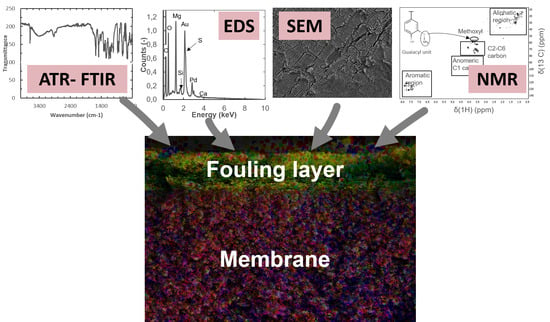
Comprehensive Analysis of Foulants in an Ultrafiltration Membrane Used for the Treatment of Bleach Plant Effluent in a Sulfite Pulp Mill
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Procedures
2.2. Analysis
2.2.1. SEM-EDS Measurements
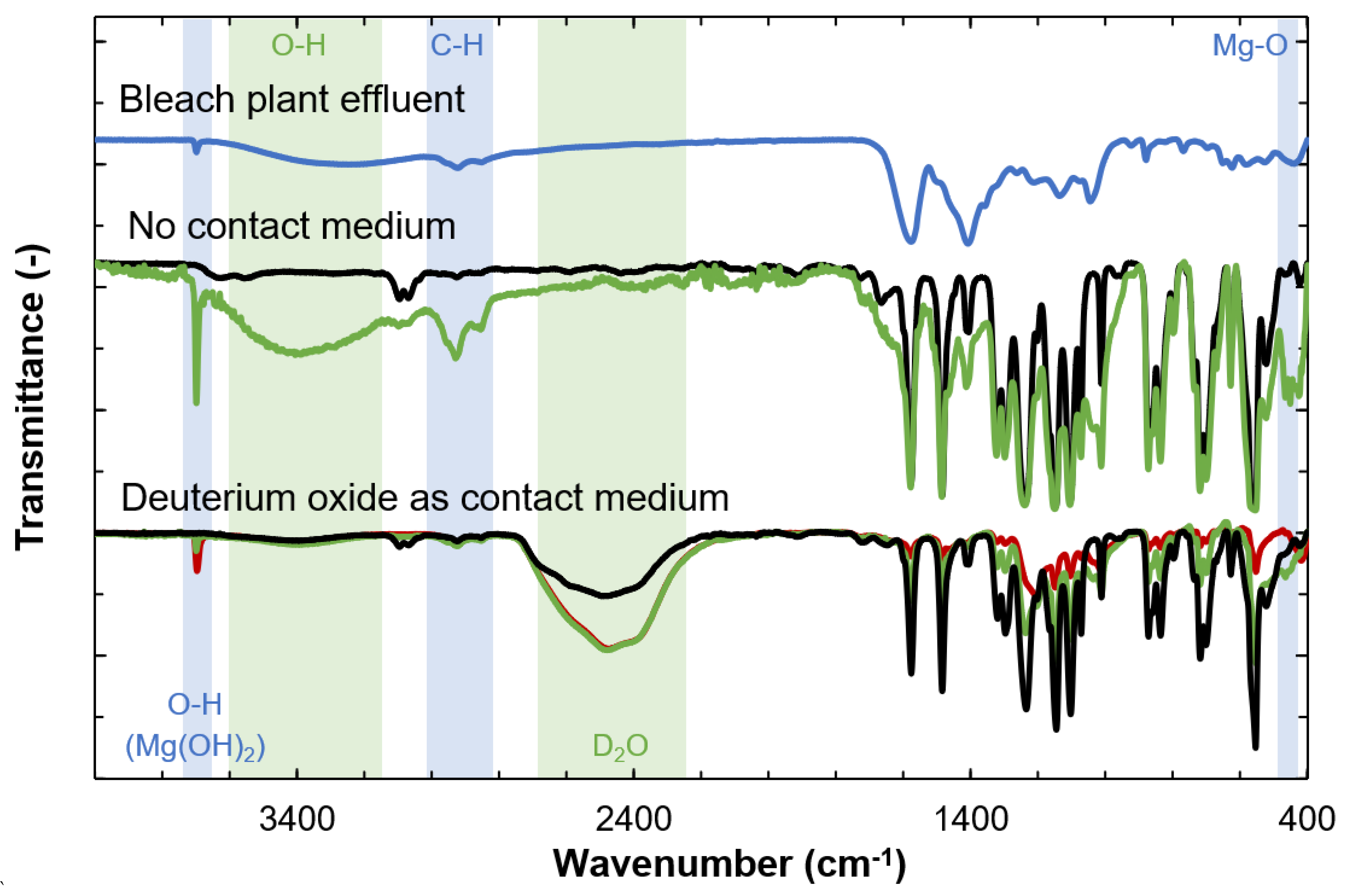
2.2.2. ATR-FTIR Measurements
2.2.3. HPAEC-PAD Measurements
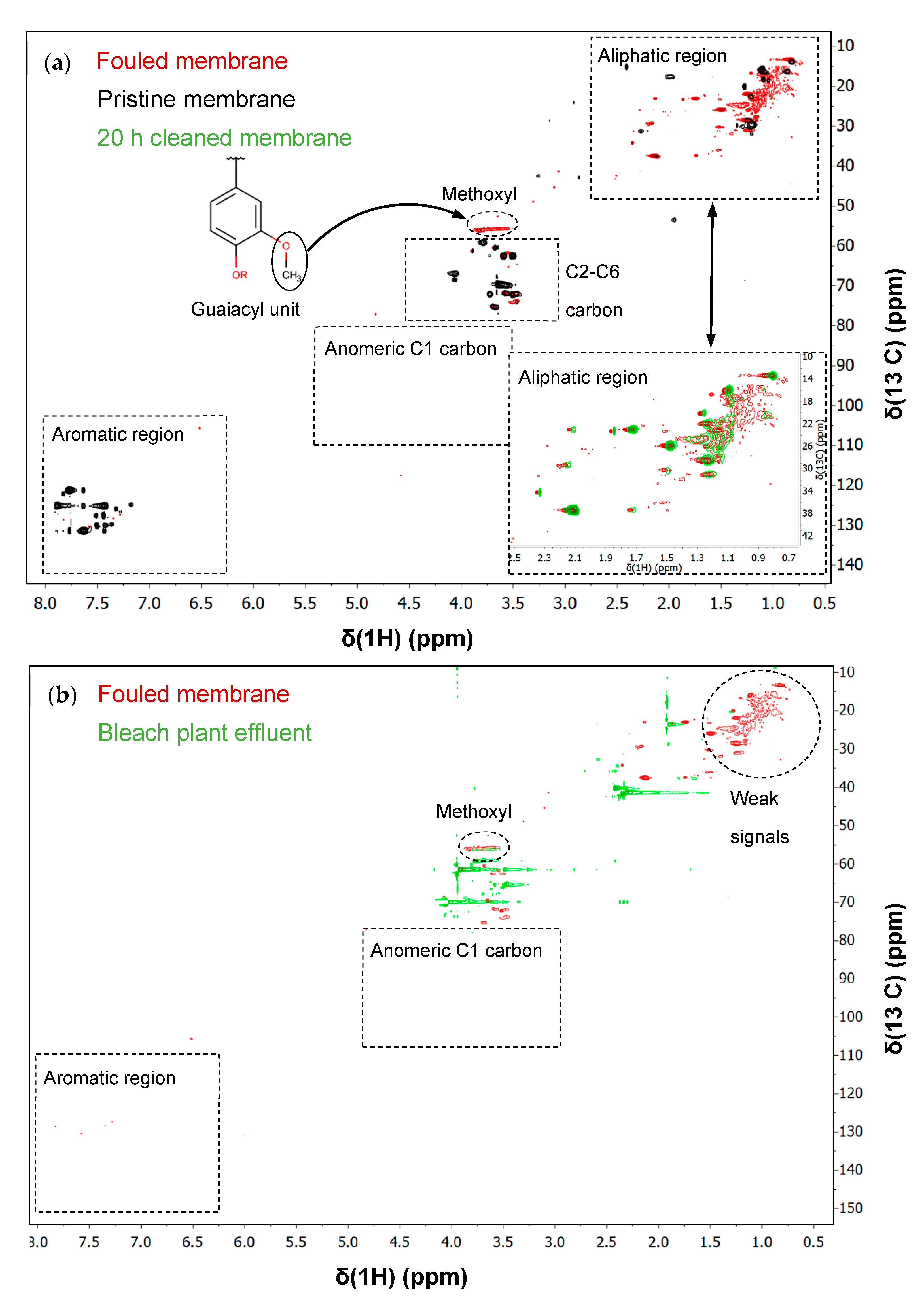
2.2.4. HSQC-2D-NMR Measurements
3. Results and Discussion
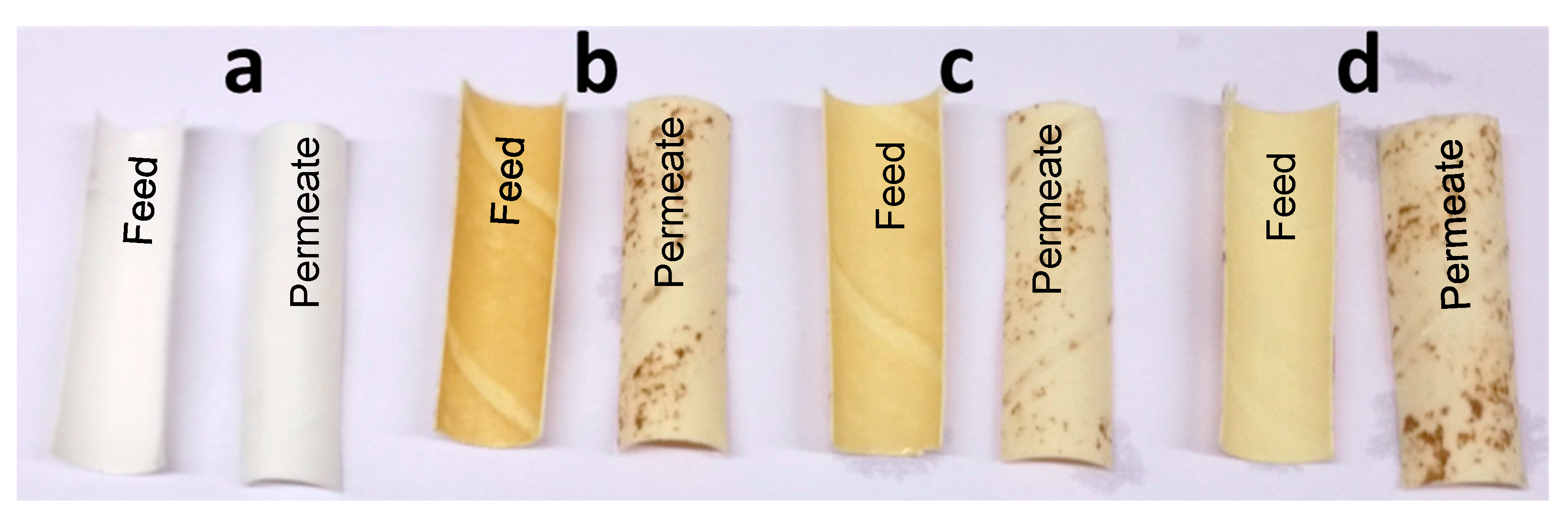
3.1. Visible Appearance
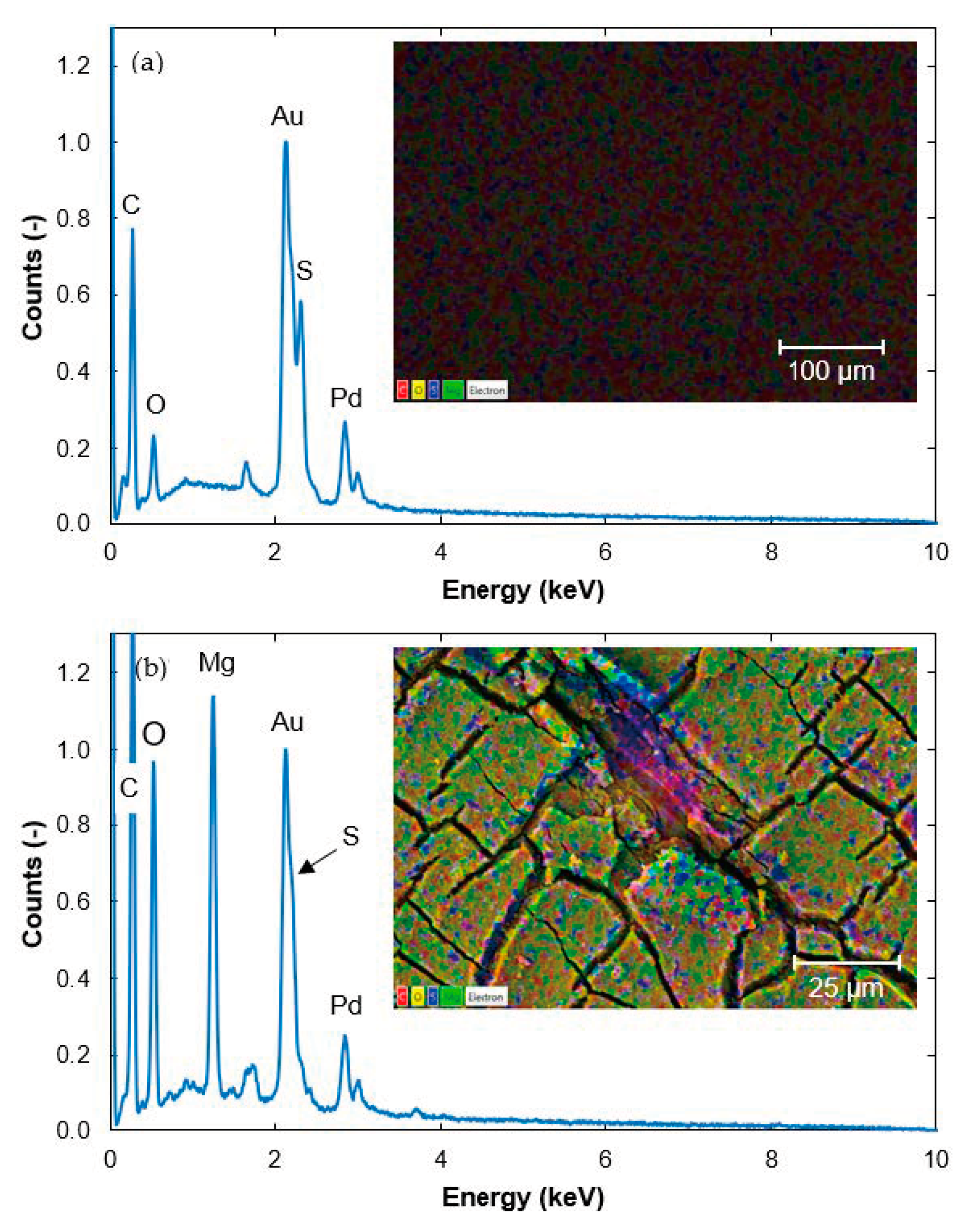
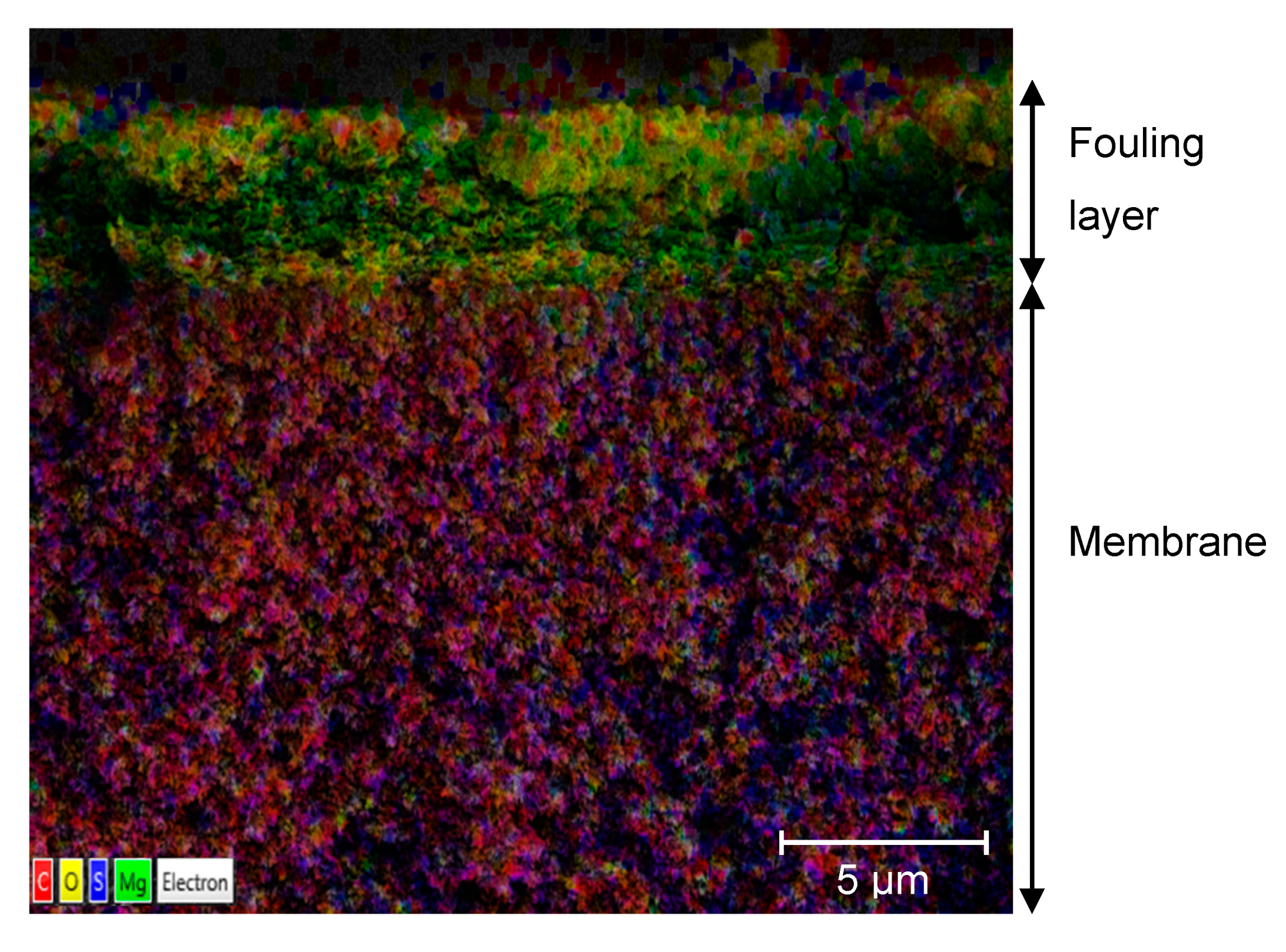
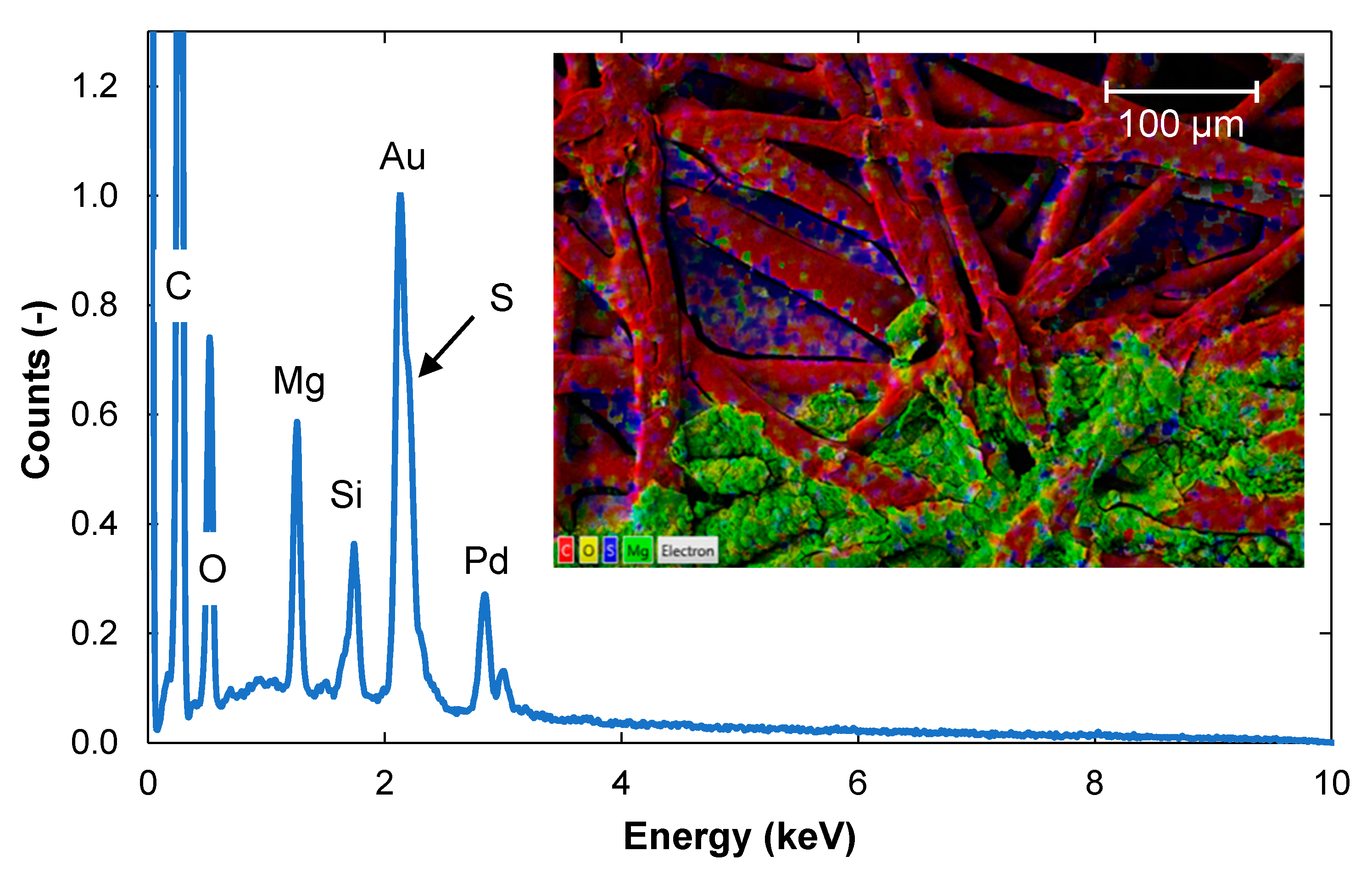
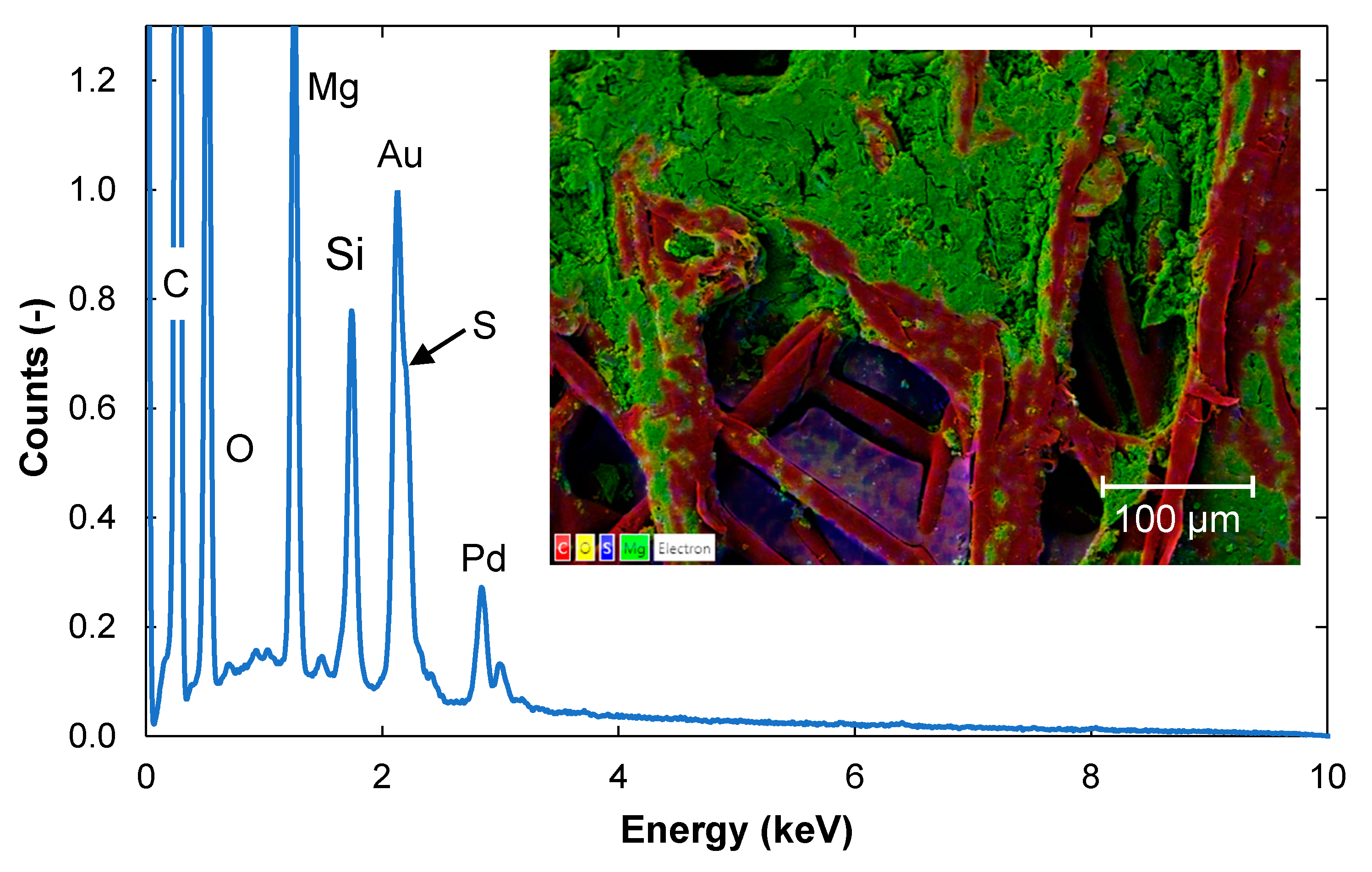
3.2. Surface Structure and Content of Elements Determined with SEM-EDS
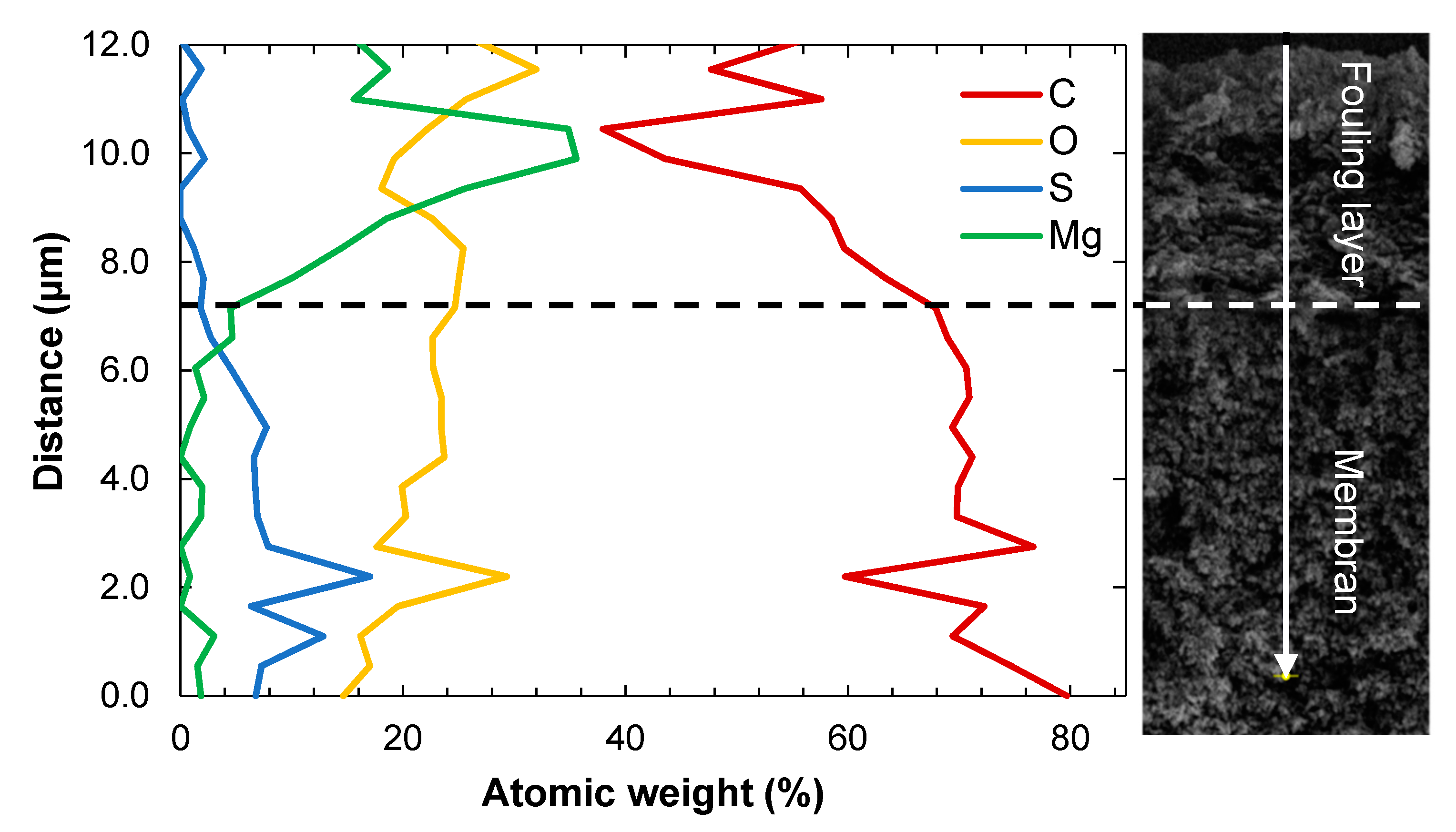
3.2.1. Contents of Elements in a Cross-Section of the Fouled Membrane
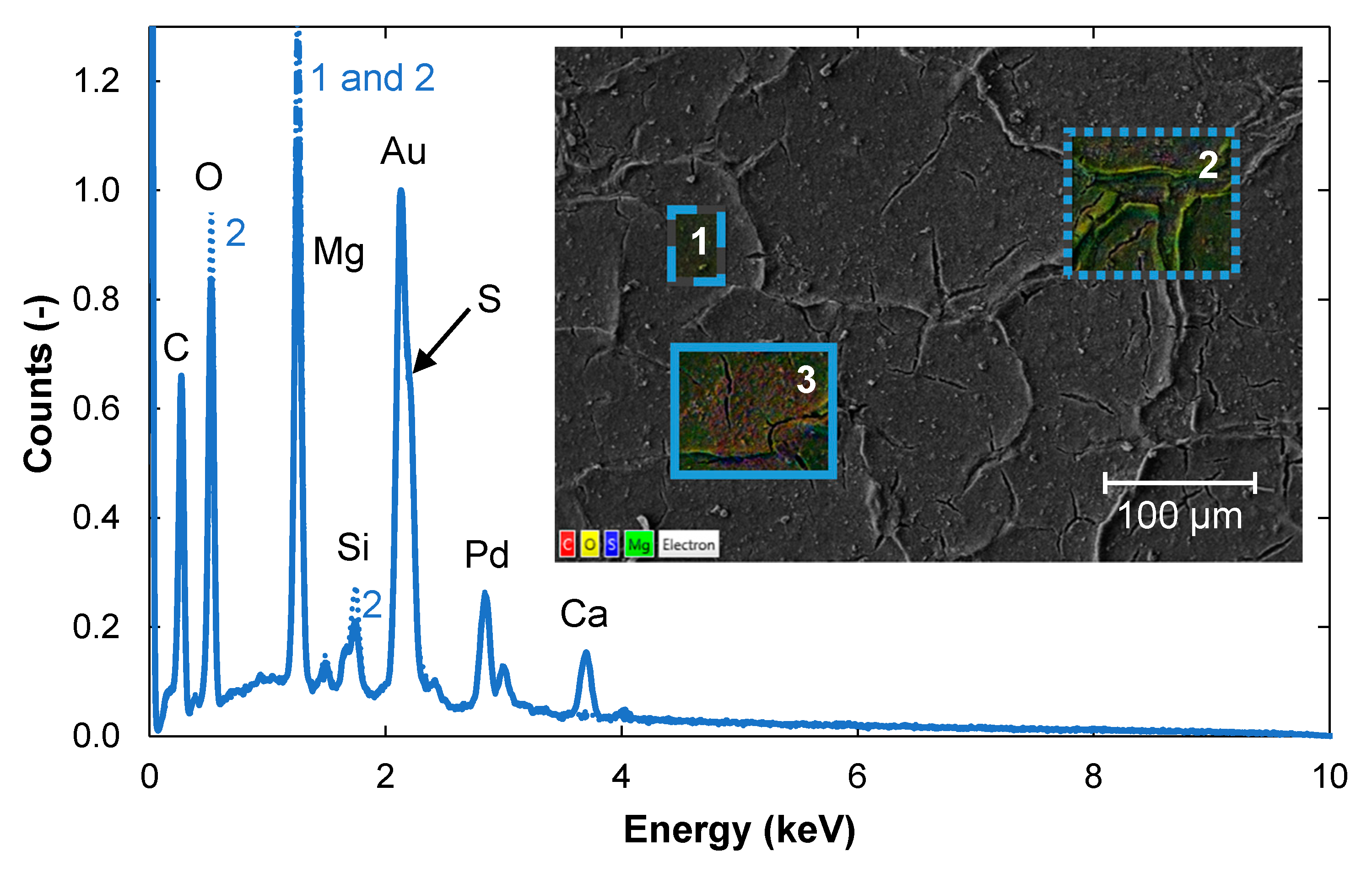
3.2.2. Contents of Elements in the Non-Woven Backing
3.2.3. Removal of Foulants by Cleaning
3.3. Identification of Foulants by ATR-FTIR
3.4. Polysaccharides Detected by HPAEC-PAD
3.5. Identification of Foulants with HSQC-2D-NMR
Analysis of a Cleaned Membrane with HSQC-2D-NMR
3.6. Remarks on the Analytical Methods Employed
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Charcosset, C. Classical and Recent Applications of Membrane Processes in the Food Industry. Food Eng. Rev. 2020, 1–22. [Google Scholar] [CrossRef]
- Zydney, A.L. New developments in membranes for bioprocessing—A review. J. Membr. Sci. 2021, 620, 118804. [Google Scholar] [CrossRef]
- Liao, B.; Bokhary, A.; Cui, L.; Lin, H. A Review of Membrane Technology for Integrated Forest Biorefinery. J. Membr. Sci. Res 2017, 3, 120–141. [Google Scholar] [CrossRef]
- Hubbe, M.; Alén, R.; Paleologou, M.; Kannangara, M.; Kihlman, J. Lignin recovery from spent alkaline pulping liquors using acidification, membrane separation, and related processing steps: A review. Bioresources 2019, 14, 2300–2351. [Google Scholar] [CrossRef]
- Maartens, A.; Jacobs, E.; Swart, P. UF of pulp and paper effluent: Membrane fouling-prevention and cleaning. J. Membr. Sci. 2002, 209, 81–92. [Google Scholar] [CrossRef]
- Trägårdh, G. Membrane cleaning. Desalination 1989, 71, 325–335. [Google Scholar] [CrossRef]
- Guo, H.; Tang, X.; Ganschow, G.; Korshin, G.V. Differential ATR FTIR spectroscopy of membrane fouling: Contributions of the substrate/fouling films and correlations with transmembrane pressure. Water Res. 2019, 161, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Thuvander, J.; Zarebska, A.; Hélix-Nielsen, C.; Jönsson, A.-S. Characterization of Irreversible Fouling after Ultrafiltration of Thermomechanical Pulp Mill Process Water. J. Wood Chem. Technol. 2018, 38, 276–285. [Google Scholar] [CrossRef]
- Gönder, Z.B.; Arayici, S.; Barlas, H. Advanced treatment of pulp and paper mill wastewater by nanofiltration process: Effects of operating conditions on membrane fouling. Sep. Purif. Technol. 2011, 76, 292–302. [Google Scholar] [CrossRef]
- Rudolph, G.; Schagerlöf, H.; Krogh, K.B.M.; Jönsson, A.-S.; Lipnizki, F. Investigations of Alkaline and Enzymatic Membrane Cleaning of Ultrafiltration Membranes Fouled by Thermomechanical Pulping Process Water. Membr. 2018, 8, 91. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cao, C.; Feng, W.; Xu, M. Chemical Cleaning of a Polyethersulfone Membrane Used for Concentrating the Effluent from Alkaline Peroxide Mechanical Pulping Plants. Environ. Eng. Sci. 2012, 29, 1020–1025. [Google Scholar] [CrossRef]
- Chen, C.; Mao, S.; Wang, J.; Bao, J.; Xu, H.; Su, W.; Dai, H. Application of Ultrafiltration in a Paper Mill: Process Water Reuse and Membrane Fouling Analysis. Bioresources 2015, 10, 2376–2391. [Google Scholar] [CrossRef]
- Puro, L.; Kallioinen, M.; Mänttäri, M.; Nyström, M. Evaluation of behavior and fouling potential of wood extractives in ultrafiltration of pulp and paper mill process water. J. Membr. Sci. 2011, 368, 150–158. [Google Scholar] [CrossRef]
- Fortunato, L.; Alshahri, A.H.; Farinha, A.S.; Zakzouk, I.; Jeong, S.; Leiknes, T. Fouling investigation of a full-scale seawater reverse osmosis desalination (SWRO) plant on the Red Sea: Membrane autopsy and pretreatment efficiency. Desalination 2020, 496, 114536. [Google Scholar] [CrossRef]
- Fernandez-Álvarez, G.; Garralón, G.; Plaza, F.; Pérez, J.I.P.; Gómez, M. Autopsy of SWRO membranes from desalination plant in Ceuta after 8years in operation. Desalination 2010, 263, 264–270. [Google Scholar] [CrossRef]
- Al-Rudainy, B.; Galbe, M.; Schagerlöf, H.; Wallberg, O. Antisolvent precipitation of hemicelluloses, lignosulfonates and their complexes from ultrafiltrated spent sulfite liquor (SSL). Holzforschung 2018, 72, 839–850. [Google Scholar] [CrossRef]
- Aminzadeh, S.; Lauberts, M.; Dobele, G.; Ponomarenko, J.; Mattsson, T.; Lindström, M.E.; Sevastyanova, O. Membrane filtration of kraft lignin: Structural charactristics and antioxidant activity of the low-molecular-weight fraction. Ind. Crops Prod. 2018, 112, 200–209. [Google Scholar] [CrossRef]
- Wickström, P. Membrane filtration in the closed-cycle bleach plant. In Working Towards Ecobalance: TAPPI Minimum Effluent Mills Symposium Proceedings, 1st ed.; Broman, P.G., Lindberg, H., Eds.; TAPPI Press: San Francisco, CA, USA, 1997; pp. 79–83. [Google Scholar]
- Nordin, A.-K.; Jönsson, A.-S. Case study of an ultrafiltration plant treating bleach plant effluent from a pulp and paper mill. Desalination 2006, 201, 277–289. [Google Scholar] [CrossRef]
- Puro, L.; Tanninen, J.; Nyström, M. Analyses of organic foulants in membranes fouled by pulp and paper mill effluent using solid-liquid extraction. Desalination 2002, 143, 1–9. [Google Scholar] [CrossRef]
- Wallberg, O.; Jönsson, A.-S.; Wickström, P. Membrane cleaning—A case study in a sulphite pulp mill bleach plant. Desalination 2001, 141, 259–268. [Google Scholar] [CrossRef]
- Jonsson, J.; Kristofersson, J.; Samuelsson, C. Energikartläggning av Integreratmassa-Och Pappersbruk: Energy Survey of Integrated Pulp and Paaper Mill; Diploma Work; Linnæus University: Växjö, Sweden, 2011. [Google Scholar]
- Pandey, K.K. A study of chemical structure of soft and hardwood and wood polymers by FTIR spectroscopy. J. Appl. Polym. Sci. 1999, 71, 1969–1975. [Google Scholar] [CrossRef]
- Litvak, I.; Anker, Y.; Cohen, H. On-line in situ determination of deuterium content in water via FTIR spectroscopy. RSC Adv. 2018, 8, 28472–28479. [Google Scholar] [CrossRef]
- Yousefi, M. A Fast Method for Synthesis Magnesium Hydroxide Nanoparticles, Thermal Stable and Flame Retardant Poly vinyl alcohol Nanocomposite. J. Nanostruct. 2014, 4, 383–388. [Google Scholar] [CrossRef]
- Palamarchuk, I.A.; Brovko, O.S.; Bogolitsyn, K.G.; Boitsova, T.A.; Ladesov, A.V.; Ivakhnov, A.D. Relationship of the structure and ion-exchange properties of polyelectrolyte complexes based on biopolymers. Russ. J. Appl. Chem. 2015, 88, 103–109. [Google Scholar] [CrossRef]
- Rönnols, J.; Schweinebarth, H.; Jacobs, A.; Stevanic, J.S.; Olsson, A.-M.; Reimann, A.; Aldaeus, F. Aldeaus Structural changes in softwood kraft lignin during nonoxidative thermal treatment. Nord. Pulp Pap. Res. J. 2015, 30, 550–561. [Google Scholar] [CrossRef]
- Faruk, O.; Sain, M. Lignin in Polymer Composites, 1st ed.; Elsevier: Cambridge, UK, 2016. [Google Scholar]
- Coates, J. Interpretation of infrared spectra, a practical approach. In Encyclopedia of Analytical Chemistry: Applications, Theory and Instrumentation; Meyers, R.A., Ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2000. [Google Scholar]
- Traoré, M.; Kaal, J.; Cortizas, A.M. Differentiation between pine woods according to species and growing location using FTIR-ATR. Wood Sci. Technol. 2018, 52, 487–504. [Google Scholar] [CrossRef] [PubMed]
- Arges, C.G.; Ramani, V. Two-dimensional NMR spectroscopy reveals cation-triggered backbone degradation in polysulfone-based anion exchange membranes. Proc. Natl. Acad. Sci. USA 2013, 110, 2490–2495. [Google Scholar] [CrossRef] [PubMed]
- Landucci, L.; Zinkel, D. The1H and13C NMR Spectra of the Abietadienoic Resin Acids. Holzforschung 1991, 45, 341–346. [Google Scholar] [CrossRef]
- Crestini, C.; Lange, H.; Sette, M.; Argyropoulos, D.S. On the structure of softwood kraft lignin. Green Chem. 2017, 19, 4104–4121. [Google Scholar] [CrossRef]

| Sample | Arabinan | Galactan | Glucan | Xylan | Mannan |
|---|---|---|---|---|---|
| Pristine | - | - | * | - | - |
| Fouled | * | * | 15 | * | * |
| 1 h cleaned | - | * | 10 | * | * |
| 20 h cleaned | - | - | 8 | * | * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rudolph, G.; Al-Rudainy, B.; Thuvander, J.; Jönsson, A.-S. Comprehensive Analysis of Foulants in an Ultrafiltration Membrane Used for the Treatment of Bleach Plant Effluent in a Sulfite Pulp Mill. Membranes 2021, 11, 201. https://doi.org/10.3390/membranes11030201
Rudolph G, Al-Rudainy B, Thuvander J, Jönsson A-S. Comprehensive Analysis of Foulants in an Ultrafiltration Membrane Used for the Treatment of Bleach Plant Effluent in a Sulfite Pulp Mill. Membranes. 2021; 11(3):201. https://doi.org/10.3390/membranes11030201
Chicago/Turabian StyleRudolph, Gregor, Basel Al-Rudainy, Johan Thuvander, and Ann-Sofi Jönsson. 2021. "Comprehensive Analysis of Foulants in an Ultrafiltration Membrane Used for the Treatment of Bleach Plant Effluent in a Sulfite Pulp Mill" Membranes 11, no. 3: 201. https://doi.org/10.3390/membranes11030201
APA StyleRudolph, G., Al-Rudainy, B., Thuvander, J., & Jönsson, A.-S. (2021). Comprehensive Analysis of Foulants in an Ultrafiltration Membrane Used for the Treatment of Bleach Plant Effluent in a Sulfite Pulp Mill. Membranes, 11(3), 201. https://doi.org/10.3390/membranes11030201