Enhanced Gas Separation Prowess Using Functionalized Lignin-Free Lignocellulosic Biomass/Polysulfone Composite Membranes
Abstract
1. Introduction
2. Membranes Mechanisms
2.1. Materials and Chemicals Used
2.2. Lignin-Free LCB Functionalization
2.3. Fabrication of Pure PSF and Lignin-Free Composite Membranes
2.4. Characterizations of Materials
2.5. Gas Separation Performance Evaluation
3. Results and Discussion
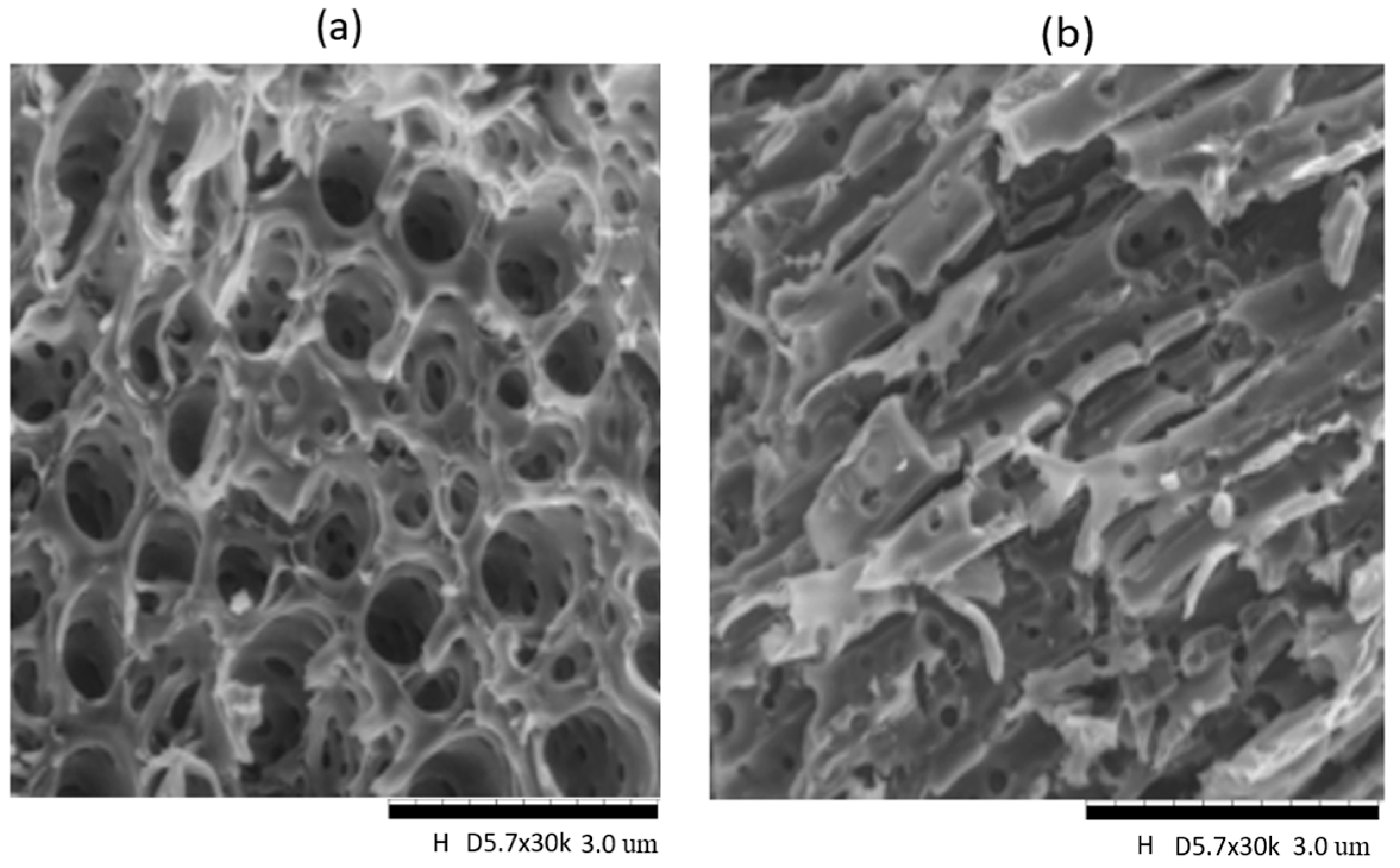
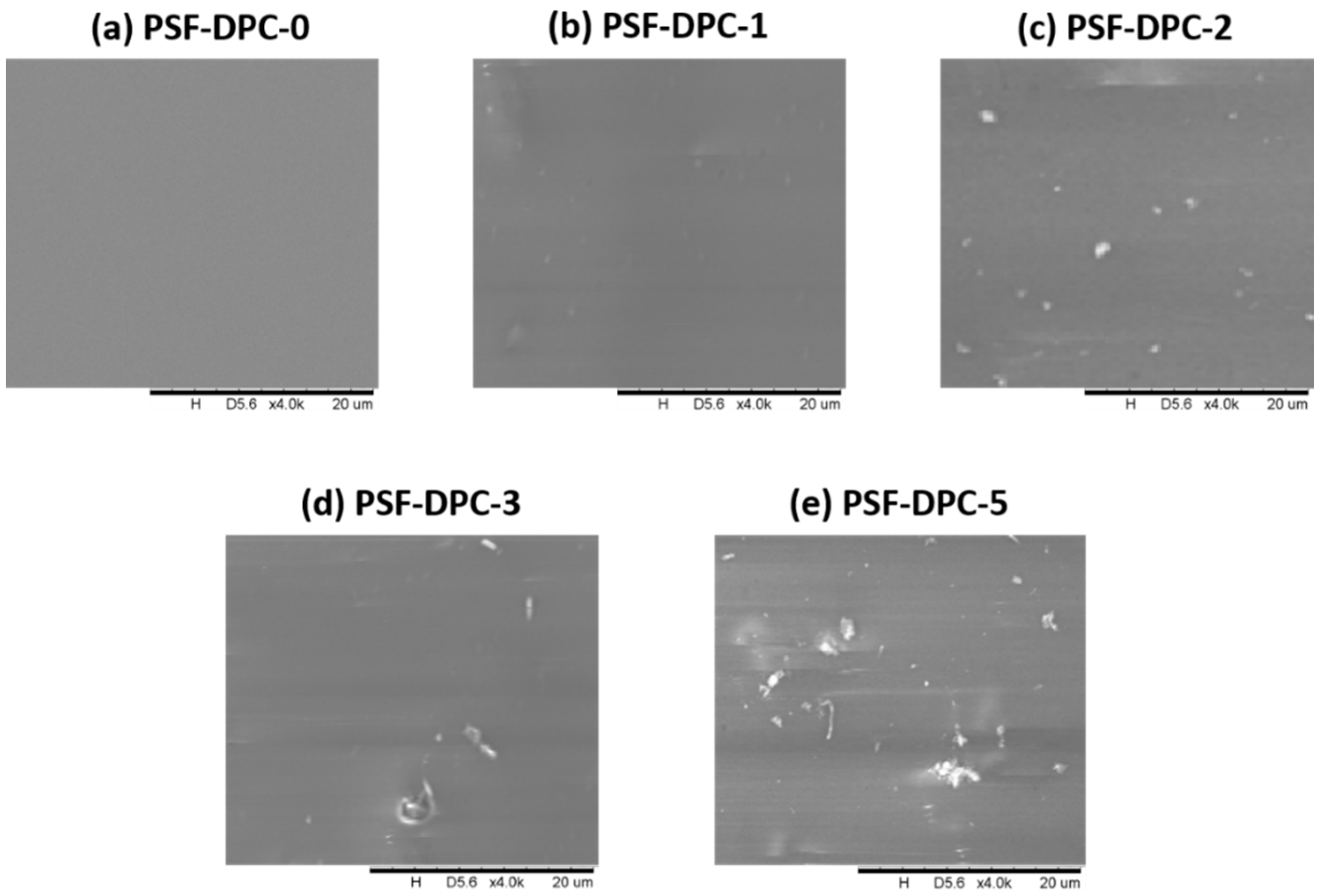
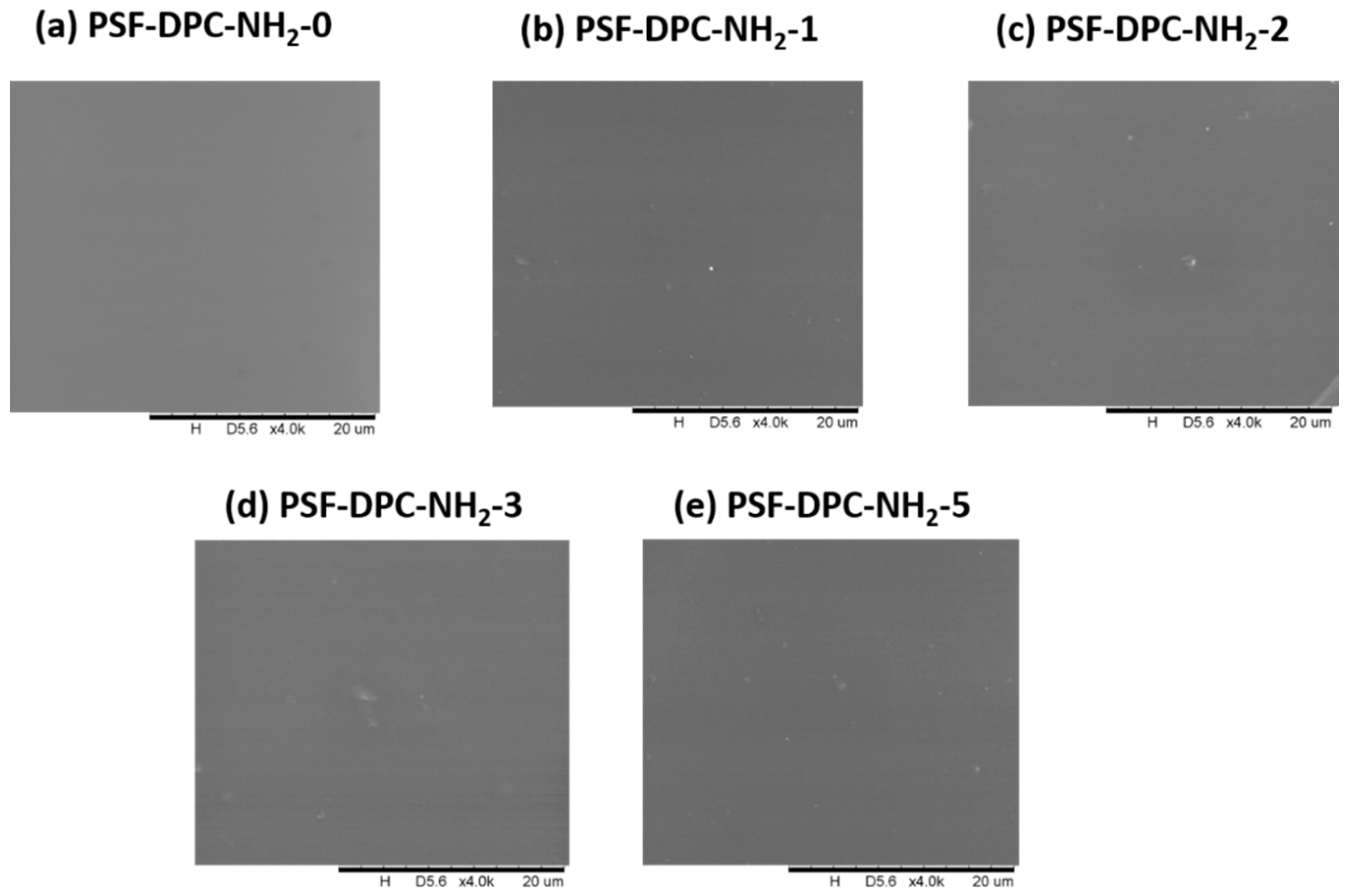
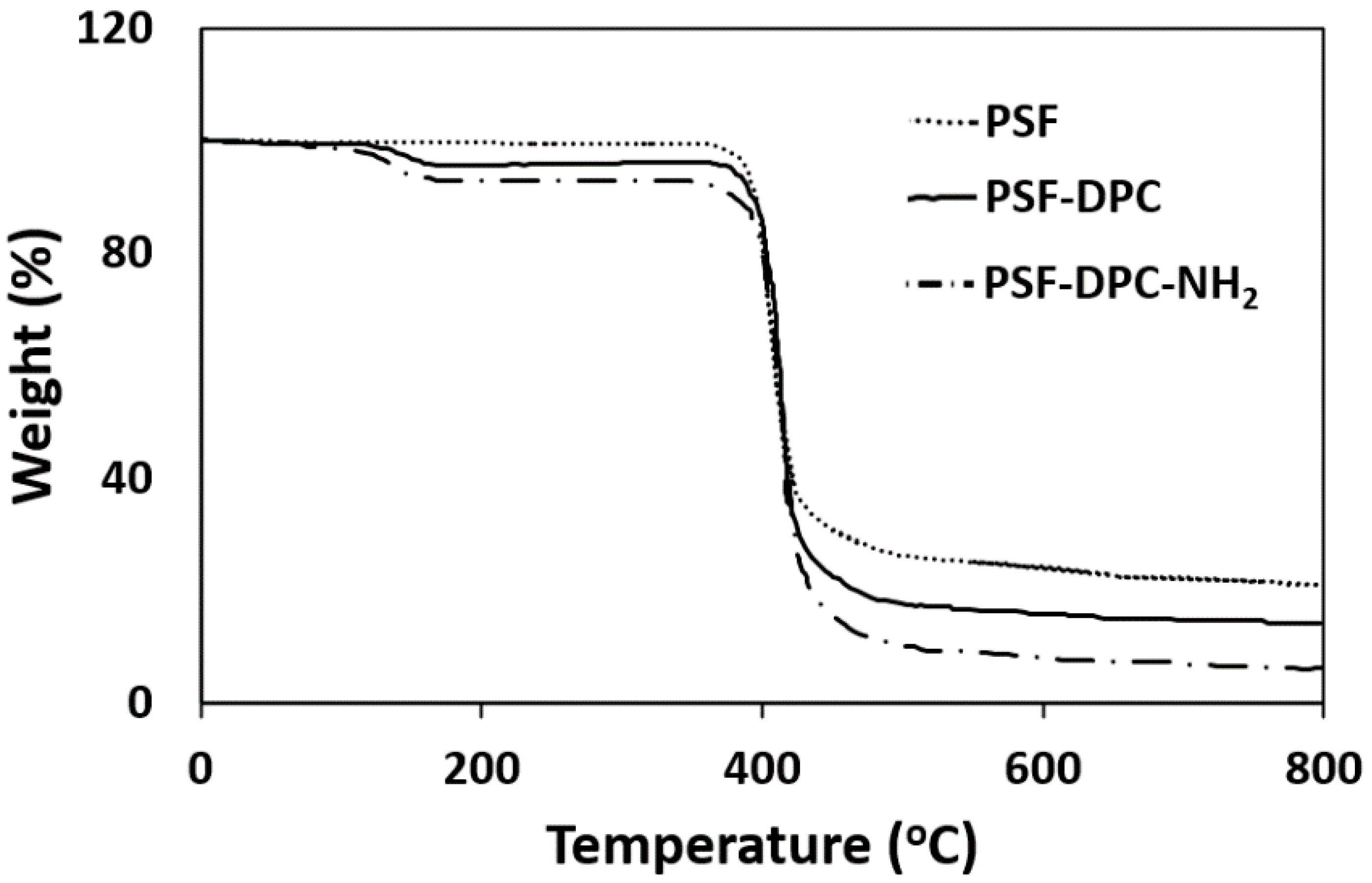
3.1. Morphological and Thermal Investigations
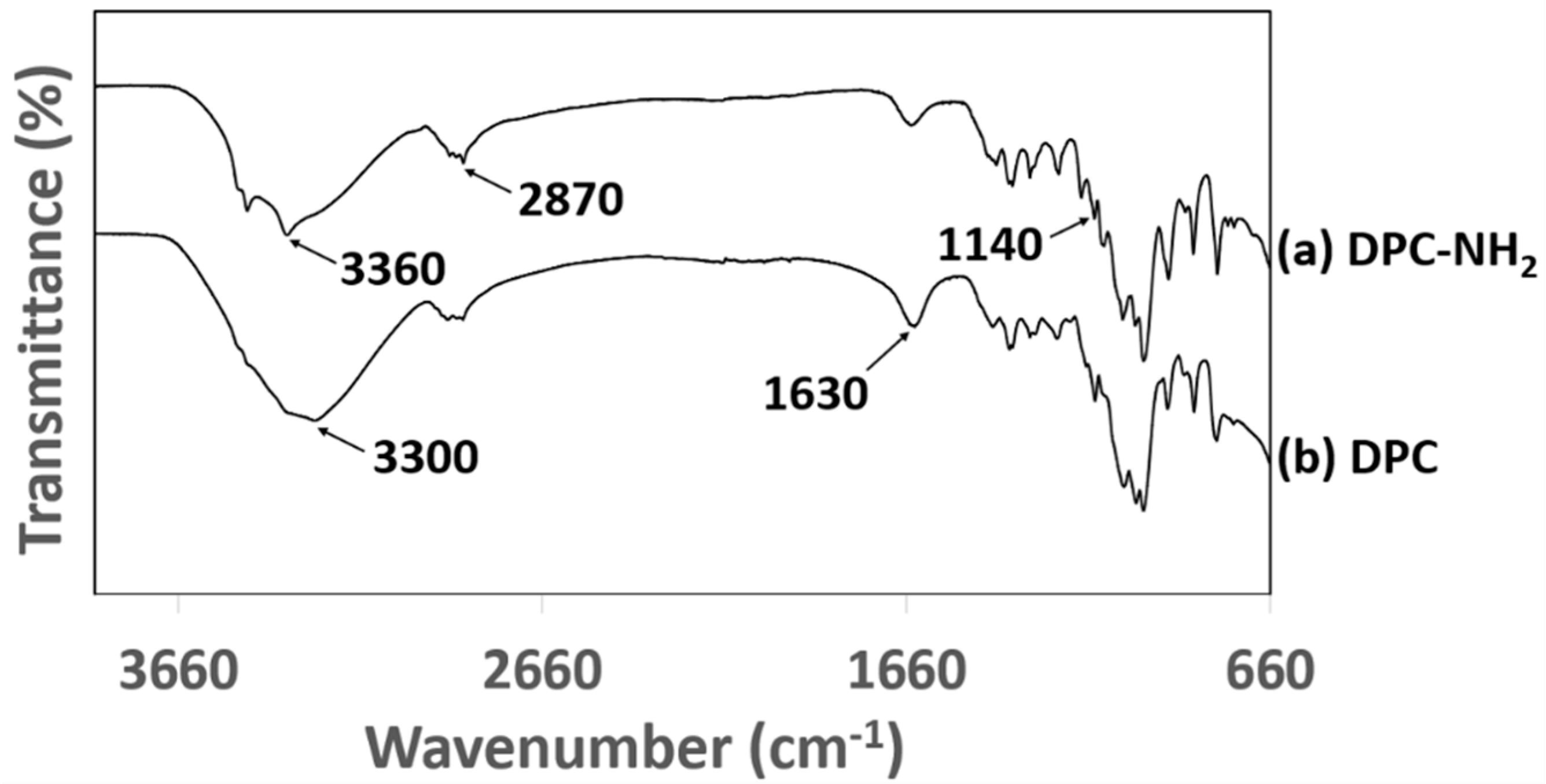
3.2. Composition and IR-Spectra Analyses
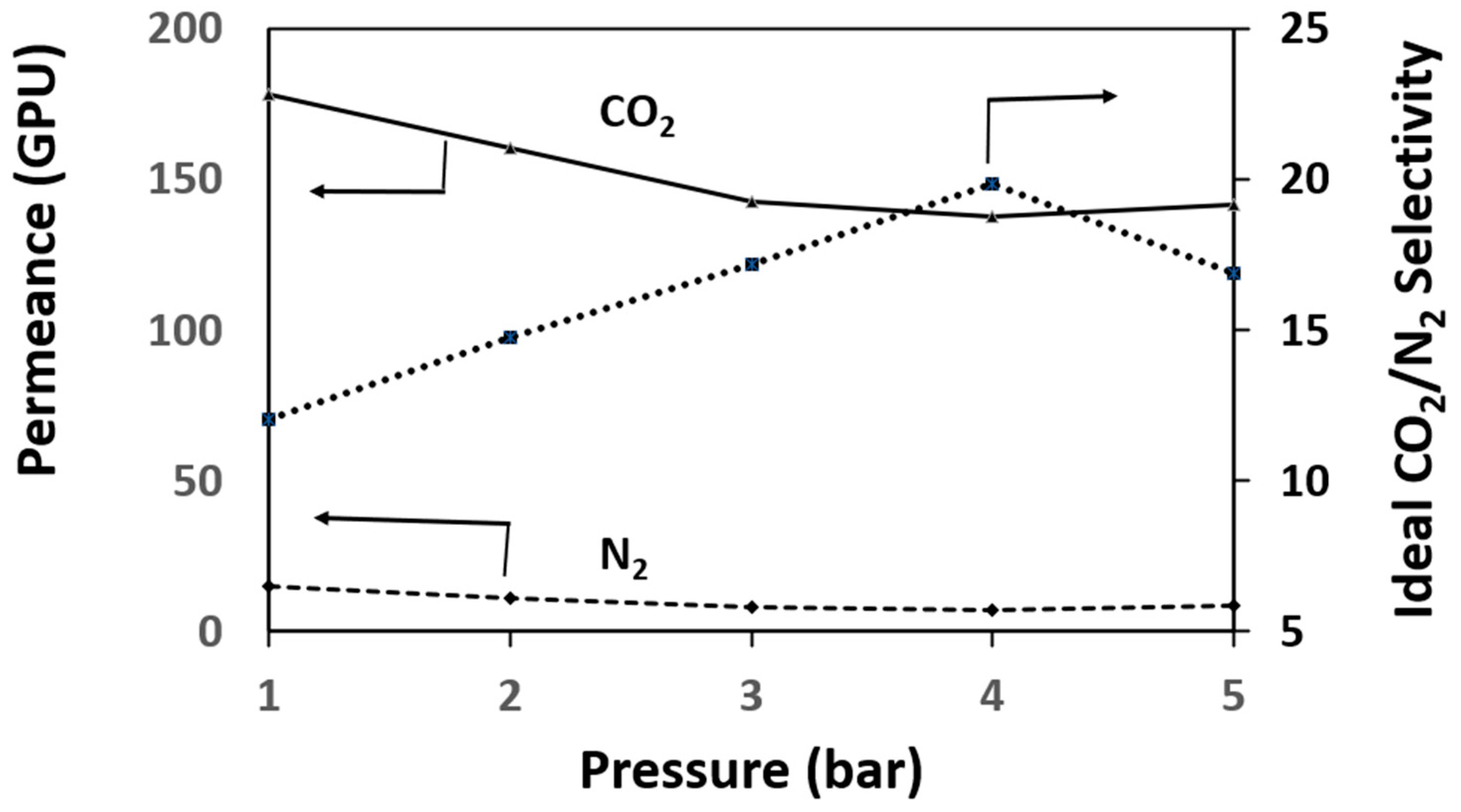
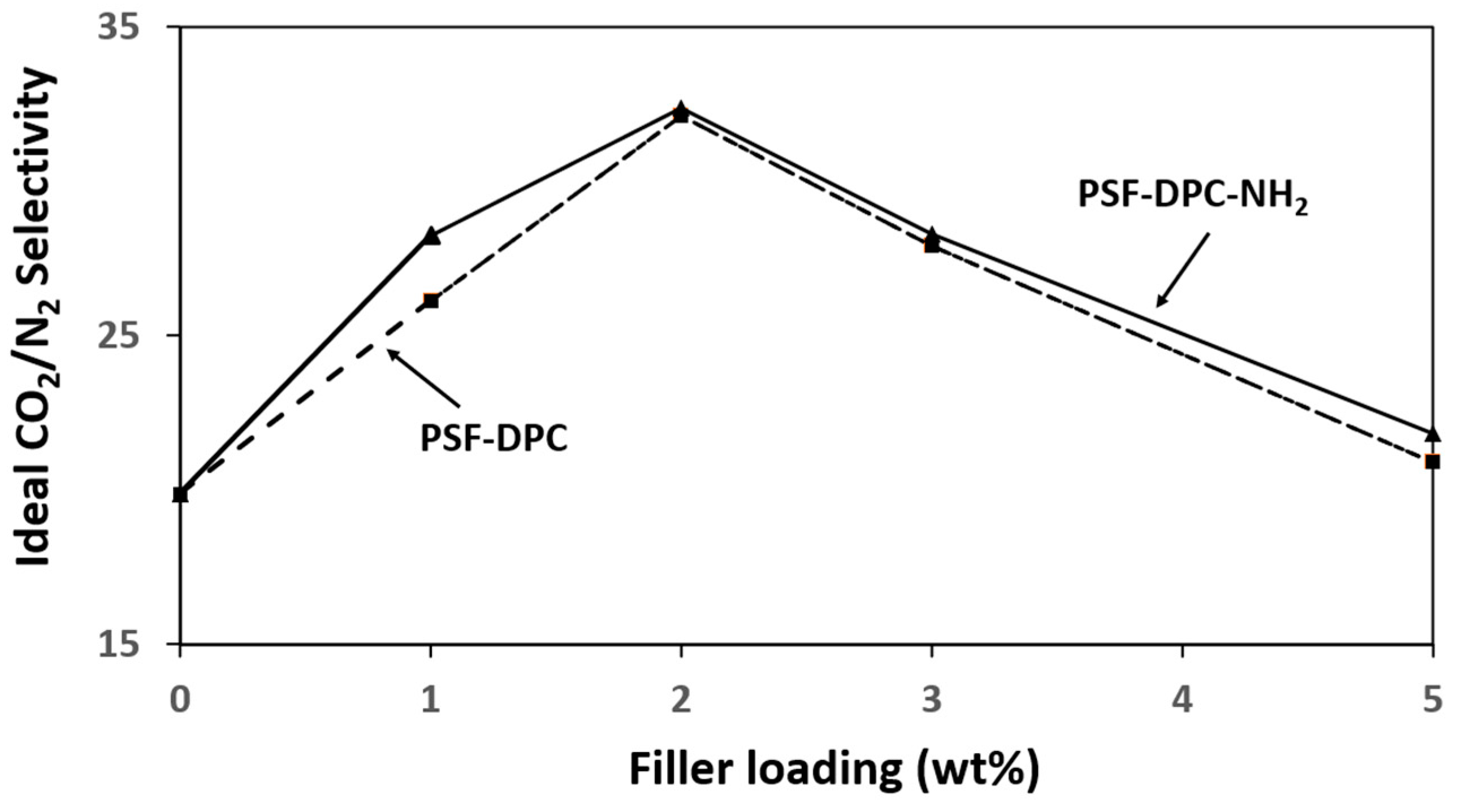
3.3. Gas Performance Measurement
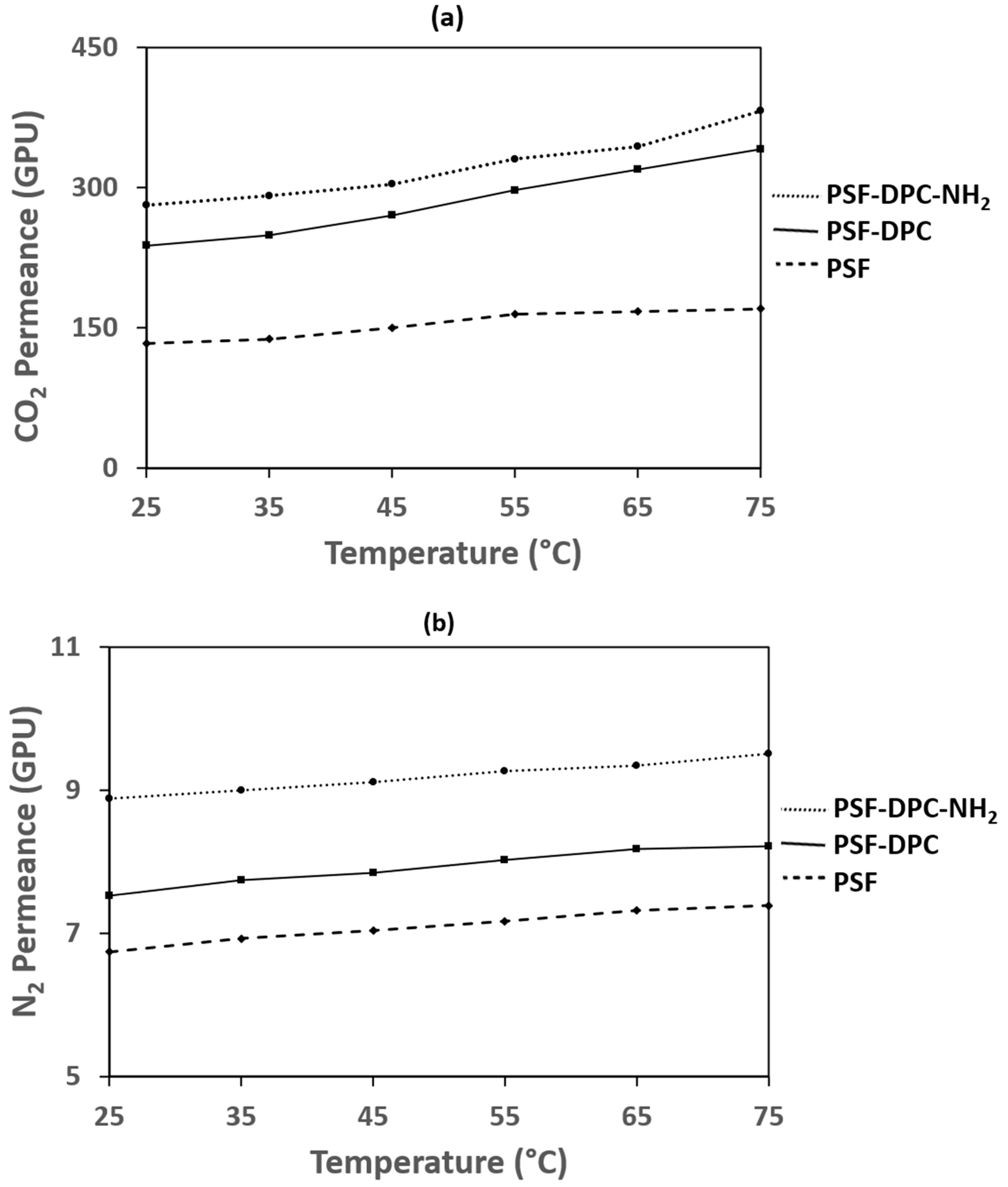
3.4. Effect of Temperature on The Optimum Membranes
3.5. Mechanical and Thermal Properties of the Optimum Membranes
3.6. Results Comparison with Literature
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, J.; Zhu, F.; Ma, X. Industrial Application of a Deep Purification Technology for Flue Gas Involving Phase-Transition Agglomeration and Dehumidification. Engineering 2018, 4, 416–420. [Google Scholar] [CrossRef]
- Tranier, J.P.; Dubettier, R.; Darde, A.; Perrin, N. Air Separation, flue gas compression and purification units for oxy-coal combustion systems. In Proceedings of the Energy Procedia; Elsevier Ltd.: Amsterdam, The Netherlands, 2011; Volume 4, pp. 966–971. [Google Scholar]
- Andersen, K.; Bræstrup, F.; Kammer Hansen, K. Fabrication of highly porous LSM/CGO cell stacks for electrochemical flue gas purification. Ceram. Int. 2013, 39, 2159–2163. [Google Scholar] [CrossRef]
- Huang, Y.; Su, W.; Wang, R.; Zhao, T. Removal of Typical Industrial Gaseous Pollutants: From Carbon, Zeolite, and Metal-organic Frameworks to Molecularly Imprinted Adsorbents. Aerosol Air Qual. Res. 2019, 19, 2130–2150. [Google Scholar] [CrossRef]
- Dalane, K.; Dai, Z.; Mogseth, G.; Hillestad, M.; Deng, L. Potential applications of membrane separation for subsea natural gas processing: A review. J. Nat. Gas Sci. Eng. 2017, 39, 101–117. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Leo, C.P.; Shukor, S.R.A. Preparation of silica/γ-alumina membrane with bimodal porous layer for improved permeation in ions separation. J. Am. Ceram. Soc. 2008, 91, 2009–2014. [Google Scholar] [CrossRef]
- Kiadehi, A.D.; Jahanshahi, M.; Rahimpour, A.; Ghoreyshi, S.A.A. The effect of functionalized carbon nano-fiber (CNF) on gas separation performance of polysulfone (PSf) membranes. Chem. Eng. Process. Process Intensif. 2015, 90, 41–48. [Google Scholar] [CrossRef]
- Bernardo, P.; Clarizia, G. 30 Years of Membrane Technology for Gas Separation. Chem. Eng. Trans. 2013, 32, 1999–2004. [Google Scholar] [CrossRef]
- Park, H.B.; Kamcev, J.; Robeson, L.M.; Elimelech, M.; Freeman, B.D. Maximizing the right stuff: The trade-off between membrane permeability and selectivity. Science 2017, 356, 1138–1148. [Google Scholar] [CrossRef]
- Amusa, A.A.; Ahmad, A.L.; Adewole, J.K. Mechanism and compatibility of pretreated lignocellulosic biomass and polymeric mixed matrix membranes: A review. Membranes 2020, 10, 370. [Google Scholar] [CrossRef]
- Najimu, M.O.; Aljundi, I.H. Separation performance of CO2 by hybrid membrane comprising nanoporous carbide derived carbon. J. Nat. Gas Sci. Eng. 2018, 59, 9–20. [Google Scholar] [CrossRef]
- Adewole, J.K. Transport properties of gases through integrally skinned asymmetric composite membranes prepared from date pit powder and polysulfone. J. Appl. Polym. Sci. 2016, 133, 1–8. [Google Scholar] [CrossRef]
- Chuah, C.Y.; Goh, K.; Yang, Y.; Gong, H.; Li, W.; Karahan, H.E.; Guiver, M.D.; Wang, R.; Bae, T.H. Harnessing filler materials for enhancing biogas separation membranes. Chem. Rev. 2018, 118, 8655–8769. [Google Scholar] [CrossRef] [PubMed]
- Tena, A.; Fernández, L.; Sánchez, M.; Palacio, L.; Lozano, A.E.; Hernández, A.; Prádanos, P. Mixed matrix membranes of 6FDA-6FpDA with surface functionalized γ-alumina particles. An analysis of the improvement of permselectivity for several gas pairs. Chem. Eng. Sci. 2010, 65, 2227–2235. [Google Scholar] [CrossRef]
- Khan, M.Y.; Khan, A.; Adewole, J.K.; Naim, M.; Basha, S.I.; Aziz, M.A. Biomass derived carboxylated carbon nanosheets blended polyetherimide membranes for enhanced CO2/CH4 separation. J. Nat. Gas Sci. Eng. 2020, 75, 103156. [Google Scholar] [CrossRef]
- Sharma, A.; Thakur, M.; Bhattacharya, M.; Mandal, T.; Goswami, S. Commercial application of cellulose nano-composites–A review. Biotechnol. Rep. 2019, 21, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Amusa, A.A.; Ahmad, A.L.; Adewole, J.K. Study on Lignin-free Lignocellulosic Biomass and PSF-PEG Membrane Compatibility. BioResource 2021, 16, 1063–1075. [Google Scholar] [CrossRef]
- Akhlaghi, S.P.; Zaman, M.; Mohammed, N.; Brinatti, C.; Batmaz, R.; Berry, R.; Loh, W.; Tam, K.C. Synthesis of amine functionalized cellulose nanocrystals: Optimization and characterization. Carbohydr. Res. 2015, 409, 48–55. [Google Scholar] [CrossRef]
- Ismail, A.F.; Lai, P.Y. Effects of phase inversion and rheological factors on formation of defect-free and ultrathin-skinned asymmetric polysulfone membranes for gas separation. Sep. Purif. Technol. 2003, 33, 127–143. [Google Scholar] [CrossRef]
- Sluiter, J.B.; Ruiz, R.O.; Scarlata, C.J.; Sluiter, A.D.; Templeton, D.W. Compositional analysis of lignocellulosic feedstocks. 1. Review and description of methods. J. Agric. Food Chem. 2010, 58, 9043–9053. [Google Scholar] [CrossRef]
- Mansor, A.M.; Lim, J.S.; Ani, F.N.; Hashim, H.; Ho, W.S. Characteristics of cellulose, hemicellulose and lignin of MD2 pineapple biomass. Chem. Eng. Trans. 2019, 72, 79–84. [Google Scholar] [CrossRef]
- Stetefeld, J.; McKenna, S.A.; Patel, T.R. Dynamic light scattering: A practical guide and applications in biomedical sciences. Biophys. Rev. 2016, 8, 409–427. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, M.; Bahkali, A.H. Valorization of date palm (Phoenix dactylifera) fruit processing by-products and wastes using bioprocess technology–Review. Saudi J. Biol. Sci. 2013, 20, 105–120. [Google Scholar] [CrossRef]
- Ghnimi, S.; Umer, S.; Karim, A.; Kamal-Eldin, A. Date fruit (Phoenix dactylifera L.): An underutilized food seeking industrial valorization. NFS J. 2017, 6, 1–10. [Google Scholar] [CrossRef]
- Elnajjar, E.; Al-Zuhair, S.; Hasan, S.; Almardeai, S.; Al Omari, S.A.B.; Hilal-Alnaqbi, A. Morphology characterization and chemical composition of United Arab Emirates date seeds and their potential for energy production. Energy 2020, 213, 118810. [Google Scholar] [CrossRef]
- Hussain, A.; Farooq, A.; Bassyouni, M.I.; Sait, H.H.; El-Wafa, M.A.; Hasan, S.W.; Ani, F.N. Pyrolysis of Saudi Arabian date palm waste: A viable option for converting waste into wealth. Life Sci. J. 2014, 11, 667–671. [Google Scholar] [CrossRef]
- Azzam, F.; Heux, L.; Putaux, J.L.; Jean, B. Preparation by grafting onto, characterization, and properties of thermally responsive polymer-decorated cellulose nanocrystals. Biomacromolecules 2010, 11, 3652–3659. [Google Scholar] [CrossRef]
- Filpponen, I.; Sadeghifar, H.; Argyropoulos, D.S. Photoresponsive cellulose nanocrystals. Nanomater. Nanotechnol. 2011, 1, 34–43. [Google Scholar] [CrossRef]
- Mahmoud, K.A.; Mena, J.A.; Male, K.B.; Hrapovic, S.; Kamen, A.; Luong, J.H.T. Effect of surface charge on the cellular uptake and cytotoxicity of fluorescent labeled cellulose nanocrystals. ACS Appl. Mater. Interfaces 2010, 2, 2924–2932. [Google Scholar] [CrossRef]
- Nielsen, L.J.; Eyley, S.; Thielemans, W.; Aylott, J.W. Dual fluorescent labelling of cellulose nanocrystals for pH sensing. Chem. Commun. 2010, 46, 8929–8931. [Google Scholar] [CrossRef]
- Pahimanolis, N.; Hippi, U.; Johansson, L.S.; Saarinen, T.; Houbenov, N.; Ruokolainen, J.; Seppälä, J. Surface functionalization of nanofibrillated cellulose using click-chemistry approach in aqueous media. Cellulose 2011, 18, 1201–1212. [Google Scholar] [CrossRef]
- Hemraz, U.D.; Boluk, Y.; Sunasee, R. Amine-decorated nanocrystalline cellulose surfaces: Synthesis, characterization, and surface properties. Can. J. Chem. 2013, 91, 974–981. [Google Scholar] [CrossRef]
- Abu-Thabit, N.Y.; Judeh, A.A.; Hakeem, A.S.; Ul-Hamid, A.; Umar, Y.; Ahmad, A. Isolation and characterization of microcrystalline cellulose from date seeds (Phoenix dactylifera L.). Int. J. Biol. Macromol. 2020, 155, 730–739. [Google Scholar] [CrossRef]
- Park, S.; Baker, J.O.; Himmel, M.E.; Parilla, P.A.; Johnson, D.K. Cellulose crystallinity index: Measurement techniques and their impact on interpreting cellulase performance. Biotechnol. Biofuels 2010, 10, 1–10. [Google Scholar] [CrossRef]
- Harris, D.; DeBolt, S. Relative Crystallinity of Plant Biomass: Studies on Assembly, Adaptation and Acclimation. PLoS ONE 2008, 3, e2897. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Xing, D.; Li, J. FTIR Studies of the Changes in Wood Chemistry from Wood Forming Tissue under Inclined Treatment. Energy Procedia 2012, 16, 758–762. [Google Scholar] [CrossRef]
- Bernard, F.L.; Rodrigues, D.M.; Polesso, B.B.; Chaban, V.V.; Seferin, M.; Vecchia, F.D.; Einloft, S. Development of inexpensive cellulosebased sorbents for carbon dioxide. Braz. J. Chem. Eng. 2019, 36, 511–521. [Google Scholar] [CrossRef]
- Pouresmaeel-Selakjani, P.; Jahanshahi, M.; Peyravi, M. Synthesis of cellulose/silica nanocomposite through electrostatic interaction to reinforce polysulfone membranes. Cellulose 2017, 24, 1333–1353. [Google Scholar] [CrossRef]
- Hägg, M.-B. Gas Permeation: Permeability, Permeance, and Separation Factor. In Encyclopedia of Membranes; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–4. [Google Scholar]
- Mason, C.R.; Maynard-Atem, L.; Heard, K.W.J.; Satilmis, B.; Budd, P.M.; Friess, K.; Lancì, M.; Bernardo, P.; Clarizia, G.; Jansen, J.C. Enhancement of CO2 affinity in a polymer of intrinsic microporosity by amine modification. Macromolecules 2014, 47, 1021–1029. [Google Scholar] [CrossRef] [PubMed]
- Ghosal, K.; Chern, R.T.; Freeman, B.D.; Daly, W.H.; Negulescu, I.I. Effect of basic substituents on gas sorption and permeation in polysulfone. Macromolecules 1996, 29, 4360–4369. [Google Scholar] [CrossRef]
- Yu, D.; Matteucci, S.; Stangland, E.; Calverley, E.; Wegener, H.; Anaya, D. Quantum chemistry calculation and experimental study of CO2/CH4 and functional group interactions for the design of solubility selective membrane materials. J. Memb. Sci. 2013, 441, 137–147. [Google Scholar] [CrossRef]
- Torstensen, J.; Helberg, R.M.L.; Deng, L.; Gregersen, Ø.W.; Syverud, K. PVA/nanocellulose nanocomposite membranes for CO2 separation from flue gas. Int. J. Greenh. Gas Control 2019, 81, 93–102. [Google Scholar] [CrossRef]
- Biondo, L.D.; Duarte, J.; Zeni, M.; Godinho, M. A dual-mode model interpretation of CO2/CH4 permeability in polysulfone membranes at low pressures. An. Acad. Bras. Cienc. 2018, 90, 1855–1864. [Google Scholar] [CrossRef]
- Ionita, M.; Vasile, E.; Crica, L.E.; Voicu, S.I.; Pandele, A.M.; Dinescu, S.; Predoiu, L.; Galateanu, B.; Hermenean, A.; Costache, M. Synthesis, characterization and in vitro studies of polysulfone/graphene oxide composite membranes. Compos. Part B Eng. 2015, 72, 108–115. [Google Scholar] [CrossRef]
- Yong, W.F.; Zhang, H. Recent advances in polymer blend membranes for gas separation and pervaporation. Prog. Mater. Sci. 2021, 116, 100713. [Google Scholar] [CrossRef]
- Khan, A.L.; Li, X.; Vankelecom, I.F.J. SPEEK/Matrimid blend membranes for CO2 separation. J. Memb. Sci. 2011, 380, 55–62. [Google Scholar] [CrossRef]
- Bai, H.; Zhou, Y.; Zhang, L. Morphology and Mechanical Properties of a New Nanocrystalline Cellulose/Polysulfone Composite Membrane. Adv. Polym. Technol. 2015, 34. [Google Scholar] [CrossRef]
- Kamal, N.; Ahzi, S.; Kochkodan, V. Polysulfone/halloysite composite membranes with low fouling properties and enhanced compaction resistance. Appl. Clay Sci. 2020, 199, 105873. [Google Scholar] [CrossRef]
- Heidary, M.; Jafari, S.H.; Khonakdar, H.A.; Wagenknecht, U.; Heinrich, G.; Boldt, R. Effect of end-capped nanosilica on mechanical properties and microstructure of LLLDPE/EVA blends. J. Appl. Polym. Sci. 2013, 127, 1172–1179. [Google Scholar] [CrossRef]
- Chapala, P.P.; Bermeshev, M.V.; Starannikova, L.E.; Shantarovich, V.P.; Gavrilova, N.N.; Avakyan, V.G.; Filatova, M.P.; Yampolskii, Y.P.; Finkelshtein, E.S. Gas-transport properties of new mixed matrix membranes based on addition poly(3-trimethylsilyltricyclononene-7) and substituted calixarenes. J. Memb. Sci. 2015, 474, 83–91. [Google Scholar] [CrossRef]
- Pathak, V.M. Navneet Review on the current status of polymer degradation: A microbial approach. Bioresour. Bioprocess. 2017, 4, 1–31. [Google Scholar] [CrossRef]
- Wang, J.; Wu, B.; Sierra, J.M.; He, C.; Hu, Z.; Wang, W. Influence of particle size distribution on anaerobic degradation of phenol and analysis of methanogenic microbial community. Environ. Sci. Pollut. Res. 2020, 27, 10391–10403. [Google Scholar] [CrossRef] [PubMed]
- Comesaña-Gándara, B.; Chen, J.; Bezzu, C.G.; Carta, M.; Rose, I.; Ferrari, M.C.; Esposito, E.; Fuoco, A.; Jansen, J.C.; McKeown, N.B. Redefining the Robeson upper bounds for CO2/CH4 and CO2/N2 separations using a series of ultrapermeable benzotriptycene-based polymers of intrinsic microporosity. Energy Environ. Sci. 2019, 12, 2733–2740. [Google Scholar] [CrossRef]
- Mozaffari, V.; Sadeghi, M.; Fakhar, A.; Khanbabaei, G.; Ismail, A.F. Gas separation properties of polyurethane/poly(ether-block-amide) (PU/PEBA) blend membranes. Sep. Purif. Technol. 2017, 185, 202–214. [Google Scholar] [CrossRef]
- Jiao, C.; Li, Z.; Li, X.; Wu, M.; Jiang, H. Improved CO2/N2 separation performance of Pebax composite membrane containing polyethyleneimine functionalized ZIF-8. Sep. Purif. Technol. 2021, 259, 118190. [Google Scholar] [CrossRef]
- Julian, H.; Sutrisna, P.D.; Hakim, A.N.; Harsono, H.O.; Hugo, Y.A.; Wenten, I.G. Nano-silica/polysulfone asymmetric mixed-matrix membranes (MMMs) with high CO2 permeance in the application of CO2/N2 separation. Polym. Technol. Mater. 2019, 58, 678–689. [Google Scholar] [CrossRef]
- Laeeq, A.; Klaysom, C.; Gahlaut, A.; Vankelecom, I.F.J. Polysulfone acrylate membranes containing functionalized mesoporous MCM-41 for CO2 separation. J. Memb. Sci. 2013, 436, 145–153. [Google Scholar] [CrossRef]
- Wijiyanti, R.; Ubaidillah, A.N.; Gunawan, T.; Karim, Z.A.; Ismail, A.F.; Smart, S.; Lin, R.; Widiastuti, N. Polysulfone mixed matrix hollow fiber membranes using zeolite templated carbon as a performance enhancement filler for gas separation. Chem. Eng. Res. Des. 2019, 150, 274–288. [Google Scholar] [CrossRef]
- Yousef, S.; Šereika, J.; Tonkonogovas, A.; Hashem, T.; Mohamed, A. CO2/CH4, CO2/N2 and CO2/H2 selectivity performance of PES membranes under high pressure and temperature for biogas upgrading systems. Environ. Technol. Innov. 2021, 21, 101339. [Google Scholar] [CrossRef]



| Samples | Carbon, C (%) | Hydrogen, H (%) | Nitrogen, N (%) | Sulfur, S (%) | Hemicellulose (wt%) | Cellulose (wt%) | Kappa Number | XRD | |
|---|---|---|---|---|---|---|---|---|---|
| 2 θ (°) | d-Spacing (Å) | ||||||||
| DPC | 54.01 ± 1.11 | 10.65 ± 0.04 | 0 | 0.79 ± 0.03 | 17.15 ± 1.40 | 79.73 ± 1.22 | 2.80 | 22.69 | 2.4 |
| DPC-NH2 | 55.03 ± 0.78 | 11.31 ± 0.25 | 2.84 ± 0.07 | 0.75 ± 0.01 | 17.21 ± 1.01 | 79.92 ± 1.05 | 2.79 | 23.87 | 2.5 |
| Filler Loading (wt%) | CO2 Gas Permeability (GPU) | N2 Gas Permeance (GPU) | ||
|---|---|---|---|---|
| PSF-DPC | PSF-DPC-NH2 | PSF-DPC | PSF-DPC-NH2 | |
| 0 | 137.65 ± 0.01 | 137.66 ± 0.03 | 6.93 ± 0.04 | 6.93 ± 0.05 |
| 1 | 164.13 ± 0.02 | 222.37 ± 0.02 | 6.29 ± 0.03 | 7.87 ± 0.05 |
| 2 | 248.84 ± 0.01 | 291.20 ± 0.01 | 7.75 ± 0.02 | 9.00 ± 0.01 |
| 3 | 322.97 ± 0.01 | 344.15 ± 0.02 | 11.58 ± 0.04 | 12.18 ± 0.03 |
| 5 | 358.32 ± 0.02 | 381.21 ± 0.01 | 17.16 ± 0.01 | 17.47 ± 0.02 |
| Membrane | Tensile Strength (MPa) | Young’s Modulus (GPa) | Tg (°C) |
|---|---|---|---|
| Pristine PSF | 2.52 ± 10.16 | 2.02 ± 8.24 | 165.3 ± 1.07 |
| PSF-DPC | 2.90 ± 10.23 | 2.51 ± 6.41 | 164.1 ± 0.98 |
| PSF-DPC-NH2 | 3.16 ± 20.41 | 2.63 ± 7.82 | 162.6 ± 0.62 |
| Polymer-Filler | Filler Loading (wt%) | Pressure (bar) | CO2 Permeance (GPU) | Ideal CO2/N2 Selectivity | Reference |
|---|---|---|---|---|---|
| PU/PEBA | 60 | 10 | ~95.00 * | ~30.00 | [55] |
| PEBAX/PEI-ZIF-8 | 5 | 30 | ~13.00 | ~49.00 | [56] |
| PSF-Nanosilica | 2 | ~2 | ~30.90 * | ~7.70 | [57] |
| PSF/fMCM-41 | 20 | 10 | ~9.13 * | ~32.97 | [58] |
| PSF/ZTC | 0.4 | 5 | 58.50 | 11.62 | [59] |
| PES/CNT | 1 | 1 | ~10.90 * | ~3.07 | [60] |
| PSF/DP | 2 | 10 | ~8.46 | ~1.65 | [12] |
| PSF/DPC | 2 | 4 | ~322.97 | ~32.11 | Current study |
| PSF/DPC-NH2 | 2 | 4 | ~344.15 | ~32.35 | Current study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amusa, A.A.; Ahmad, A.L.; Jimoh, A.K. Enhanced Gas Separation Prowess Using Functionalized Lignin-Free Lignocellulosic Biomass/Polysulfone Composite Membranes. Membranes 2021, 11, 202. https://doi.org/10.3390/membranes11030202
Amusa AA, Ahmad AL, Jimoh AK. Enhanced Gas Separation Prowess Using Functionalized Lignin-Free Lignocellulosic Biomass/Polysulfone Composite Membranes. Membranes. 2021; 11(3):202. https://doi.org/10.3390/membranes11030202
Chicago/Turabian StyleAmusa, Abiodun Abdulhameed, Abdul Latif Ahmad, and Adewole Kayode Jimoh. 2021. "Enhanced Gas Separation Prowess Using Functionalized Lignin-Free Lignocellulosic Biomass/Polysulfone Composite Membranes" Membranes 11, no. 3: 202. https://doi.org/10.3390/membranes11030202
APA StyleAmusa, A. A., Ahmad, A. L., & Jimoh, A. K. (2021). Enhanced Gas Separation Prowess Using Functionalized Lignin-Free Lignocellulosic Biomass/Polysulfone Composite Membranes. Membranes, 11(3), 202. https://doi.org/10.3390/membranes11030202







