency. Sci. Total Environ. 2021, 773, 145678. [Google Scholar] [CrossRef]
- Kim, D.; Kwak, M.; Kim, K.; Chang, Y.K. Turbulent jet-assisted microfiltration for energy efficient harvesting of microalgae. J. Memb. Sci. 2019, 575, 170–178. [Google Scholar] [CrossRef]
- Ismail, I.; Kurnia, K.A.; Samsuri, S.; Bilad, M.R.; Marbelia, L.; Ismail, N.M.; Khan, A.L.; Budiman, A.; Susilawati, S. Energy efficient harvesting of Spirulina sp. from the growth medium using a tilted panel membrane filtration. Bioresour. Technol. Reports 2021, 15, 100697. [Google Scholar] [CrossRef]
- Lau, A.K.S.; Bilad, M.R.; Nordin, N.A.H.M.; Faungnawakij, K.; Narkkun, T.; Wang, D.K.; Mahlia, T.M.I.; Jaafar, J. Effect of membrane properties on tilted panel performance of microalgae biomass filtration for biofuel feedstock. Renew. Sustain. Energy Rev. 2020, 120, 109666. [Google Scholar] [CrossRef]
- Hwang, T.; Oh, Y.K.; Kim, B.; Han, J.I. Dramatic improvement of membrane performance for microalgae harvesting with a simple bubble-generator plate. Bioresour. Technol. 2015, 186, 343–347. [Google Scholar] [CrossRef]
- Razak, N.N.A.N.; Rahmawati, R.; Bilad, M.R.; Pratiwi, A.E.; Elma, M.; Nawi, N.I.M.; Jaafar, J.; Lam, M.K. Finned spacer for enhancing the impact of air bubbles for membrane fouling control in Chlorella vulgaris filtration. Bioresour. Technol. Reports 2020, 11, 100429. [Google Scholar] [CrossRef]
- Yang, L.; Wang, L.; Ren, S.; Pan, B.; Li, J.; Zhang, X.; Chen, Y.; Hu, Q. Harvesting of Scenedesmus acuminatus using ultrafiltration membranes operated in alternative feed directions. J. Biosci. Bioeng. 2019, 128, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Mo, W.; Soh, L.; Werber, J.R.; Elimelech, M.; Zimmerman, J.B. Application of membrane dewatering for algal biofuel. Algal Res. 2015, 11, 1–12. [Google Scholar] [CrossRef]
- Nurra, C.; Clavero, E.; Salvadó, J.; Torras, C. Vibrating membrane filtration as improved technology for microalgae dewatering. Bioresour. Technol. 2014, 157, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Hapońska, M.; Clavero, E.; Salvadó, J.; Torras, C. Application of ABS membranes in dynamic filtration for Chlorella sorokiniana dewatering. Biomass Bioenergy 2018, 111, 224–231. [Google Scholar] [CrossRef]
- Bilad, M.R.; Discart, V.; Vandamme, D.; Foubert, I.; Muylaert, K.; Vankelecom, I.F.J. Harvesting microalgal biomass using a magnetically induced membrane vibration (MMV) system: Filtration performance and energy consumption. Bioresour. Technol. 2013, 138, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Chu, H.; Tan, X.; Zhang, Y.; Yang, L.; Zhou, X.; Zhao, J. Comparison of axial vibration membrane and submerged aeration membrane in microalgae harvesting. Bioresour. Technol. 2016, 208, 178–183. [Google Scholar] [CrossRef]
- Zhao, F.; Li, Z.; Han, X.; Zhou, X.; Zhang, Y.; Jiang, S.; Yu, Z.; Zhou, X.; Liu, C.; Chu, H. The interaction between microalgae and membrane surface in filtration by uniform shearing vibration membrane. Algal Res. 2020, 50, 102012. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Xu, R.; Ma, C.; Wang, X.; Fu, Q. Algal fouling control in a hollow fiber module during ultrafiltration by angular vibrations. J. Memb. Sci. 2019, 569, 200–208. [Google Scholar] [CrossRef]
- Cho, H.; Mushtaq, A.; Hwang, T.; Kim, H.S.; Han, J.I. Orifice-based membrane fouling inhibition employing in-situ turbulence for efficient microalgae harvesting. Sep. Purif. Technol. 2020, 251, 117277. [Google Scholar] [CrossRef]
- Won, Y.J.; Jung, S.Y.; Jang, J.H.; Lee, J.W.; Chae, H.R.; Choi, D.C.; Hyun Ahn, K.; Lee, C.H.; Park, P.K. Correlation of membrane fouling with topography of patterned membranes for water treatment. J. Memb. Sci. 2016, 498, 14–19. [Google Scholar] [CrossRef]
- Zhao, Z.; Muylaert, K.; Szymczyk, A.; Vankelecom, I.F.J. Harvesting microalgal biomass using negatively charged polysulfone patterned membranes: Influence of pattern shapes and mechanism of fouling mitigation. Water Res. 2021, 188, 116530. [Google Scholar] [CrossRef]
- Zhao, Z.; Muylaert, K.; Vankelecom, I.F.J. Combining patterned membrane filtration and flocculation for economical microalgae harvesting. Water Res. 2021, 117181. [Google Scholar] [CrossRef]
- Li, H.; Li, Z.; Xiong, S.; Zhang, H.; Li, N.; Zhou, S.; Liu, Y.; Huang, Z. Pilot-scale isolation of bioactive extracellular polymeric substances from cell-free media of mass microalgal cultures using tangential-flow ultrafiltration. Process Biochem. 2011, 46, 1104–1109. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Garcia-Depraect, O.; Leon-Becerril, E.; Cassano, A. Conidi, C. Fíla, V. Recovery of protein-based compounds from meat by-products by membrane-assisted separations: A review. J. Chem. Technol. Biotechnol. 2021, in press. [Google Scholar] [CrossRef]
- Hwang, T.; Kotte, M.R.; Han, J.I.; Oh, Y.K.; Diallo, M.S. Microalgae recovery by ultrafiltration using novel fouling-resistant PVDF membranes with in situ PEGylated polyethyleneimine particles. Water Res. 2015, 73, 181–192. [Google Scholar] [CrossRef]
- Purnima, M.; Arul Manikandan, N.; Pakshirajan, K.; Pugazhenthi, G. Recovery of microalgae from its broth solution using kaolin based tubular ceramic membranes prepared with different binders. Sep. Purif. Technol. 2020, 250, 117212. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, Y.; Zang, X.; Zhang, X. Harvesting of Microcystis aeruginosa using membrane filtration: Influence of pore structure on fouling kinetics, algogenic organic matter retention and cake formation. Algal Res. 2020, 52, 102112. [Google Scholar] [CrossRef]
- Wang, K.; Saththasivam, J.; Yiming, W.; Loganathan, K.; Liu, Z. Fast and efficient separation of seawater algae using a low-fouling micro/nano-composite membrane. Desalination 2018, 433, 108–112. [Google Scholar] [CrossRef]
- Bilad, M.R.; Azizo, A.S.; Wirzal, M.D.H.; Jia Jia, L.; Putra, Z.A.; Nordin, N.A.H.M.; Mavukkandy, M.O.; Jasni, M.J.F.; Yusoff, A.R.M. Tackling membrane fouling in microalgae filtration using nylon 6,6 nanofiber membrane. J. Environ. Manag. 2018, 223, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Liu, Z.; Yan, B.; Li, Y.; Li, H.; Liu, D.; Wang, P.; Cui, F.; Shi, W. Layer-by-layer assembly of high negatively charged polycarbonate membranes with robust antifouling property for microalgae harvesting. J. Memb. Sci. 2020, 595, 117488. [Google Scholar] [CrossRef]
- Hwang, T.; Park, S.J.; Oh, Y.K.; Rashid, N.; Han, J.I. Harvesting of Chlorella sp. KR-1 using a cross-flow membrane filtration system equipped with an anti-fouling membrane. Bioresour. Technol. 2013, 139, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Gerardo, M.L.; Oatley-Radcliffe, D.L.; Lovitt, R.W. Integration of membrane technology in microalgae biorefineries. J. Memb. Sci. 2014, 464, 86–99. [Google Scholar] [CrossRef]
- Haupt, A.; Lerch, A. Forward osmosis application in manufacturing industries: A short review. Membranes 2018, 8, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.; Jung, J.Y.; Shin, H.; Choi, S.A.; Kim, D.; Bai, S.C.; Chang, Y.K.; Han, J.I. Harvesting of Scenedesmus obliquus using dynamic filtration with a perforated disk. J. Memb. Sci. 2016, 517, 14–20. [Google Scholar] [CrossRef]
- Soydemir, G.; Gurol, M.D.; Hocaog, S.M.; Karagündüz, A. Fouling mechanisms of membrane filtration of mixed microalgal biomass grown in wastewater. Water Sci. Technol. 2020, 81, 2127–2139. [Google Scholar] [CrossRef]
- Hafiz, M.A.; Hawari, A.H.; Das, P.; Khan, S.; Altaee, A. Comparison of dual stage ultrafiltration and hybrid ultrafiltration-forward osmosis process for harvesting microalgae (Tetraselmis sp.) biomass. Chem. Eng. Process. Process Intensif. 2020, 157, 108112. [Google Scholar] [CrossRef]
- Honda, R.; Rukapan, W.; Komura, H.; Teraoka, Y.; Noguchi, M.; Hoek, E.M.V. Effects of membrane orientation on fouling characteristics of forward osmosis membrane in concentration of microalgae culture. Bioresour. Technol. 2015, 197, 429–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Q.; Yang, Y.; Wang, X.; Wang, Q.; Wang, S.; Gao, X.; Gao, C. Harvesting microalgae biomass using sulfonated polyethersulfone (SPES)/PES porous membranes in forward osmosis processes. J. Ocean Univ. China 2020, 19, 1345–1352. [Google Scholar] [CrossRef]
- Munshi, F.M.; Church, J.; McLean, R.; Maier, N.; Sadmani, A.H.M.A.; Duranceau, S.J.; Lee, W.H. Dewatering algae using an aquaporin-based polyethersulfone forward osmosis membrane. Sep. Purif. Technol. 2018, 204, 154–161. [Google Scholar] [CrossRef]
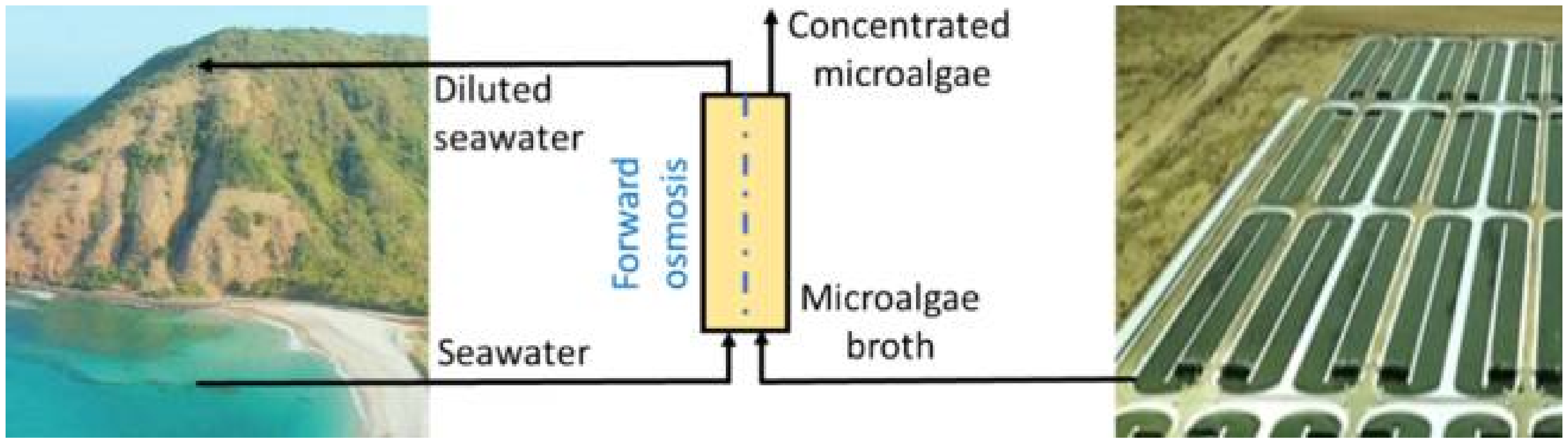
- Nawi, N.I.M.; Arifin, S.N.H.M.; Hizam, S.M.; Rampun, E.L.A.; Bilad, M.R.; Elma, M.; Khan, A.L.; Wibisono, Y.; Jaafar, J. Chlorella vulgaris broth harvesting via standalone forward osmosis using seawater draw solution. Bioresour. Technol. Reports 2020, 9. [Google Scholar] [CrossRef]
- Ryu, H.; Kim, K.; Cho, H.; Park, E.; Chang, Y.K.; Han, J.I. Nutrient-driven forward osmosis coupled with microalgae cultivation for energy efficient dewatering of microalgae. Algal Res. 2020, 48, 101880. [Google Scholar] [CrossRef]
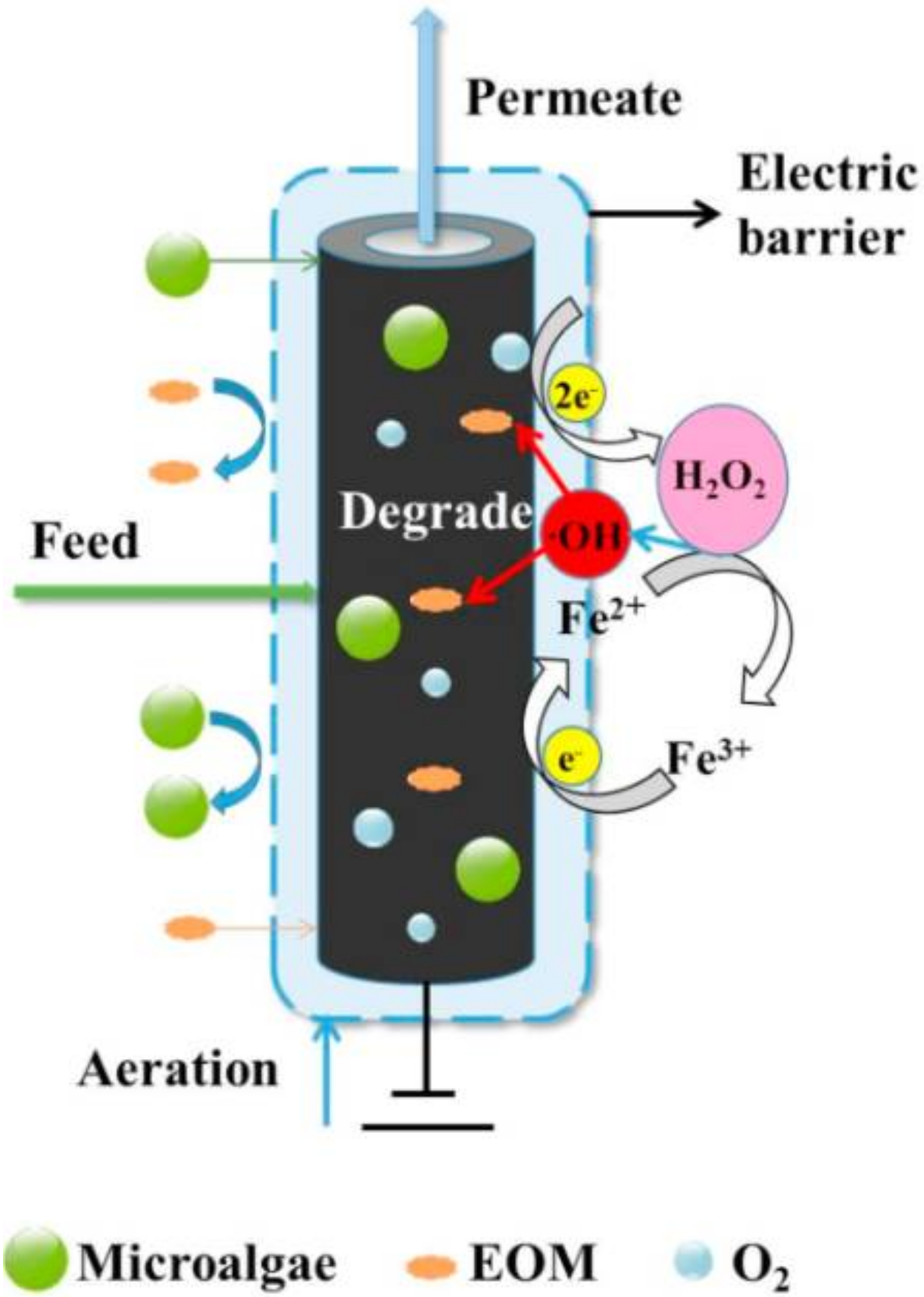
- Zheng, M.; Yang, Y.; Qiao, S.; Zhou, J.; Quan, X. A porous carbon-based electro-Fenton hollow fiber membrane with good antifouling property for microalgae harvesting. J. Memb. Sci. 2021, 626, 119189. [Google Scholar] [CrossRef]
- Gerardo, M.L.; Zanain, M.A.; Lovitt, R.W. Pilot-scale cross-flow microfiltration of Chlorella minutissima: A theoretical assessment of the operational parameters on energy consumption. Chem. Eng. J. 2015, 280, 505–513. [Google Scholar] [CrossRef] [Green Version]
- Chew, K.W.; Yap, J.Y.; Show, P.L.; Suan, N.H.; Juan, J.C.; Ling, T.C.; Lee, D.J.; Chang, J.S. Microalgae biorefinery: High value products perspectives. Bioresour. Technol. 2017, 229, 53–62. [Google Scholar] [CrossRef]
- Mehariya, S.; Goswami, R.K.; Karthikeysan, O.P.; Verma, P. Microalgae for high-value products: A way towards green nutraceutical and pharmaceutical compounds. Chemosphere 2021, 280, 130553. [Google Scholar] [CrossRef] [PubMed]
- Soto-Sierra, L.; Stoykova, P.; Nikolov, Z.L. Extraction and fractionation of microalgae-based protein products. Algal Res. 2018, 36, 175–192. [Google Scholar] [CrossRef]
- Laamanen, C.A.; Desjardins, S.M.; Senhorinho, G.N.A.; Scott, J.A. Harvesting microalgae for health beneficial dietary supplements. Algal Res. 2021, 54, 102189. [Google Scholar] [CrossRef]
- Marcati, A.; Ursu, A.V.; Laroche, C.; Soanen, N.; Marchal, L.; Jubeau, S.; Djelveh, G.; Michaud, P. Extraction and fractionation of polysaccharides and B-phycoerythrin from the microalga Porphyridium cruentum by membrane technology. Algal Res. 2014, 5, 258–263. [Google Scholar] [CrossRef]
- Safi, C.; Olivieri, G.; Campos, R.P.; Engelen-Smit, N.; Mulder, W.J.; van den Broek, L.A.M.; Sijtsma, L. Biorefinery of microalgal soluble proteins by sequential processing and membrane filtration. Bioresour. Technol. 2017, 225, 151–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gifuni, I.; Lavenant, L.; Pruvost, J.; Masse, A. Recovery of microalgal protein by three-steps membrane filtration: Advancements and feasibility. Algal Res. 2020, 51, 102082. [Google Scholar] [CrossRef]
- Böcker, L.; Bertsch, P.; Wenner, D.; Teixeira, S.; Bergfreund, J.; Eder, S.; Fischer, P.; Mathys, A. Effect of Arthrospira platensis microalgae protein purification on emulsification mechanism and efficiency. J. Colloid Interface Sci. 2021, 584, 344–353. [Google Scholar] [CrossRef]
- Chaiklahan, R.; Chirasuwan, N.; Loha, V.; Tia, S.; Bunnag, B. Separation and purification of phycocyanin from Spirulina sp. using a membrane process. Bioresour. Technol. 2011, 102, 7159–7164. [Google Scholar] [CrossRef]
- Lauceri, R.; Chini Zittelli, G.; Maserti, B.; Torzillo, G. Purification of phycocyanin from Arthrospira platensis by hydrophobic interaction membrane chromatography. Algal Res. 2018, 35, 333–340. [Google Scholar] [CrossRef]
- Mittal, R.; Lamdande, A.G.; Sharma, R.; Raghavarao, K.S.M.S. Membrane processing for purification of R-Phycoerythrin from marine macro-alga, Gelidium pusillum and process integration. Sep. Purif. Technol. 2020, 252, 117470. [Google Scholar] [CrossRef]
- Denis, C.; Massé, A.; Fleurence, J.; Jaouen, P. Concentration and pre-purification with ultrafiltration of a R-phycoerythrin solution extracted from macro-algae Grateloupia turuturu: Process definition and up-scaling. Sep. Purif. Technol. 2009, 69, 37–42. [Google Scholar] [CrossRef]
- Rumin, J.; Nicolau, E.; de Oliveira, R.G.; Fuentes-Grünewald, C.; Picot, L. Analysis of Scientific Research Driving Microalgae Market Opportunities in Europe. Mar. Drugs 2020, 18, 264. [Google Scholar] [CrossRef] [PubMed]
- Zaouk, L.; Massé, A.; Bourseau, P.; Taha, S.; Rabiller-Baudry, M.; Jubeau, S.; Teychené, B.; Pruvost, J.; Jaouen, P. Filterability of exopolysaccharides solutions from the red microalga Porphyridium cruentum by tangential filtration on a polymeric membrane. Environ. Technol. 2020, 41, 1167–1184. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.K.; Laroche, C.; Marcati, A.; Ursu, A.V.; Jubeau, S.; Marchal, L.; Petit, E.; Djelveh, G.; Michaud, P. Separation and fractionation of exopolysaccharides from Porphyridium cruentum. Bioresour. Technol. 2013, 145, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Balti, R.; Le Balc’h, R.; Brodu, N.; Gilbert, M.; Le Gouic, B.; Le Gall, S.; Sinquin, C.; Massé, A. Concentration and purification of Porphyridium cruentum exopolysaccharides by membrane filtration at various cross-flow velocities. Process Biochem. 2018, 74, 175–184. [Google Scholar] [CrossRef] [Green Version]
- Pierre, G.; Delattre, C.; Dubessay, P.; Jubeau, S.; Vialleix, C.; Cadoret, J.P.; Probert, I.; Michaud, P. What is in store for EPS microalgae in the next decade? Molecules 2019, 24, 4296. [Google Scholar] [CrossRef] [Green Version]
- Lourenço-Lopes, C.; Fraga-Corral, M.; Jimenez-Lopez, C.; Pereira, A.G.; Garcia-Oliveira, P.; Carpena, M.; Prieto, M.A.; Simal-Gandara, J. Metabolites from macroalgae and its applications in the cosmetic industry: A circular economy approach. Resources 2020, 9, 101. [Google Scholar] [CrossRef]
- Clavijo Rivera, E.; Villafaña-López, L.; Liu, S.; Vinoth Kumar, R.; Viau, M.; Bourseau, P.; Monteux, C.; Frappart, M.; Couallier, E. Cross-flow filtration for the recovery of lipids from microalgae aqueous extracts: Membrane selection and performances. Process Biochem. 2020, 89, 199–207. [Google Scholar] [CrossRef] [Green Version]
- Villafaña-López, L.; Clavijo Rivera, E.; Liu, S.; Couallier, E.; Frappart, M. Shear-enhanced membrane filtration of model and real microalgae extracts for lipids recovery in biorefinery context. Bioresour. Technol. 2019, 288, 121539. [Google Scholar] [CrossRef] [Green Version]
- Mat Aron, N.S.; Khoo, K.S.; Chew, K.W.; Veeramuthu, A.; Chang, J.S.; Show, P.L. Microalgae cultivation in wastewater and potential processing strategies using solvent and membrane separation technologies. J. Water Process Eng. 2021, 39, 101701. [Google Scholar] [CrossRef]
| Process | Pore Size (nm) | Pressure Requirement (bar) | Separation Mechanism |
|---|---|---|---|
| MF | 100–10,000 | 0.1–2 | Molecular sieving |
| UF | 2–100 | 0.1–7 | Molecular sieving |
| NF | 0.5–2 | 3–25 | Sieving/molecular interactions |
| Microalgae | Technology | Membrane | Average Membrane Flux (L/m2 h) | Biomass Recovery | Total Harvesting Cost/Energy Consumption | Ref. |
|---|---|---|---|---|---|---|
| Scenedesmus acuminatus | Cross-flow UF air-assisted backwashing system (53-m3 pilot scale) | Polyvinylchloride (PVC) hollow fiber membrane (cut-off 50 kDa) | 56 | 93% (concentration factor of 145 and final dry weight of 136 g/L) | USD 0.30/kg dry biomass | [28] |
| Dictyosphaerium sp. | Magnetically induced membrane vibration system | 12% polyvinylidene difluoride (PVDF) Mw ~543 kDa | 46 | Harvesting efficiency higher than 97% | 0.21 KWh/m3 | [31] |
| Nannochloropsis sp. | Cross-flow UF | Antifouling Polyethersulfone (PES) membrane with carbon nanotubes and lithium bromide | 28.9 | 100% harvesting efficiency, final concentration of 28 g/L | - | [32] |
| Dunaliella salina | Cross-flow UF | PES capillary membrane (cut-off 150 kDa) | 31 | Concentration factor of 5.9 | - | [34] |
| Picochlorum sp. (Tetraselmis sp.) | Pilot-scale cross-flow | Polyacrylonitrile (PAN) hollow fiber (weight cut-off 10 kDa) | 37.7 (33.8) | Final concentration of 28 g/L 27.1 g/L (22.0 g/L) | 1.81 kWh/m3 (3.3 kWh/m3) | [43] |
| Dictyosphaerium sp. (Chlorella vulgaris) | Dynamic filtration combined with flocculation | PVDF-12% (0.013 µm) | 78 (85) | - | - | [49] |
| Chlorella vulgaris | Tilted panel NF | Treated nylon 6,6 nanofiber | 37.9 | 379.5 L/m2 h bar | - | [51] |
| Chlorella vulgaris | Turbulent jet-assisted MF | PVDF hollow fiber membrane (0.2 µm) | 104 | - | - | [54] |
| Spirulina sp. | Tilted panel MF | PVDF (0.42 µm) | 55.4 | 554 L/m2 h bar | 0.20 KWh/m3 | [55] |
| Chlorella sp. | Cross-flow MF with a bubble-generator plate | PVDF (0.2) | - | 105 L/m2 h bar, 100% harvesting efficiency, 1.3 concentration factor | - | [57] |
| Chlorella vulgaris | Submerged filtration system | Pristine nylon 6,6 nanofiber | 28.6 | 286 L/m2 h bar | 4.16 KWh/m3 | [58] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro-Muñoz, R.; García-Depraect, O. Membrane-Based Harvesting Processes for Microalgae and Their Valuable-Related Molecules: A Review. Membranes 2021, 11, 585. https://doi.org/10.3390/membranes11080585
Castro-Muñoz R, García-Depraect O. Membrane-Based Harvesting Processes for Microalgae and Their Valuable-Related Molecules: A Review. Membranes. 2021; 11(8):585. https://doi.org/10.3390/membranes11080585
Chicago/Turabian StyleCastro-Muñoz, Roberto, and Octavio García-Depraect. 2021. "Membrane-Based Harvesting Processes for Microalgae and Their Valuable-Related Molecules: A Review" Membranes 11, no. 8: 585. https://doi.org/10.3390/membranes11080585
APA StyleCastro-Muñoz, R., & García-Depraect, O. (2021). Membrane-Based Harvesting Processes for Microalgae and Their Valuable-Related Molecules: A Review. Membranes, 11(8), 585. https://doi.org/10.3390/membranes11080585







