Concentration of Municipal MBBR Effluent by FO for Resource Recovery: Batch Experiments in Side-Stream Configuration
Abstract
1. Introduction
- To demonstrate what membrane performance can be expected,
- To assess flux decline due to membrane fouling using different strategies for pre-treatment,
- To assess flux recovery by applying high-rate water flushing, and
- To assess the recovery potential for nutrients and carbon.
2. Materials and Methods
2.1. MWW Supply and Pre-Treatment of FO Feedwater
2.2. FO Lab Scale Unit
2.3. Water Quality Analyses
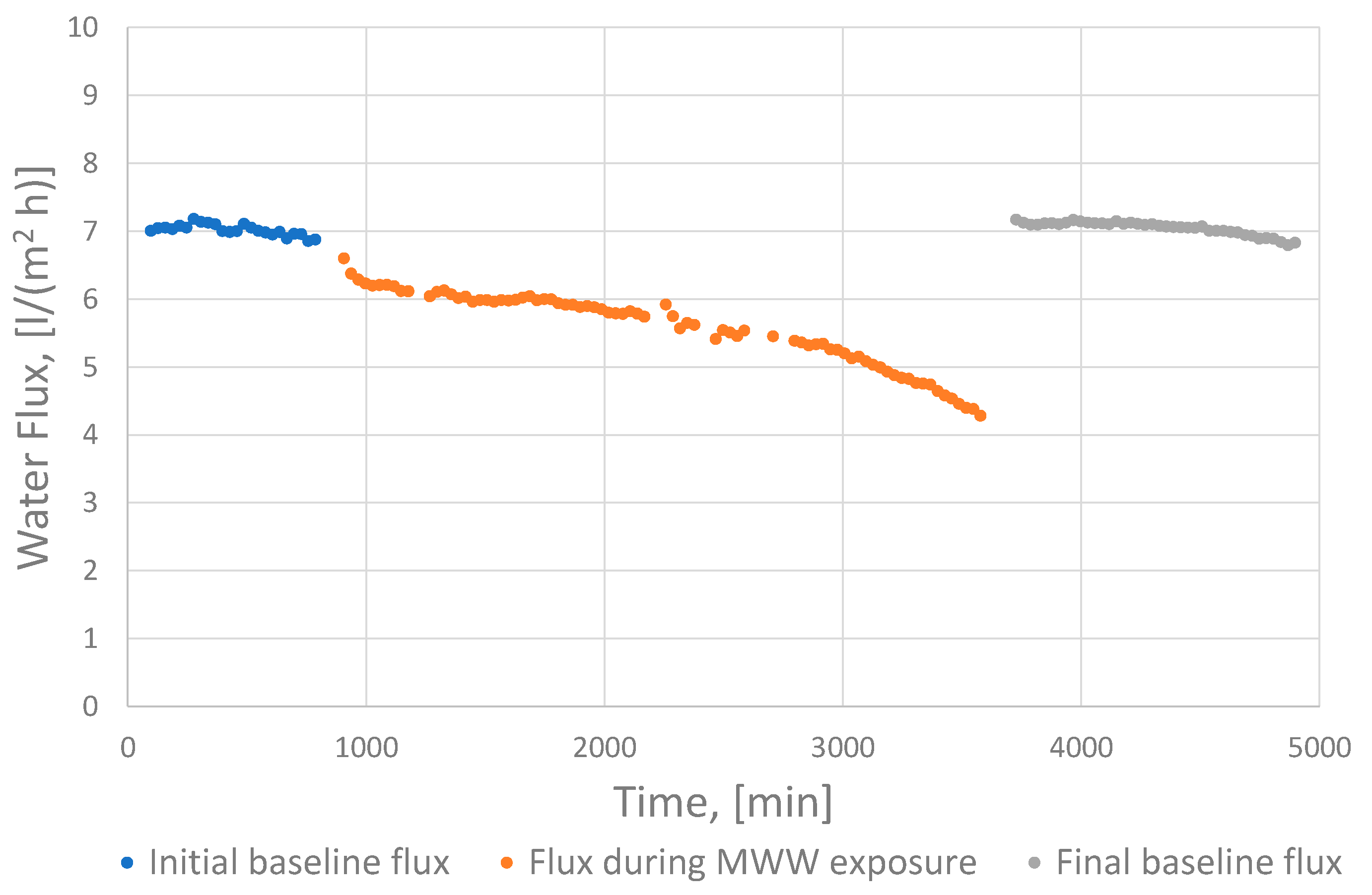
2.4. Baseline-Corrected Water Flux
2.5. Concentration Factors
2.6. Scanning Electron Microscopy
3. Results
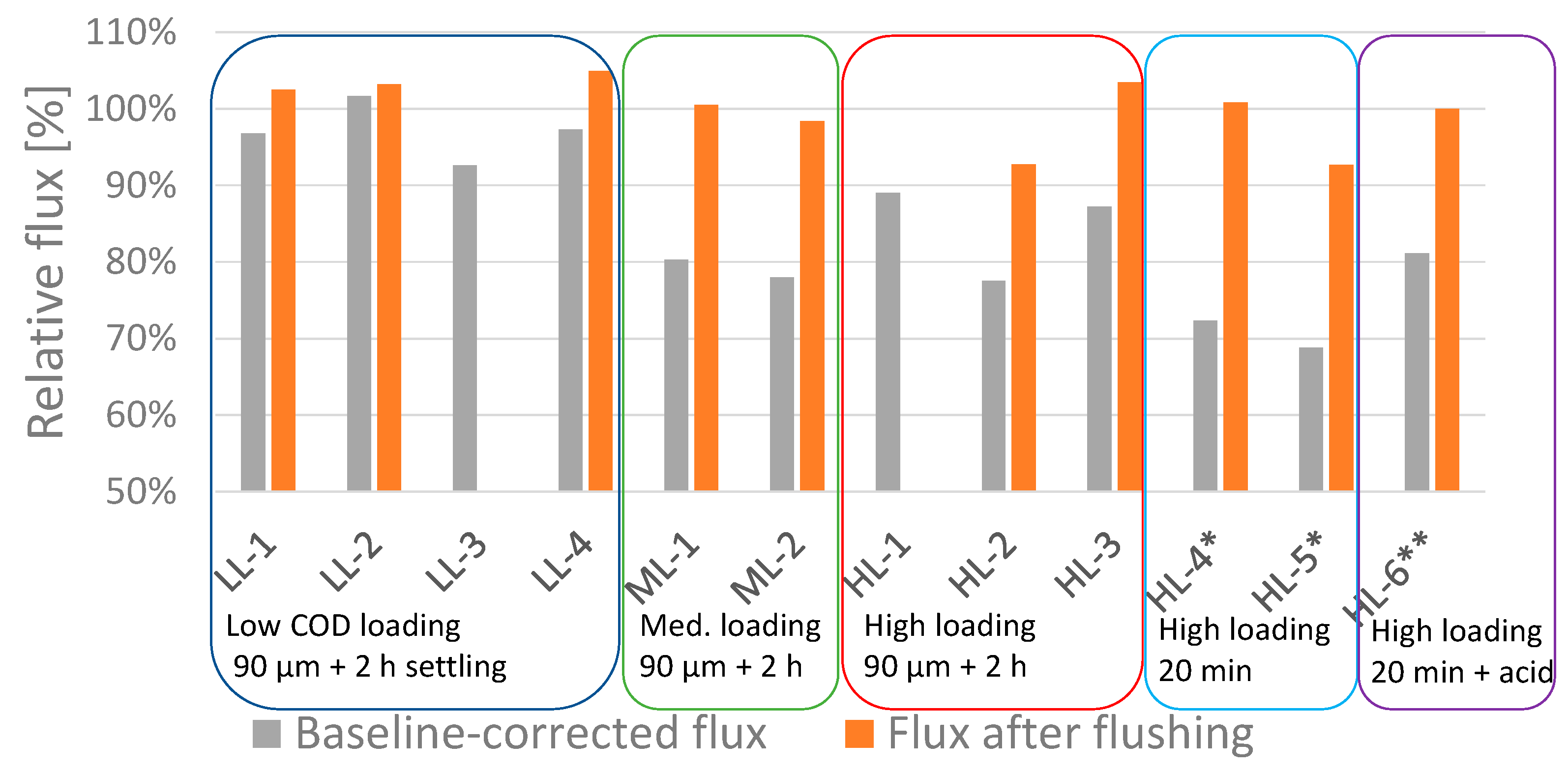
3.1. Membrane Performance
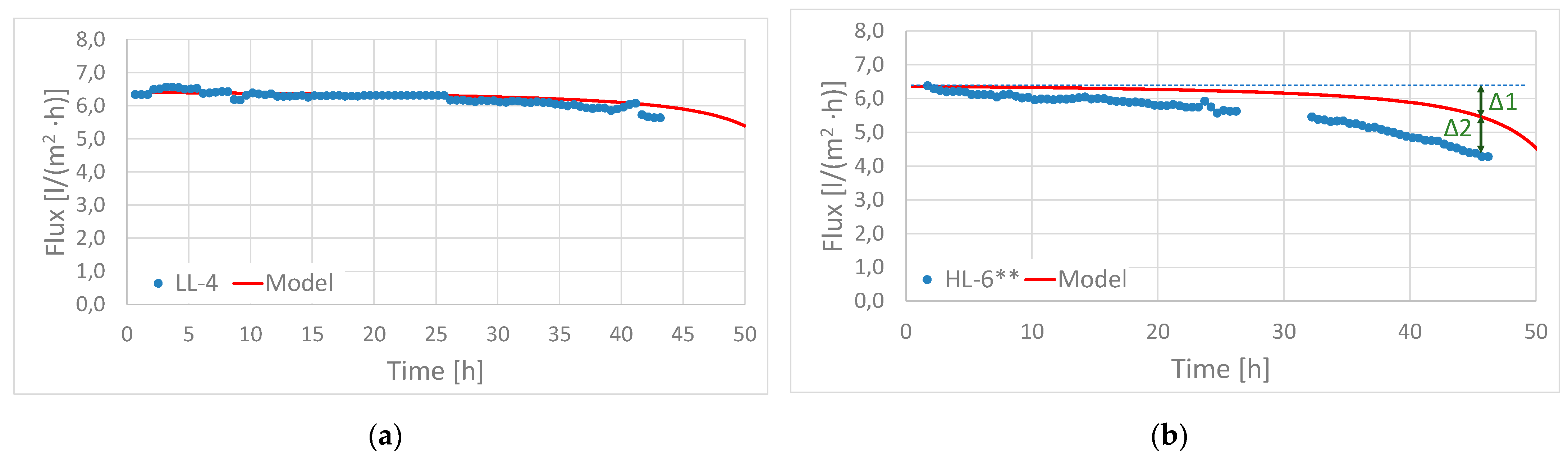
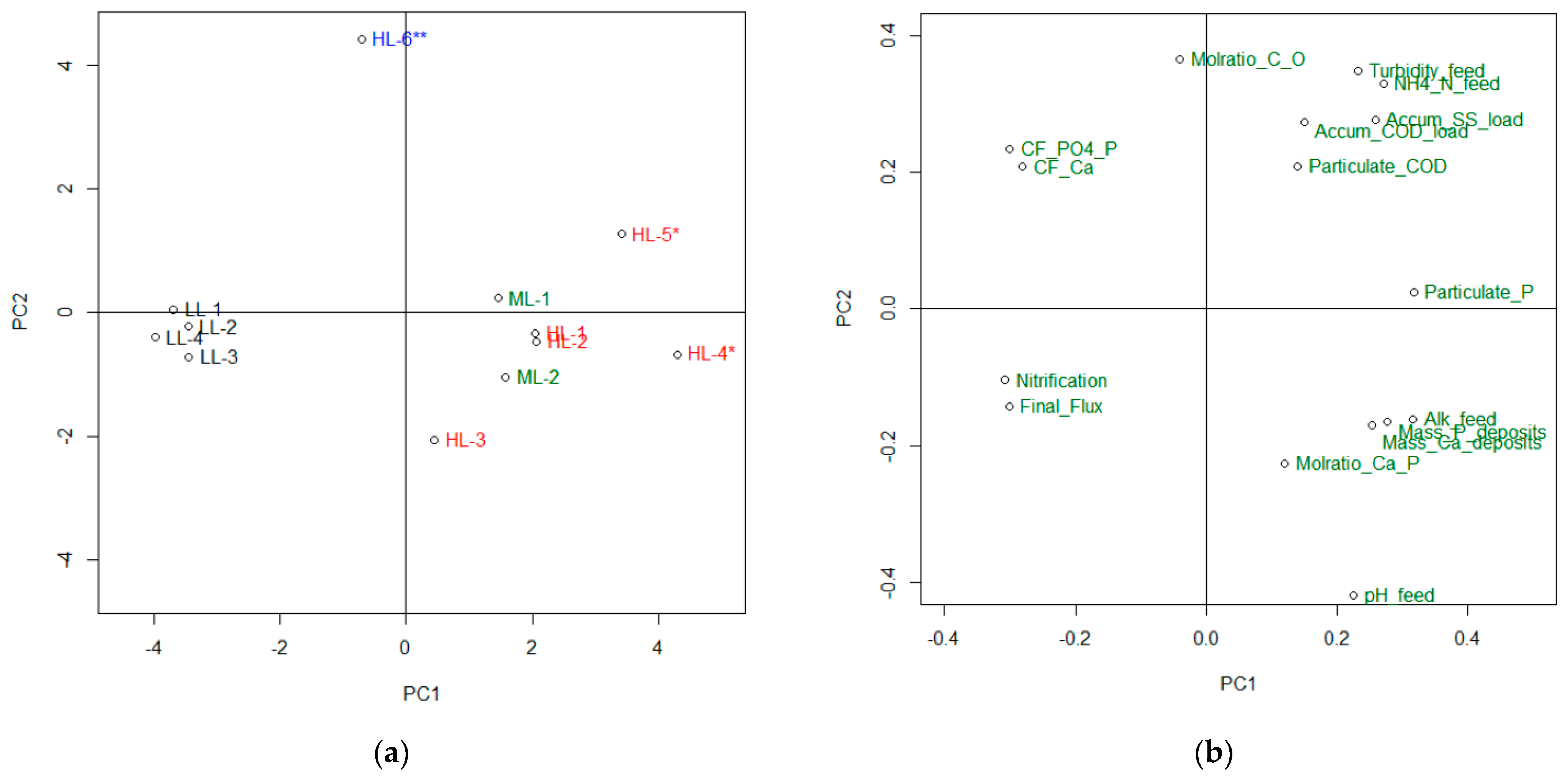
3.2. Factors Influencing Fouling and Importance of Pre-Treatment Strategy
3.3. Resource Recovery from FO Feed
3.3.1. Recovery Potential for Phosphorous in FO-Feed
3.3.2. Conditions for Optimizing Phosphorous Recovery
3.3.3. Recovery Potential of Nitrogen and COD
4. Discussion
- Applying MBBR effluent from reactors with a sufficiently low organic loading rate to achieve high nitrification, thereby reducing alkalinity and pH and consequently achieving a lower scaling potential for both CaCO3 and CaHPO4;
- Reduce pH sufficiently to avoid CaCO3 and CaHPO4 scaling by dosing a strong acid.
5. Conclusions
- Demonstration of membrane performance:
- The observed initial water fluxes when using a 35 g/L NaCl draw solution were in the range of 6.3 to 8.0 L/(m2·h).
- The flux declines due to fouling beingobserved at volumetric concentration factors of 3.5 to 8.5 were in the range of 0 to 7% for experiments performed with low-loaded MBBR effluent and in the range of 11 to 31% for experiments performed with high-loaded MBBR effluent.
- Assessment of the impact of pre-treatment strategy with respect to membrane fouling:
- The observed flux decline due to fouling was caused by a combination of organic/particulate fouling and the precipitation of CaHPO4 and CaCO3. The importance of the different forms of deposits varied among the different experiments as a result of different feed characteristics and operating conditions.
- Lowering of pH was important for avoiding precipitation of the phosphate and carbonate scale. Two feasible pre-treatment strategies for pH reduction were demonstrated; (1) operating the MBBR stage at a low loading rate to achieve high nitrification and (2) adding a strong acid.
- Reducing the concentration of organics and particles in the feedwater was important for reducing organic/particulate fouling. The operation of the MBBR stage at a low loading rate was demonstrated as one means of reducing the potential for organic/particulate fouling. In general, more efficient particle removal applied upstream the FO will be beneficial for reducing organic particulate scaling.
- Assessment of recovery potential:
- This study confirmed that the recovery potential of phosphorous and COD in the FO-feed can be close to 100%, which is in line with previous studies [12,30]. However, further studies are required to assess the actual recovery of energy and nutrients, which must be obtained by the further processing of the FO concentrate.
- Assessment of high-rate flushing as a flux recovery measure:
- High-rate flushing with water was found to fully recover the initial water flux at most conditions.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Daneshgar, S.; Callegari, A.; Capodaglio, A.G.; Vaccari, D. The Potential Phosphorus Crisis: Resource Conservation and Possible Escape Technologies: A Review. Resources 2018, 7, 37. [Google Scholar] [CrossRef]
- Rosso, D.; Stenstrom, M.K. The carbon-sequestration potential of municipal wastewater treatment. Chemosphere 2008, 70, 1468–1475. [Google Scholar] [CrossRef]
- Kaszycki, P.; Głodniok, M.; Petryszak, P. Towards a bio-based circular economy in organic waste management and wastewater treatment—The Polish perspective. New Biotechnol. 2021, 61, 80–89. [Google Scholar] [CrossRef]
- European Commission. FUTURE BRIEF: Innovation in the European Water Sector. 2015. Available online: https://ec.europa.eu/environment/integration/research/newsalert/pdf/innovation_european_water_sector_FB10_en.pdf (accessed on 10 March 2021).
- Melia, P.M.; Cundy, A.B.; Sohi, S.P.; Hooda, P.S.; Busquets, R. Trends in the recovery of phosphorus in bioavailable forms from wastewater. Chemosphere 2017, 186, 381–395. [Google Scholar] [CrossRef]
- Yaqub, M.; Lee, W. Zero-liquid discharge (ZLD) technology for resource recovery from wastewater: A review. Sci. Total. Environ. 2019, 681, 551–563. [Google Scholar] [CrossRef]
- Ramasahayam, S.K.; Guzman, L.; Gunawan, G.; Viswanathan, T. A Comprehensive Review of Phosphorus Removal Technologies and Processes. J. Macromol. Sci. Part A 2014, 51, 538–545. [Google Scholar] [CrossRef]
- Tian, Y.; Hu, H.; Zhang, J. Solution to water resource scarcity: Water reclamation and reuse. Environ. Sci. Pollut. Res. 2017, 24, 5095–5097. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Gu, J.; Zhao, Q.; Liu, Y. COD capture: A feasible option towards energy self-sufficient domestic wastewater treatment. Sci. Rep. 2016, 6, 25054. [Google Scholar] [CrossRef] [PubMed]
- Lutchmiah, K.; Verliefde, A.; Roest, K.; Rietveld, L.; Cornelissen, E. Forward osmosis for application in wastewater treatment: A review. Water Res. 2014, 58, 179–197. [Google Scholar] [CrossRef]
- Ansari, A.J.; Hai, F.I.; Price, W.E.; Drewes, J.E.; Nghiem, L.D. Forward osmosis as a platform for resource recovery from municipal wastewater - A critical assessment of the literature. J. Membr. Sci. 2017, 529, 195–206. [Google Scholar] [CrossRef]
- Wang, Z.; Zheng, J.; Tang, J.; Wang, X.; Wu, Z. A pilot-scale forward osmosis membrane system for concentrating low-strength municipal wastewater: Performance and implications. Sci. Rep. 2016, 6, 21653. [Google Scholar] [CrossRef] [PubMed]
- Qiu, G.; Zhang, S.; Raghavan, D.S.S.; Das, S.; Ting, Y.-P. The potential of hybrid forward osmosis membrane bioreactor (FOMBR) processes in achieving high throughput treatment of municipal wastewater with enhanced phosphorus recovery. Water Res. 2016, 105, 370–382. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Tobino, T.; Nakajima, F.; Yamamoto, K. Seawater-driven forward osmosis for enriching nitrogen and phosphorous in treated municipal wastewater: Effect of membrane properties and feed solution chemistry. Water Res. 2015, 69, 120–130. [Google Scholar] [CrossRef]
- Achilli, A.; Cath, T.Y.; Marchand, E.A.; Childress, A.E. The forward osmosis membrane bioreactor: A low fouling alternative to MBR processes. Desalination 2009, 239, 10–21. [Google Scholar] [CrossRef]
- Smol, M. The use of membrane processes for the removal of phosphorus from wastewater. DESALINATION Water Treat. 2018, 128, 397–406. [Google Scholar] [CrossRef]
- Holloway, R.W.; Regnery, J.; Nghiem, L.D.; Cath, T.Y. Removal of Trace Organic Chemicals and Performance of a Novel Hybrid Ultrafiltration-Osmotic Membrane Bioreactor. Environ. Sci. Technol. 2014, 48, 10859–10868. [Google Scholar] [CrossRef]
- Alturki, A.; McDonald, J.; Khan, S.J.; Hai, F.I.; Price, W.E.; Nghiem, L.D. Performance of a novel osmotic membrane bioreactor (OMBR) system: Flux stability and removal of trace organics. Bioresour. Technol. 2012, 113, 201–206. [Google Scholar] [CrossRef]
- Yang, S.; Gao, B.; Jang, A.; Shon, H.K.; Yue, Q. Municipal wastewater treatment by forward osmosis using seawater concentrate as draw solution. Chemosphere 2019, 237, 124485. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Hai, F.I.; Price, W.E.; Guo, W.; Ngo, H.H.; Yamamoto, K.; Nghiem, L.D. Phosphorus and water recovery by a novel osmotic membrane bioreactor–reverse osmosis system. Bioresour. Technol. 2016, 200, 297–304. [Google Scholar] [CrossRef]
- Nguyen, N.C.; Nguyen, H.T.; Chen, S.-S.; Ngo, H.H.; Guo, W.; Chan, W.H.; Ray, S.S.; Li, C.-W.; Hsu, H.-T. A novel osmosis membrane bioreactor-membrane distillation hybrid system for wastewater treatment and reuse. Bioresour. Technol. 2016, 209, 8–15. [Google Scholar] [CrossRef]
- Chekli, L.; Phuntsho, S.; Kim, J.E.; Kim, J.; Choi, J.Y.; Choi, J.-S.; Kim, S.; Kim, J.H.; Hong, S.; Sohn, J.; et al. A comprehensive review of hybrid forward osmosis systems: Performance, applications and future prospects. J. Membr. Sci. 2016, 497, 430–449. [Google Scholar] [CrossRef]
- She, Q.; Wang, R.; Fane, A.G.; Tang, C.Y. Membrane fouling in osmotically driven membrane processes: A review. J. Membr. Sci. 2016, 499, 201–233. [Google Scholar] [CrossRef]
- Sun, Y.; Tian, J.; Zhao, Z.; Shi, W.; Liu, D.; Cui, F. Membrane fouling of forward osmosis (FO) membrane for municipal wastewater treatment: A comparison between direct FO and OMBR. Water Res. 2016, 104, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Boo, C.; Elimelech, M.; Hong, S. Comparison of fouling behavior in forward osmosis (FO) and reverse osmosis (RO). J. Membr. Sci. 2010, 365, 34–39. [Google Scholar] [CrossRef]
- Bell, E.A.; Holloway, R.W.; Cath, T.Y. Evaluation of forward osmosis membrane performance and fouling during long-term osmotic membrane bioreactor study. J. Membr. Sci. 2016, 517, 1–13. [Google Scholar] [CrossRef]
- Luo, W.; Hai, F.I.; Price, W.E.; Guo, W.; Ngo, H.H.; Yamamoto, K.; Nghiem, L.D. High retention membrane bioreactors: Challenges and opportunities. Bioresour. Technol. 2014, 167, 539–546. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.; Yuan, B.; Li, X.; Ren, Y. Impacts of sludge retention time on sludge characteristics and membrane fouling in a submerged osmotic membrane bioreactor. Bioresour. Technol. 2014, 161, 340–347. [Google Scholar] [CrossRef]
- Melin, E.; Leiknes, T.; Helness, H.; Rasmussen, V.; Ødegaard, H. Effect of organic loading rate on a wastewater treatment process combining moving bed biofilm and membrane reactors. Water Sci. Technol. 2005, 51, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Holloway, R.W.; Wait, A.S.; da Silva, A.F.; Herron, J.; Schutter, M.D.; Lampi, K.; Cath, T.Y. Long-term pilot scale investigation of novel hybrid ultrafiltration-osmotic membrane bioreactors. Desalination 2015, 363, 64–74. [Google Scholar] [CrossRef]
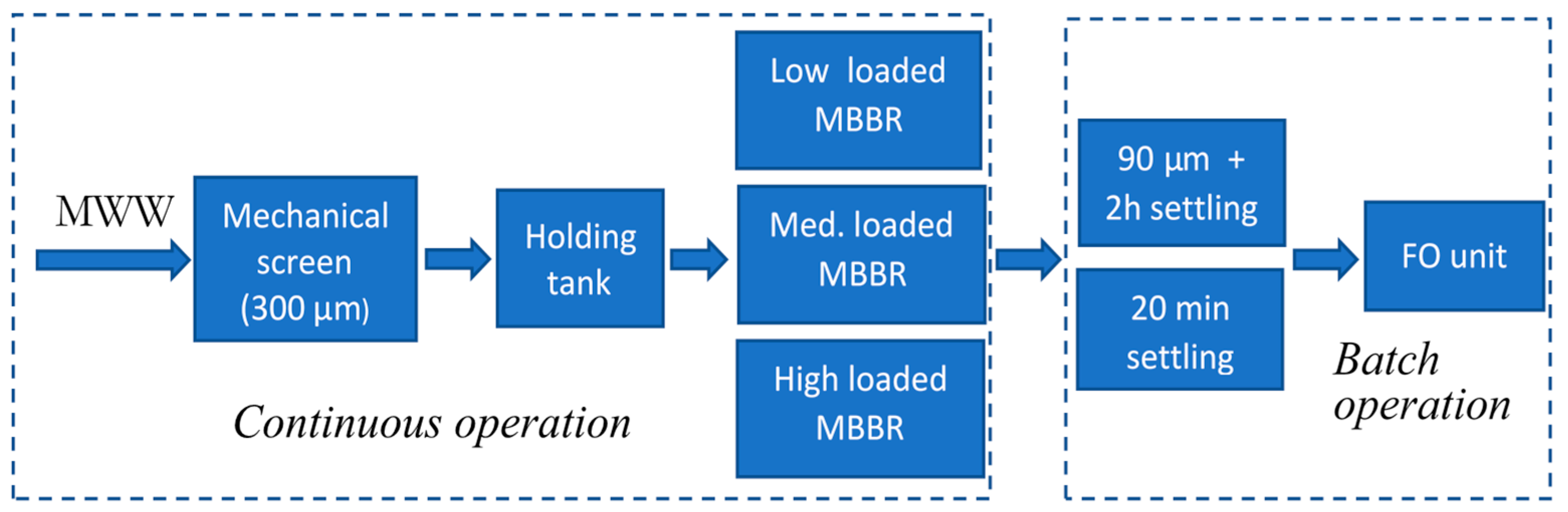
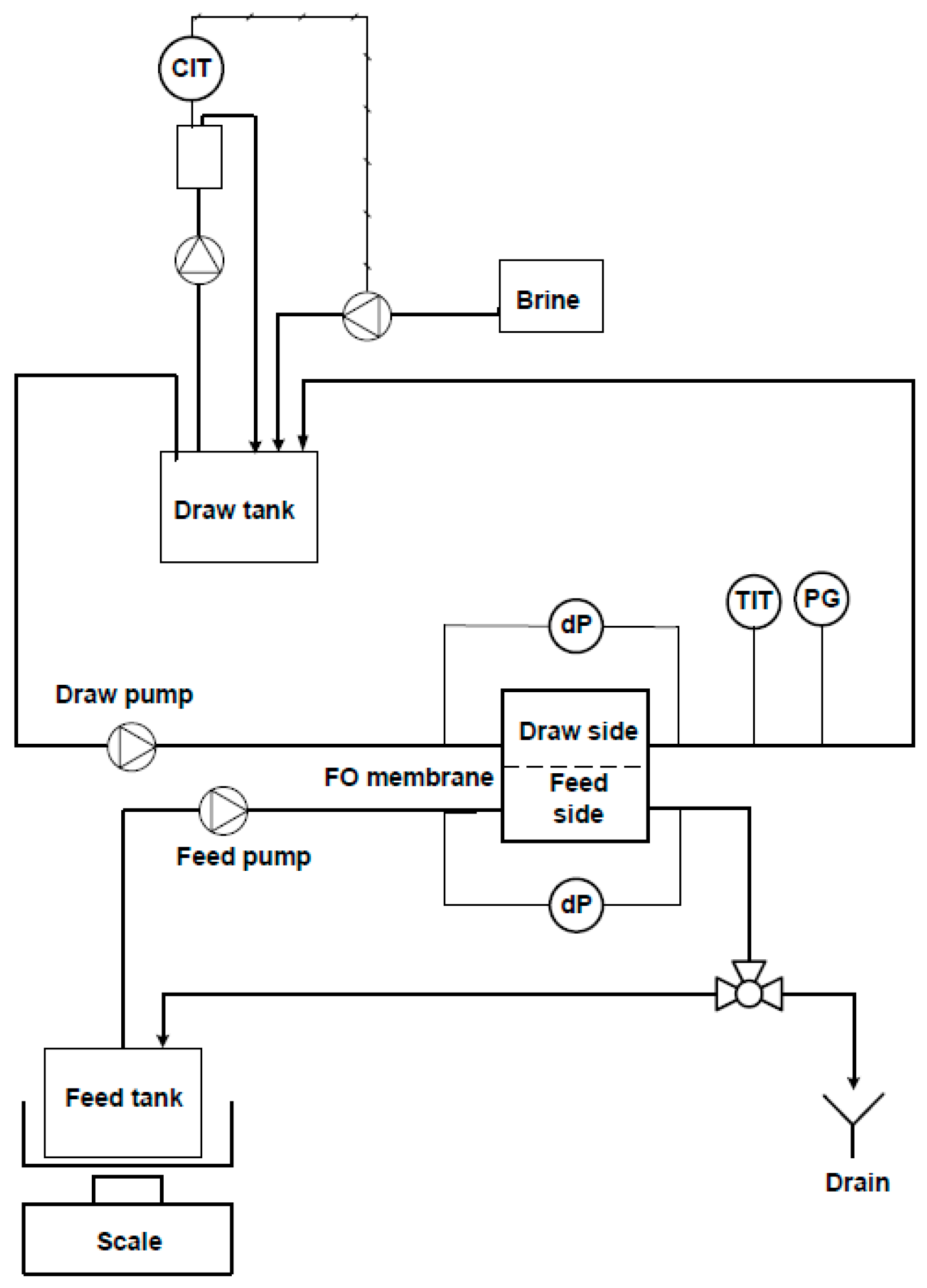

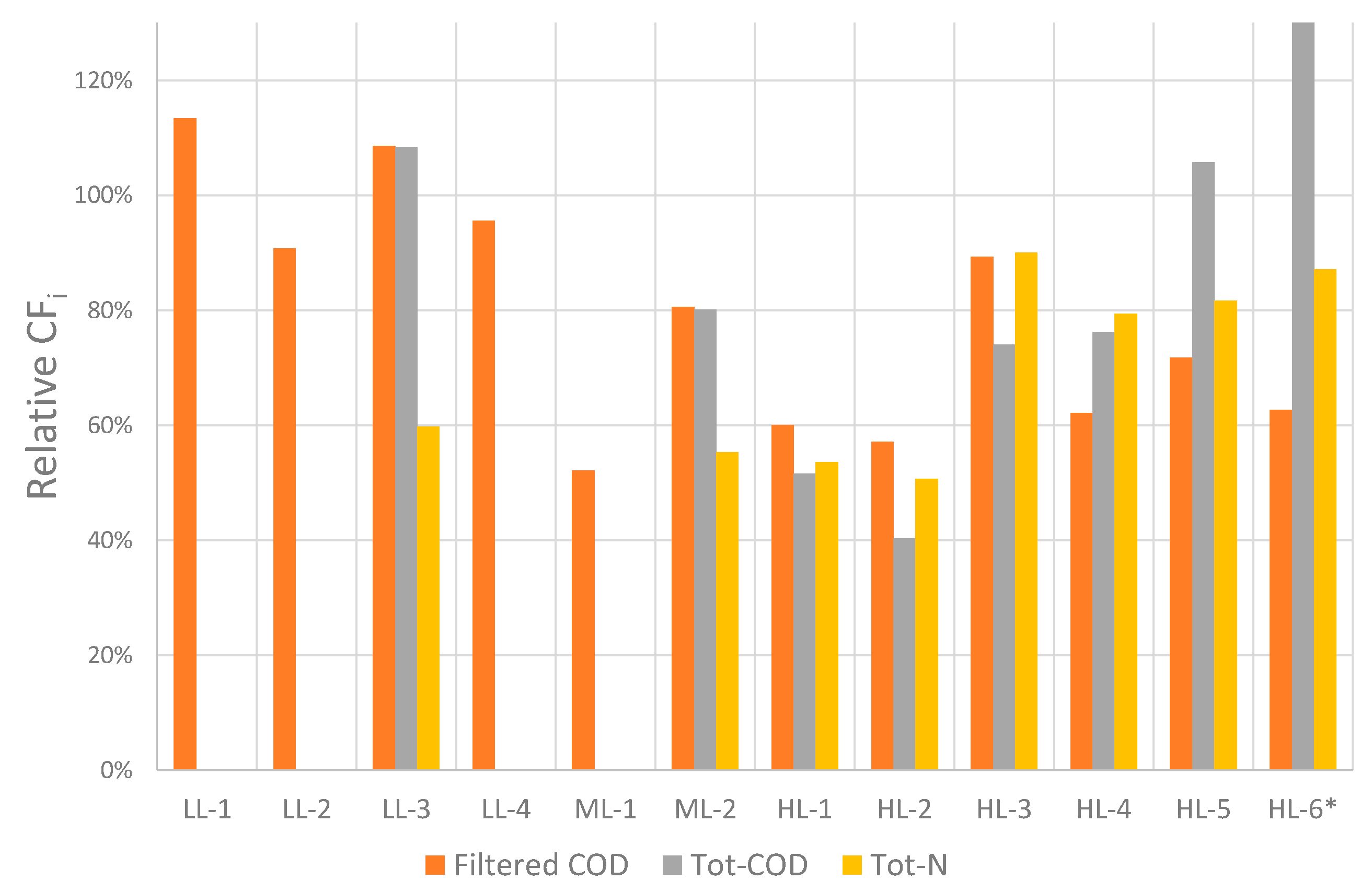
| Experiment ID | MBBR Loading Rate | Particle Removal | pH Adjustment |
|---|---|---|---|
| LL–1 | 1.2 | 90 μm + 2 h settling | No |
| LL–2 | 0.7 | 90 μm + 2 h settling | No |
| LL–3 | 0.5 | 90 μm + 2 h settling | No |
| LL–4 | 0.5 | 90 μm + 2 h settling | No |
| ML–1 | 4.8 | 90 μm + 2 h settling | No |
| ML–2 | 3.0 | 90 μm + 2 h settling | No |
| HL–1 | 5.8 | 90 μm + 2 h settling | No |
| HL–2 | 7.1 | 90 μm + 2 h settling | No |
| HL–3 | 6.5 | 90 μm + 2 h settling | No |
| HL–4* | 10.0 | 20 min settling | No |
| HL–5* | 15.4 | 20 min settling | No |
| HL–6** | 14.2 | 20 min settling | Yes (HCl) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thelin, W.R.; Sivertsen, E.; Raspati, G.; Azrague, K.; Helness, H. Concentration of Municipal MBBR Effluent by FO for Resource Recovery: Batch Experiments in Side-Stream Configuration. Membranes 2021, 11, 278. https://doi.org/10.3390/membranes11040278
Thelin WR, Sivertsen E, Raspati G, Azrague K, Helness H. Concentration of Municipal MBBR Effluent by FO for Resource Recovery: Batch Experiments in Side-Stream Configuration. Membranes. 2021; 11(4):278. https://doi.org/10.3390/membranes11040278
Chicago/Turabian StyleThelin, Willy Røstum, Edvard Sivertsen, Gema Raspati, Kamal Azrague, and Herman Helness. 2021. "Concentration of Municipal MBBR Effluent by FO for Resource Recovery: Batch Experiments in Side-Stream Configuration" Membranes 11, no. 4: 278. https://doi.org/10.3390/membranes11040278
APA StyleThelin, W. R., Sivertsen, E., Raspati, G., Azrague, K., & Helness, H. (2021). Concentration of Municipal MBBR Effluent by FO for Resource Recovery: Batch Experiments in Side-Stream Configuration. Membranes, 11(4), 278. https://doi.org/10.3390/membranes11040278






