Substrate-Independent, Regenerable Anti-Biofouling Coating for Polymeric Membranes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
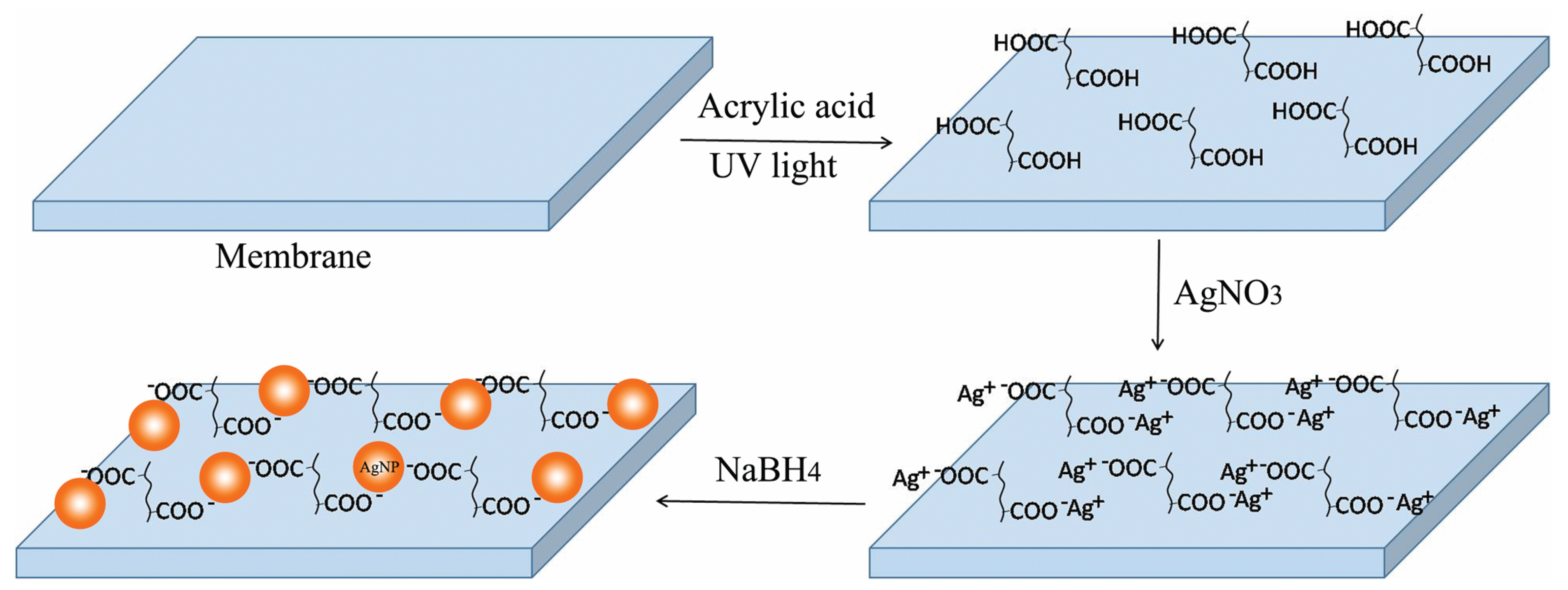
2.2. Surface Functionalization
2.3. In Situ Immobilization of Silver Nanoparticles
2.4. Membrane Characterizations
3. Results and Discussion
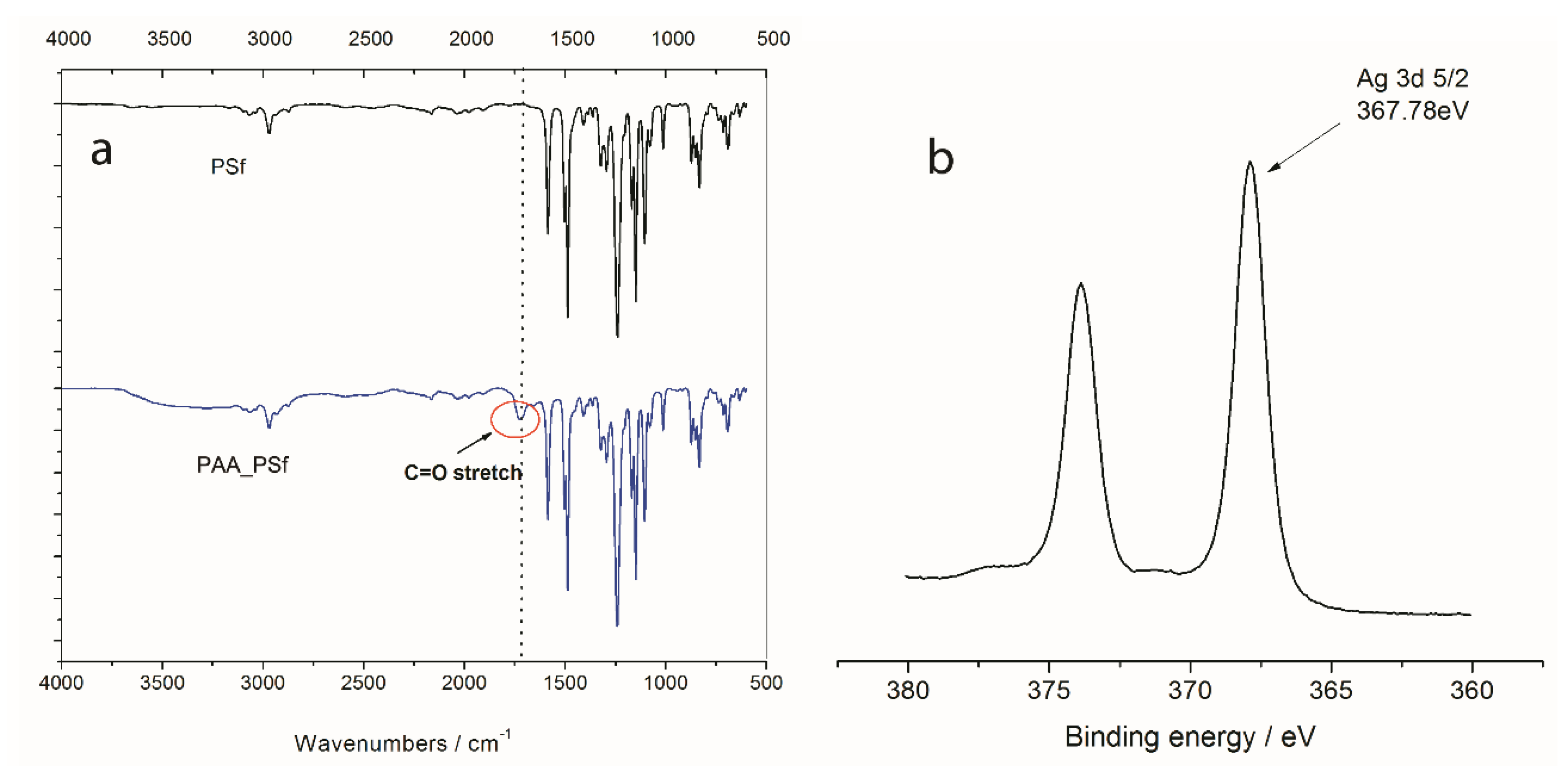
3.1. Membrane Surface Composition and Morphology
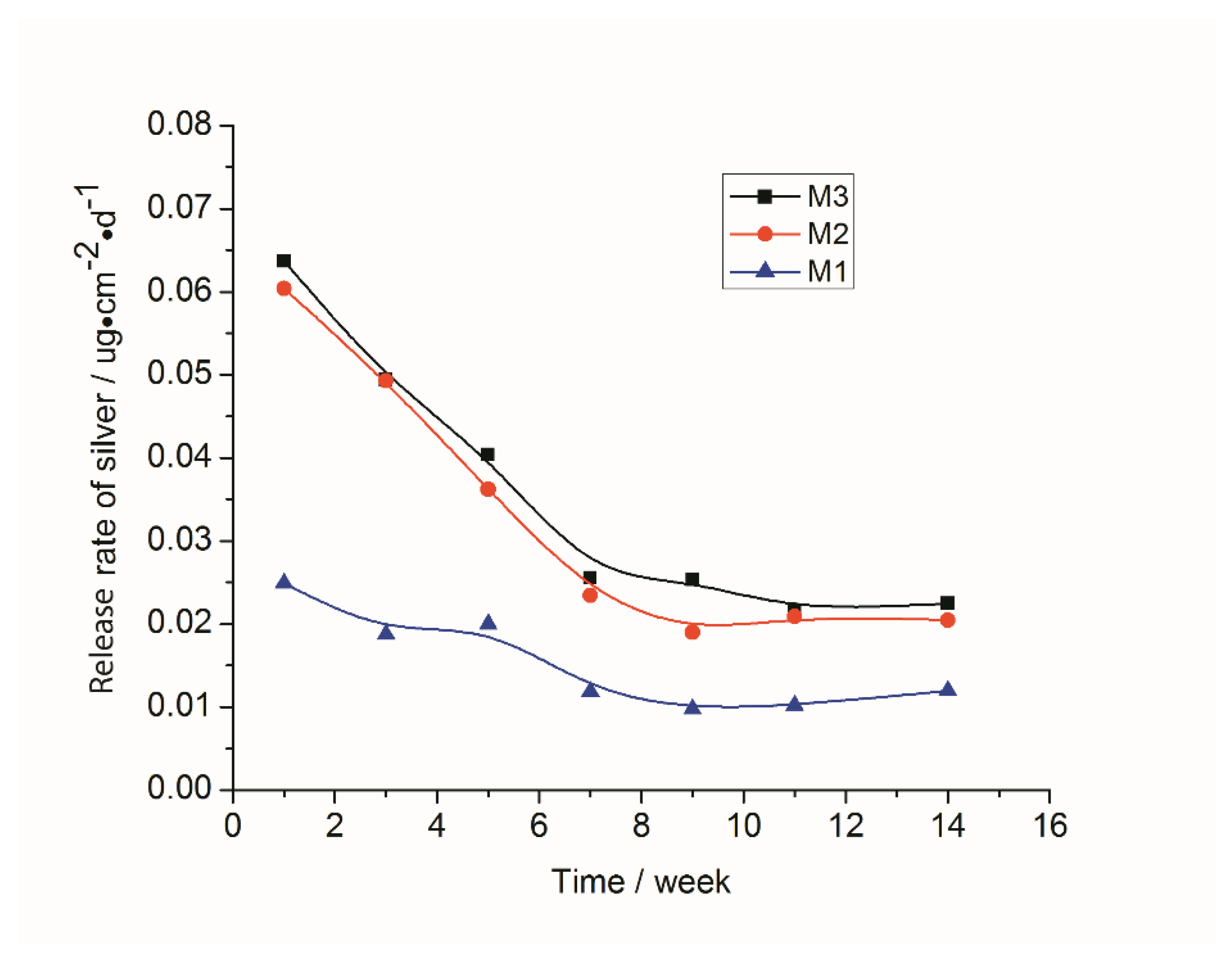
3.2. Stability of the Immobilized Silver Nanoparticles
3.3. Restoration of Silver Nanoparticles
3.4. Surface Hydrophilicity
3.5. Water Permeability and BSA Rejection
3.6. Anti-Biofouling Performance
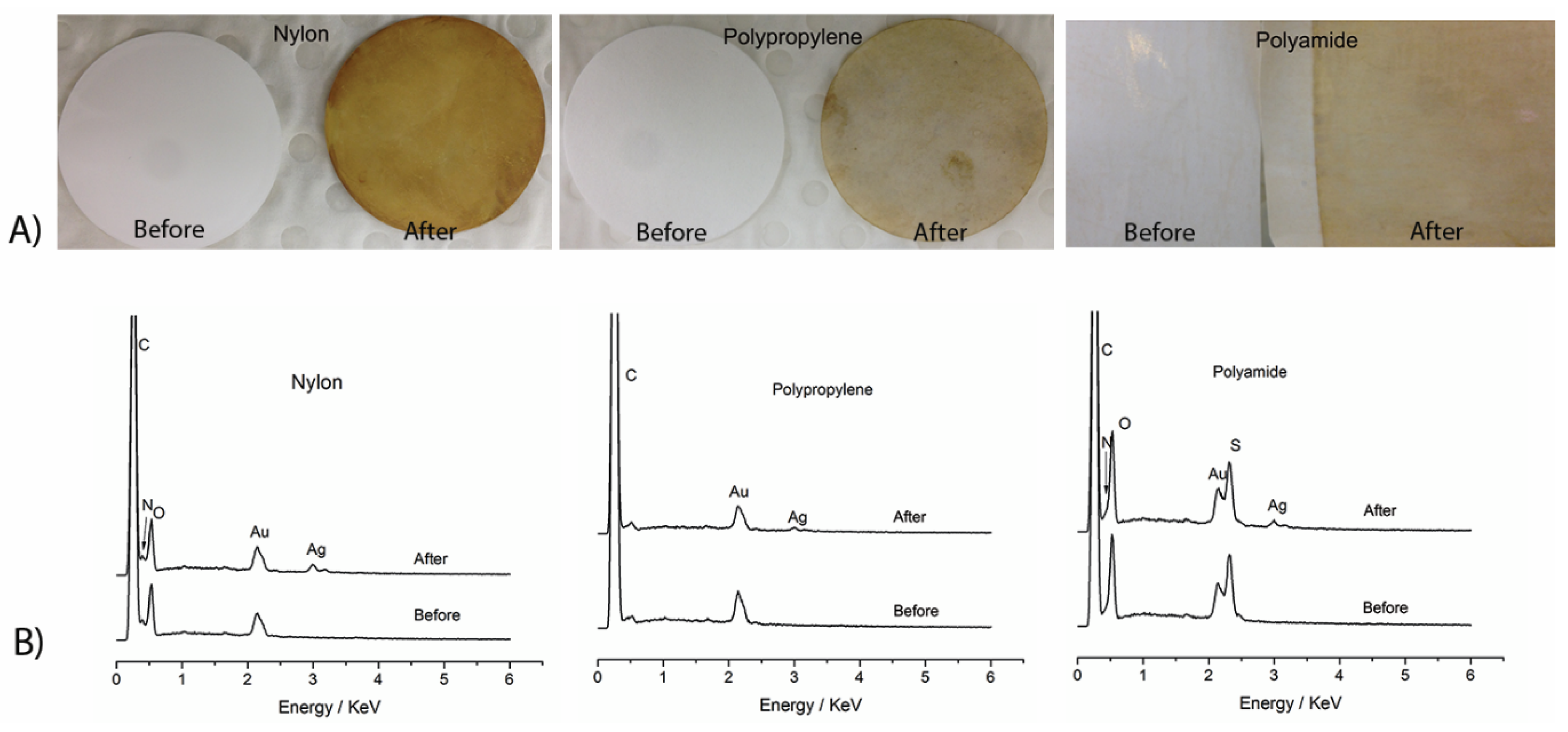
3.7. Immobilization of AgNPs on Different Membrane Surfaces
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Guo:, W.; Ngo, H.-H.; Li, J. A mini-review on membrane fouling. Bioresour. Technol. 2012, 122, 27–34. [Google Scholar] [CrossRef]
- Yang, R.; Jang, H.; Stocker, R.; Gleason, K.K. Synergistic Prevention of Biofouling in Seawater Desalination by Zwitterionic Surfaces and Low-Level Chlorination. Adv. Mater. 2014, 26, 1711–1718. [Google Scholar] [CrossRef]
- Ramesh, A.; Lee, D.J.; Wang, M.L.; Hsu, J.P.; Juang, R.-S.; Hwang, K.J.; Liu, J.C.; Tseng, S.J. Biofouling in Membrane Bioreactor. Sep. Sci. Technol. 2006, 41, 1345–1370. [Google Scholar] [CrossRef]
- Chen, P.; Cui, L.; Zhang, K. Surface-enhanced Raman spectroscopy monitoring the development of dual-species biofouling on membrane surfaces. J. Membr. Sci 2015, 473, 36–44. [Google Scholar] [CrossRef]
- Bogler, A.; Rice, D.; Perreault, F.; Bar-Zeev, E. Comparing membrane and spacer biofouling by Gram-negative Pseudomonas aeruginosa and Gram-positive Anoxybacillus sp. in forward osmosis. Biofouling 2019, 35, 104–116. [Google Scholar] [CrossRef]
- Khan, M.M.T.; Stewart, P.S.; Moll, D.J.; Mickols, W.E.; Nelson, S.E.; Camper, A.K. Characterization and effect of biofouling on polyamide reverse osmosis and nanofiltration membrane surfaces. Biofouling 2011, 27, 173–183. [Google Scholar] [CrossRef]
- Vrouwenvelder, J.; Van Loosdrecht, M.; Kruithof, J. Early warning of biofouling in spiral wound nanofiltration and reverse osmosis membranes. Desalination 2011, 265, 206–212. [Google Scholar] [CrossRef]
- Pichardo-Romero, D.; Garcia-Arce, Z.P.; Zavala-Ramirez, A.; Castro-Munoz, R. Current Advances in Biofouling Mitigation in Membranes for Water Treatment: An Overview. Processes 2020, 8, 182. [Google Scholar] [CrossRef]
- Kappachery, S.; Paul, D.; Yoon, J.; Kweon, J.H. Vanillin, a potential agent to prevent biofouling of reverse osmosis membrane. Biofouling 2010, 26, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.C.; Schaule, G.; Griebe, T.; Schmitt, J.; Tamachkiarowa, A. Biofouling—The Achilles heel of membrane processes. Desalination 1997, 113, 215–225. [Google Scholar] [CrossRef]
- Saeed, M.O.; Jamaluddin, A.; Tisan, I.; Lawrence, D.; Al-Amri, M.; Chida, K. Biofouling in a seawater reverse osmosis plant on the Red Sea coast, Saudi Arabia. Desalination 2000, 128, 177–190. [Google Scholar] [CrossRef]
- Koseoglu-Imer, D.Y.; Kose, B.; Altinbas, M.; Koyuncu, I. The production of polysulfone (PS) membrane with silver nanoparticles (AgNP): Physical properties, filtration performances, and biofouling resistances of membranes. J. Membr. Sci. 2013, 428, 620–628. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, K.; De Gusseme, B.; Verstraete, W. Biogenic silver nanoparticles (bio-Ag0) decrease biofouling of bio-Ag0/PES nanocomposite membranes. Water Res. 2012, 46, 2077–2087. [Google Scholar] [CrossRef] [PubMed]
- Pounraj, S.; Somu, P.; Paul, S. Chitosan and graphene oxide hybrid nanocomposite film doped with silver nanoparticles efficiently prevents biofouling. Appl. Surf. Sci 2018, 452, 487–497. [Google Scholar] [CrossRef]
- Harrison, J.J.; Ceri, H.; Stremick, C.A.; Turner, R.J. Biofilm susceptibility to metal toxicity. Environ. Microbiol. 2004, 6, 1220–1227. [Google Scholar] [CrossRef]
- Zhang, M.; Field, R.W.; Zhang, K. Biogenic silver nanocomposite polyethersulfone UF membranes with antifouling properties. J. Membr. Sci. 2014, 471, 274–284. [Google Scholar] [CrossRef]
- Chou, W.-L.; Yu, D.-G.; Yang, M.-C. The preparation and characterization of silver-loading cellulose acetate hollow fiber membrane for water treatment. Polym. Adv. Technol. 2005, 16, 600–607. [Google Scholar] [CrossRef]
- Yu, D.G.; Teng, M.Y.; Chou, W.L.; Yang, M.C. Characterization and inhibitory effect of antibacterial PAN-based hollow fiber loaded with silver nitrate. J. Membr. Sci. 2003, 225, 115–123. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, H.J.; Patel, R.; Im, S.J.; Kim, J.H.; Min, B.R. Silver nanoparticles immobilized on thin film composite polyamide membrane: Characterization, nanofiltration, antifouling properties. Polym. Adv. Technol. 2007, 18, 562–568. [Google Scholar] [CrossRef]
- Rana, D.; Kim, Y.; Matsuura, T.; Arafat, H.A. Development of antifouling thin-film-composite membranes for seawater desalination. J. Membr. Sci. 2011, 367, 110–118. [Google Scholar] [CrossRef]
- Yin, J.; Yang, Y.; Hu, Z.; Deng, B. Attachment of silver nanoparticles (AgNPs) onto thin-film composite (TFC) membranes through covalent bonding to reduce membrane biofouling. J. Membr. Sci. 2013, 441, 73–82. [Google Scholar] [CrossRef]
- Gunawan, P.; Guan, C.; Song, X.; Zhang, Q.; Leong, S.S.J.; Tang, C.; Chen, Y.; Chan-Park, M.B.; Chang, M.W.; Wang, K.; et al. Hollow Fiber Membrane Decorated with Ag/MWNTs: Toward Effective Water Disinfection and Biofouling Control. ACS Nano 2011, 5, 10033–10040. [Google Scholar] [CrossRef]
- Mauter, M.S.; Wang, Y.; Okemgbo, K.C.; Osuji, C.O.; Giannelis, E.P.; Elimelech, M. Antifouling Ultrafiltration Membranes via Post-Fabrication Grafting of Biocidal Nanomaterials. ACS Appl. Mater. Interfaces 2011, 3, 2861–2868. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Bai, R.; Wee, K.-H.; Liu, C.; Tang, S.-L. Membrane surfaces immobilized with ionic or reduced silver and their anti-biofouling performances. J. Membr. Sci. 2010, 363, 278–286. [Google Scholar] [CrossRef]
- Cao, X.; Tang, M.; Liu, F.; Nie, Y.; Zhao, C. Immobilization of silver nanoparticles onto sulfonated polyethersulfone membranes as antibacterial materials. Colloids Surf. B. 2010, 81, 555–562. [Google Scholar] [CrossRef]
- Yang, H.-L.; Lin, J.C.-T.; Huang, C. Application of nanosilver surface modification to RO membrane and spacer for mitigating biofouling in seawater desalination. Water Res. 2009, 43, 3777–3786. [Google Scholar] [CrossRef]
- Ben-Sasson, M.; Lu, X.; Bar-Zeev, E.; Zodrow, K.R.; Nejati, S.; Qi, G.; Giannelis, E.P.; Elimelech, M. In situ formation of silver nanoparticles on thin-film composite reverse osmosis membranes for biofouling mitigation. Water Res. 2014, 62, 260–270. [Google Scholar] [CrossRef]
- Ping, X.; Wang, M.; Ge, X. Surface modification of poly(ethylene terephthalate) (PET) film by gammaray induced grafting of poly(acrylic acid) and its application in antibacterial hybrid film. Radiat. Phys. Chem. 2011, 80, 567–572. [Google Scholar] [CrossRef]
- Li, J.-H.; Shao, X.-S.; Zhou, Q.; Li, M.-Z.; Zhang, Q.-Q. The double effects of silver nanoparticles on the PVDF membrane: Surface hydrophilicity and antifouling performance. Appl. Surf. Sci. 2013, 265, 663–670. [Google Scholar] [CrossRef]
- Bojarska, M.; Piątkiewicz, W. Antibacterial properties of membranes modified by acrylic acid with silver nanoparticles. Desalination Water Treat. 2014, 56, 1–7. [Google Scholar] [CrossRef]
- Loo, S.-L.; Fane, A.G.; Lim, T.-T.; Krantz, W.B.; Liang, Y.-N.; Liu, X.; Hu, X. Superabsorbent Cryogels Decorated with Silver Nanoparticles as a Novel Water Technology for Point-of-Use Disinfection. Environ. Sci. Technol. 2013, 47, 9363–9371. [Google Scholar] [CrossRef] [PubMed]
- Hussain, I.; Brust, M.; Papworth, A.J.; Cooper, A.I. Preparation of Acrylate-Stabilized Gold and Silver Hydrosols and Gold−Polymer Composite Films. Langmuir 2003, 19, 4831–4835. [Google Scholar] [CrossRef]
- Nishimura, S.; Mott, D.; Takagaki, A.; Maenosono, S.; Ebitani, K. Role of base in the formation of silver nanoparticles synthesized using sodium acrylate as a dual reducing and encapsulating agent. Phys. Chem. Chem. Phys. 2011, 13, 9335–9343. [Google Scholar] [CrossRef]
- Wang, T.C.; Rubner, M.F.; Cohen, R.E. Polyelectrolyte multilayer nanoreactors for preparing silver nanoparticle composites: Controlling metal concentration and nanoparticle size. Langmuir 2002, 18, 3370–3375. [Google Scholar] [CrossRef]
- Joly, S.; Kane, R.; Radzilowski, L.; Wang, T.; Wu, A.; Cohen, R.E.; Thomas, E.L.; Rubner, M.F. Multilayer nanoreactors for metallic and semiconducting particles. Langmuir 2000, 16, 1354–1359. [Google Scholar] [CrossRef]
- Li, Z.; Lee, D.; Sheng, X.; Cohen, R.E.; Rubner, M.F. Two-level antibacterial coating with both release-killing and contact-killing capabilities. Langmuir 2006, 22, 9820–9823. [Google Scholar] [CrossRef]
- WHO. Guidelines for Drinking Water Quality; WHO: Geneva, Switzerland, 2004. [Google Scholar]
- Zhu, X.; Tang, L.; Wee, K.-H.; Zhao, Y.-H.; Bai, R. Immobilization of silver in polypropylene membrane for anti-biofouling performance. Biofouling 2011, 27, 773–786. [Google Scholar] [CrossRef]



| Sample ID | Pristine Membrane | 2.5% AA Modified Membrane | AgNO3 Concentration (mM) | NaBH4 Concentration (mM) |
|---|---|---|---|---|
| M0 | ✓ | - | - | |
| M0_AgNP | ✓ | 5 | 5 | |
| PAA_PSf | ✓ | - | - | |
| M1 | ✓ | 1 | 5 | |
| M2 | ✓ | 3 | 5 | |
| M3 | ✓ | 5 | 5 |
| Sample ID | M0 | PAA_PSf | M0_AgNP | M1 | M2 | M3 |
|---|---|---|---|---|---|---|
| O (At. %) | 8.4 | 12.2 | - | - | - | - |
| Ag (At. %) | 0 | - | 0.19 | 3.45 | 5.50 | 9.06 |
| Sample ID | Total Ag Loaded (μg·cm−2) | Release Rate * (μg·cm−2·d−1) | Estimated Lasting Time (Days) | After Ag Regenerating (μg·cm−2) |
|---|---|---|---|---|
| M1 | 4.75 | 0.010 | 474 | 8.12 |
| M2 | 10.88 | 0.020 | 543 | 13.92 |
| M3 | 15.20 | 0.024 | 633 | 19.32 |
| Sample ID | M0 | PAA_PSf | M1 | M2 | M3 |
|---|---|---|---|---|---|
| Contac t angle (°) | 62.0 ± 5.2 | 55.1 ± 1.6 | 54.3 ± 1.8 | 49.4 ± 6.3 | 50.4 ± 3.1 |
| Membrane permeability (L·m−2·h−1·bar−1) | 131.1 ± 2.4 | 63.2 ± 1.9 | 40.0 ± 3.6 | 36.2 ± 4.8 | 36.8 ± 1.3 |
| BSA rejection (%) | 75.8 ± 1.1 | 89.8 ± 0.9 | 92.3 ± 1.5 | 90.3 ± 0.6 | 91.7 ± 0.5 |
| Sample ID | PP Membrane (Filtered at 0.2 bar) | Nylon Membrane (Filtered at 0.2 bar) | Polyamide RO Membrane (Filtered at 15 bar) | |||
|---|---|---|---|---|---|---|
| Pristine | With AgNPs | Pristine | With AgNPs | Pristine | With AgNPs | |
| Water permeability(L·m−2·h−1) | 616 ± 18 | 623 ± 23 | 484 ± 24 | 274 ± 16 | 39.3 ± 3.6 | 33.6 ± 4.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Wang, G.; Zhang, J.; Xu, Z.; Zhao, Y.; Wang, Y.; She, F.; Gray, S.; Kong, L. Substrate-Independent, Regenerable Anti-Biofouling Coating for Polymeric Membranes. Membranes 2021, 11, 205. https://doi.org/10.3390/membranes11030205
Zhang J, Wang G, Zhang J, Xu Z, Zhao Y, Wang Y, She F, Gray S, Kong L. Substrate-Independent, Regenerable Anti-Biofouling Coating for Polymeric Membranes. Membranes. 2021; 11(3):205. https://doi.org/10.3390/membranes11030205
Chicago/Turabian StyleZhang, Juan, Guang Wang, Jianhua Zhang, Zhiguang Xu, Yan Zhao, Yichao Wang, Fenghua She, Stephen Gray, and Lingxue Kong. 2021. "Substrate-Independent, Regenerable Anti-Biofouling Coating for Polymeric Membranes" Membranes 11, no. 3: 205. https://doi.org/10.3390/membranes11030205
APA StyleZhang, J., Wang, G., Zhang, J., Xu, Z., Zhao, Y., Wang, Y., She, F., Gray, S., & Kong, L. (2021). Substrate-Independent, Regenerable Anti-Biofouling Coating for Polymeric Membranes. Membranes, 11(3), 205. https://doi.org/10.3390/membranes11030205











