Chitosan/PVA Based Membranes Processed by Gamma Radiation as Scaffolding Materials for Skin Regeneration
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Chitosan/PVA Based Membranes
2.3. Evaluation of Membranes’ Physicochemical Properties
2.3.1. Thermal Stability
2.3.2. Structural Characterization
2.3.3. Morphological Characterization
2.3.4. Hydrophilicity
2.3.5. In vitro degradation
2.4. In Vitro Evaluation of Membranes’ Biological Properties
2.4.1. Cell Viability Assay (almarBlue®)
2.4.2. Cytochemistry
3. Results and Discussion
3.1. Thermal Stability
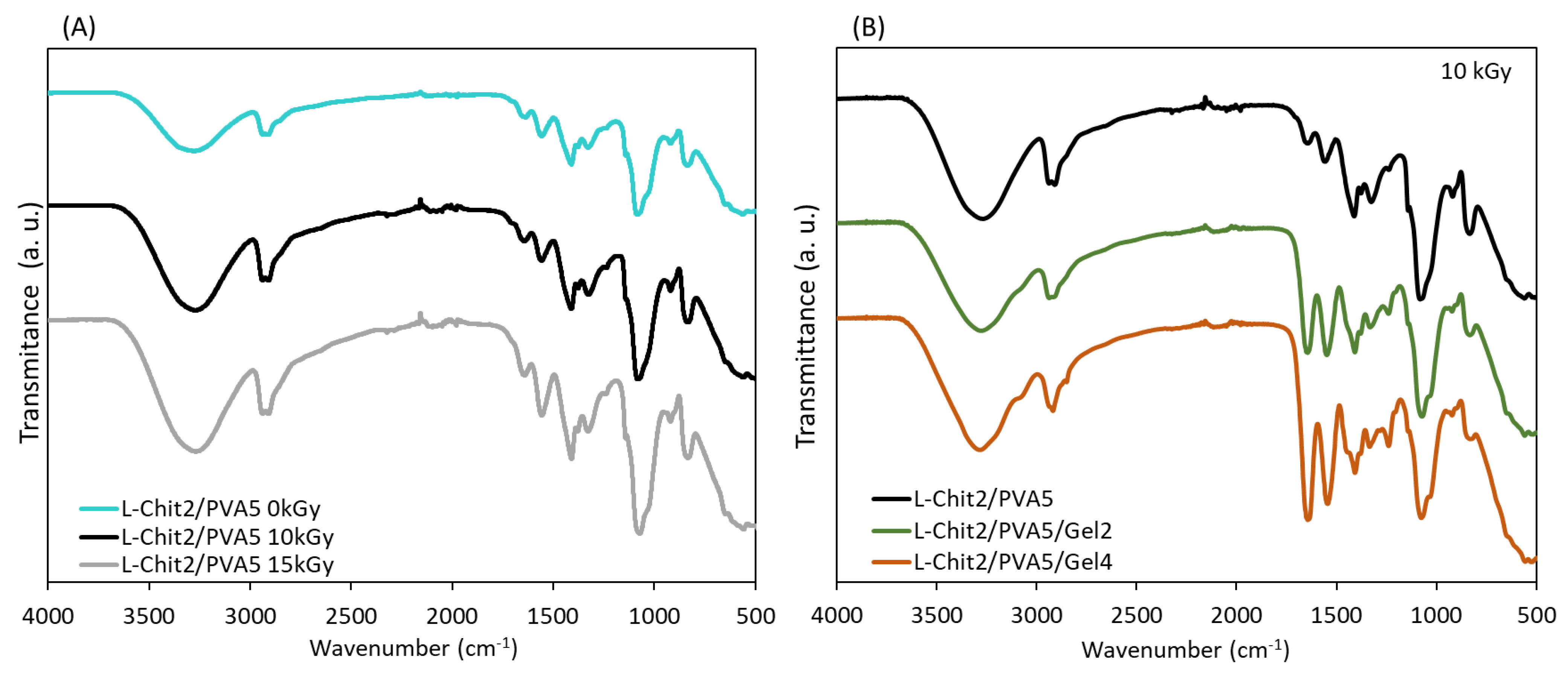
3.2. Structural Characterization
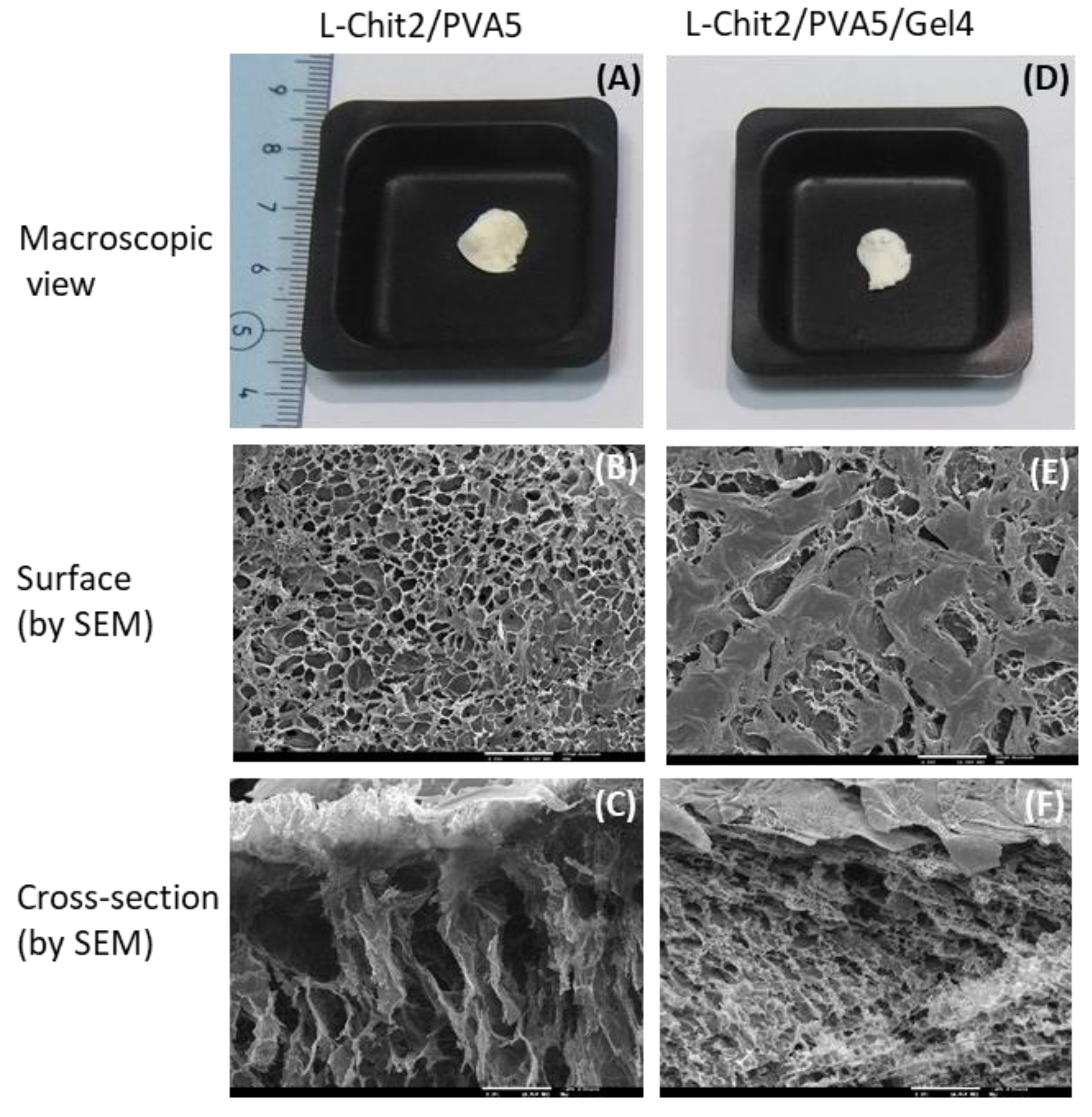
3.3. Morphological Characterization
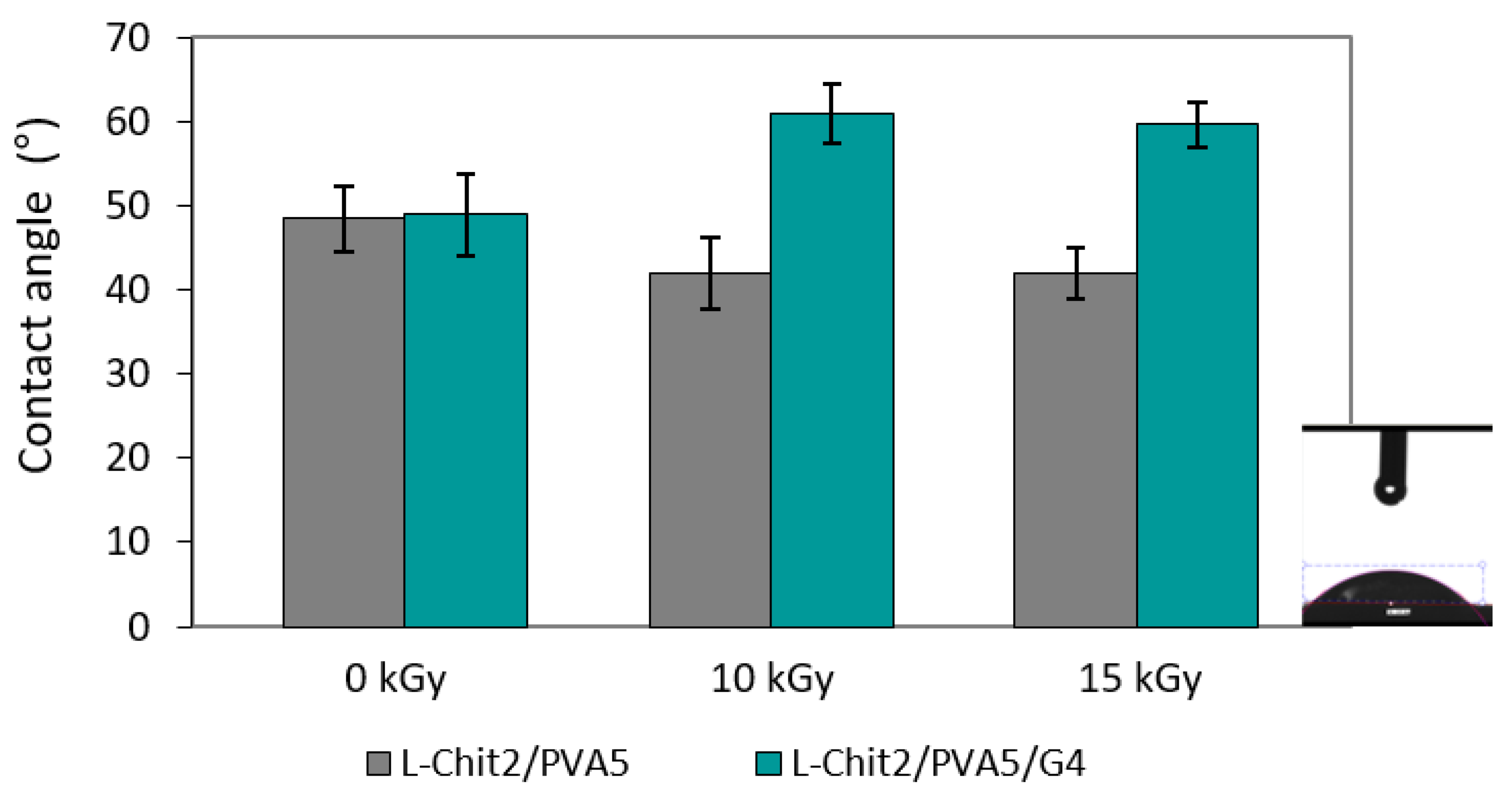
3.4. Hydrophililicity
3.5. In Vitro Degradation
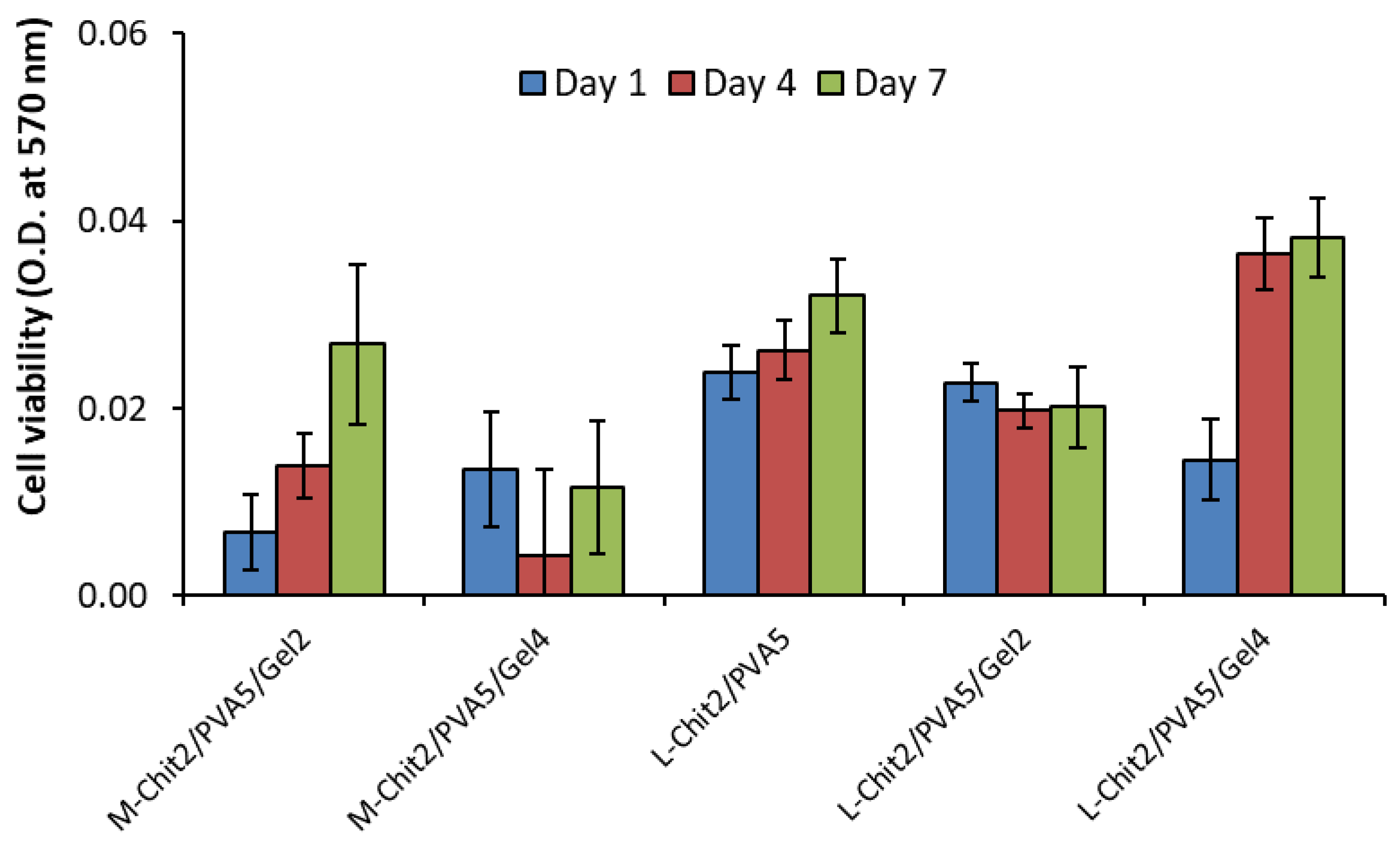
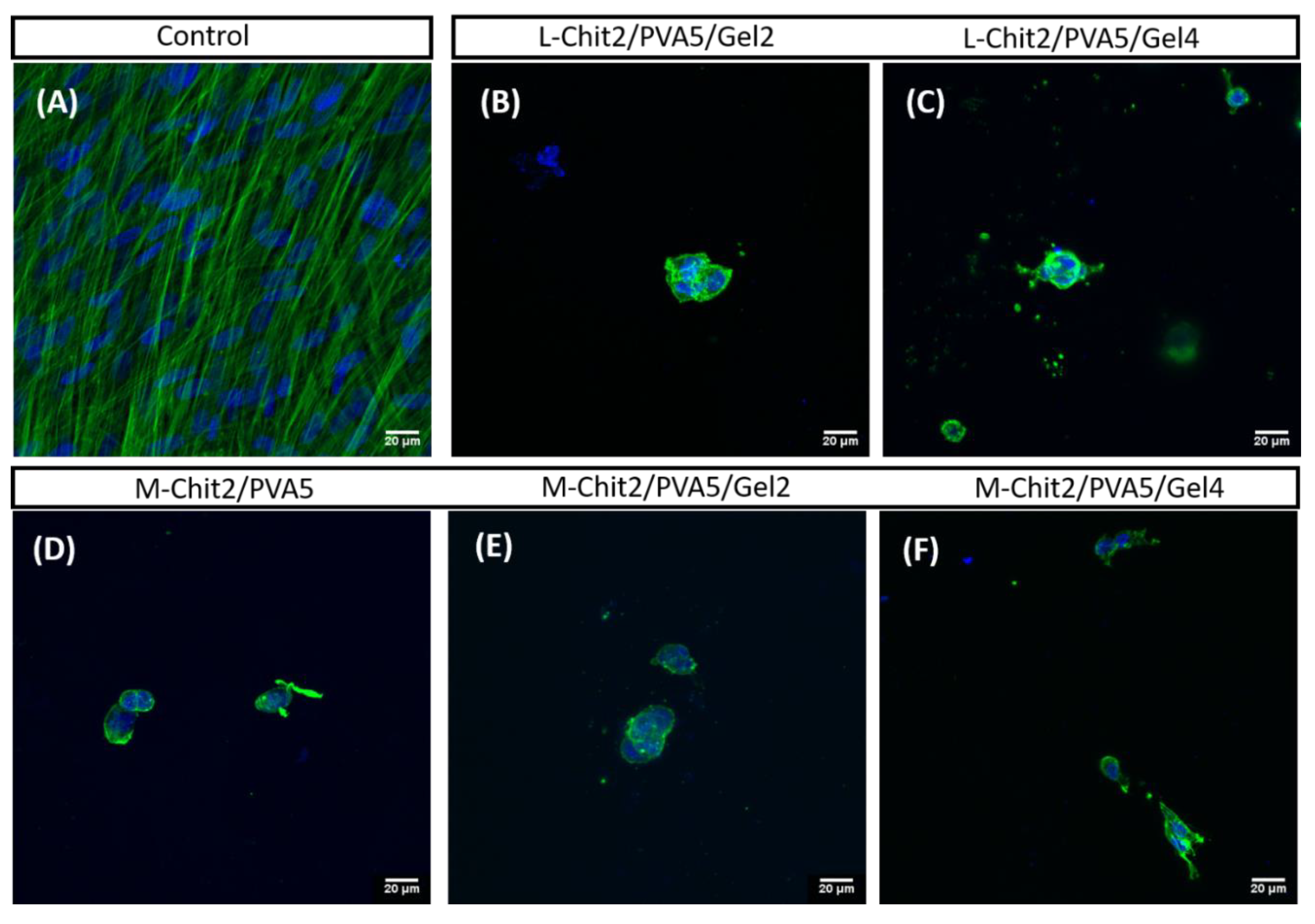
3.6. Biological Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Wilkinson, P.F.; Millington, R. Skin; Cambridge University Press: Cambridge, UK, 2009; pp. 49–50. [Google Scholar]
- Gottschlich, M.M. Fat-soluble vitamins and wound healing. In Nutrition and Wound Healing; Molnar, J.A., Ed.; CRC Press: Boca Raton, FL, USA, 2007; p. 106. [Google Scholar]
- Augustine, R.; Kalarikkal, N.; Thomas, S. Advancement of wound care from grafts to bioengineered smart skin substitutes. Prog. Biomater. 2014, 3, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Yildirimer, L.; Thanh, N.T.K.; Seifalian, A.M. Skin regeneration scaffolds: A multimodal bottom-up approach. Trends Biotechnol. 2012, 30, 638–648. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Hydrogels for tissue engineering. Chem. Rev. 2001, 101, 1869–1879. [Google Scholar] [CrossRef] [PubMed]
- Dhandayuthapani, B.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Polymeric Scaffolds in Tissue Engineering Application: A Review. Int. J. Polym. Sci. 2011, 2011, 290602. [Google Scholar] [CrossRef]
- Pauchot, J.; Guichard, A.; Lihoreau, T.; Elkhyat, A.; Mac-Mary, S.; Humbert, P. Mechanical properties of three different types of skin graft. In Agache’s Measuring the Skin; Humbert, P., Fanian, F., Maibach, H., Agache, P., Eds.; Springer: Cham, Switzerland, 2016; pp. 1–15. [Google Scholar]
- Casimiro, M.H.; Ferreira, L.M.; Leal, J.P.; Pereira, C.C.L.; Monteiro, B. Ionizing Radiation for Preparation and Functionalization of Membranes and Their Biomedical and Environmental Applications. Membranes 2019, 9, 163. [Google Scholar] [CrossRef] [PubMed]
- Muzzarelli, R.A.A. Chitins and chitosans for the repair of wounded skin, nerve, cartilage and bone. Carbohydr. Polym. 2009, 76, 167–182. [Google Scholar] [CrossRef]
- Mittal, H.; Ray, S.S.; Kaith, B.S.; Bhatia, J.K.; Sukriti; Sharma, J.; Alhassan, S.M. Recent progress in the structural modification of chitosan for applications in diversified biomedical fields. Eur. Polym. J. 2018, 109, 402–434. [Google Scholar] [CrossRef]
- Casimiro, M.H.; Lancastre, J.J.H.; Rodrigues, A.P.; Gomes, S.R.; Rodrigues, G.; Ferreira, L.M. Chitosan-Based Matrices Prepared by Gamma Irradiation for Tissue Regeneration: Structural Properties vs. Preparation Method. Top. Curr. Chem. 2017, 374, 121–143. [Google Scholar]
- Casimiro, M.H.; Gomes, S.R.; Rodrigues, G.; Leal, J.P.; Ferreira, L.M. Chitosan/Poly(vinylpyrrolidone) Matrices Obtained by Gamma-Irradiation for Skin Scaffolds: Characterization and Preliminary Cell Response Studies. Materials 2018, 11, 2535. [Google Scholar] [CrossRef]
- Croisier, F.; Jérôme, C. Chitosan-based biomaterials for tissue engineering. Eur. Polym. J. 2013, 49, 780–792. [Google Scholar] [CrossRef]
- Kim, Y.; Ko, H.; Kwon, I.K.; Shin, K. Extracellular Matrix Revisited: Roles in Tissue Engineering. Int. Neurourol. J. 2016, 20 (Suppl. 1), S23–S29. [Google Scholar] [CrossRef] [PubMed]
- Hoshiba, T.; Yamaoka, T. Chapter 1: Extracellular matrix scaffolds for tissue engineering and biological research. In Decellularized Extracellular Matrix: Characterization, Fabrication and Applications; Yamaoka, T., Hoshiba, T., Eds.; RCS Books: Kerala, India, 2019; pp. 1–14. [Google Scholar]
- Ferreira, L.M.; Falcao, A.N.; Gil, M.H. Elemental and topographic characterization of LDPE based copolymeric films obtained by gamma irradiation. Nucl. Instrum. Methods B 2007, 265, 193–197. [Google Scholar] [CrossRef][Green Version]
- Hegazy, E.-S.A.; AbdEl-Rehim, H.A.; Kamal, H.; Kandeel, K.A. Advances in radiation grafting. Nucl. Instrum. Methods B 2011, 185, 235–240. [Google Scholar] [CrossRef]
- Casimiro, M.H.; Silva, A.G.; Pinto, J.V.; Ramos, A.M.; Vital, J.; Ferreira, L.M. Catalytic poly(vinyl alcohol) functionalized membranes obtained by gamma irradiation. Radiat. Phys. Chem. 2012, 81, 1314–1318. [Google Scholar] [CrossRef]
- Ferreira, L.M.; Leal, J.P.; Casimiro, M.H.; Cruz, C.; Lancastre, J.J.; Falcao, A.N. Evidence of structural order recovery in LDPE based copolymers prepared by gamma irradiation. Radiat. Phys. Chem. 2014, 94, 31–35. [Google Scholar] [CrossRef]
- Darwins, D.; Abbas, E.B.; Nurlidar, F.; Putra, D.P. Radiation processing of polymers for medical and pharmaceutical applications. Macromol. Symp. 2015, 353, 15–23. [Google Scholar] [CrossRef]
- Malafaya, P.B.; Silva, G.A.; Reis, R.L. Natural-origin Polymers as Carriers and Scaffolds for Biomolecules and Cell Delivery in Tissue Engineering Applications. Adv. Drug. Deliv. Rev. 2007, 59, 207–233. [Google Scholar] [CrossRef] [PubMed]
- Bello, A.B.; Kim, D.; Kim, D.; Park, H.; Lee, S.-H. Engineering and Functionalization of Gelatin Biomaterials: From Cell Culture to Medical Applications. Tissue Eng. Part B 2020, 26, 164–180. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-M. Thermal analysis. In Materials Science and Engineering of Carbon: Characterization; Inagaki, M., Kang, F., Eds.; Butterworth-Heinemann: Oxford, UK, 2016; pp. 249–272. [Google Scholar]
- Sadtler Research Laboratories, Division of Bio-Rad Laboratories, Inc. The Infrared Spectra Atlas of Monomers and Polymers; Sadtler Research Laboratories: Philadelphia, PA, USA, 1980. [Google Scholar]
- Hebbar, R.S.; Isloor, A.M.; Ismail, A.F. Chapter 12-Contact angle measurements. In Membrane Characterization; Hilal, A., Ismail, A.F., Matsuura, T., Oatley-Radcliffe, D., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 219–255. [Google Scholar]
- Wang, H.; Boerman, O.C.; Sariibrahimoglu, K.; Li, Y.; Jansen, J.A.; Leeuwenburg, S.C.C. Comparison of Micro Vs. Nanostructured Colloidal Gelatin Gels for Sustainable Delivery of Osteogenic Proteins: Bone Morphogenetic Protein-2 and alkaline Phosphatase. Biomaterials 2012, 33, 8695–8703. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casimiro, M.H.; Pereira, A.; Leal, J.P.; Rodrigues, G.; Ferreira, L.M. Chitosan/PVA Based Membranes Processed by Gamma Radiation as Scaffolding Materials for Skin Regeneration. Membranes 2021, 11, 561. https://doi.org/10.3390/membranes11080561
Casimiro MH, Pereira A, Leal JP, Rodrigues G, Ferreira LM. Chitosan/PVA Based Membranes Processed by Gamma Radiation as Scaffolding Materials for Skin Regeneration. Membranes. 2021; 11(8):561. https://doi.org/10.3390/membranes11080561
Chicago/Turabian StyleCasimiro, Maria Helena, Andreia Pereira, João P. Leal, Gabriela Rodrigues, and Luís M. Ferreira. 2021. "Chitosan/PVA Based Membranes Processed by Gamma Radiation as Scaffolding Materials for Skin Regeneration" Membranes 11, no. 8: 561. https://doi.org/10.3390/membranes11080561
APA StyleCasimiro, M. H., Pereira, A., Leal, J. P., Rodrigues, G., & Ferreira, L. M. (2021). Chitosan/PVA Based Membranes Processed by Gamma Radiation as Scaffolding Materials for Skin Regeneration. Membranes, 11(8), 561. https://doi.org/10.3390/membranes11080561






