Seawater Desalination by Modified Membrane Distillation: Effect of Hydrophilic Surface Modifying Macromolecules Addition into PVDF Hollow Fiber Membrane
Abstract
:1. Introduction
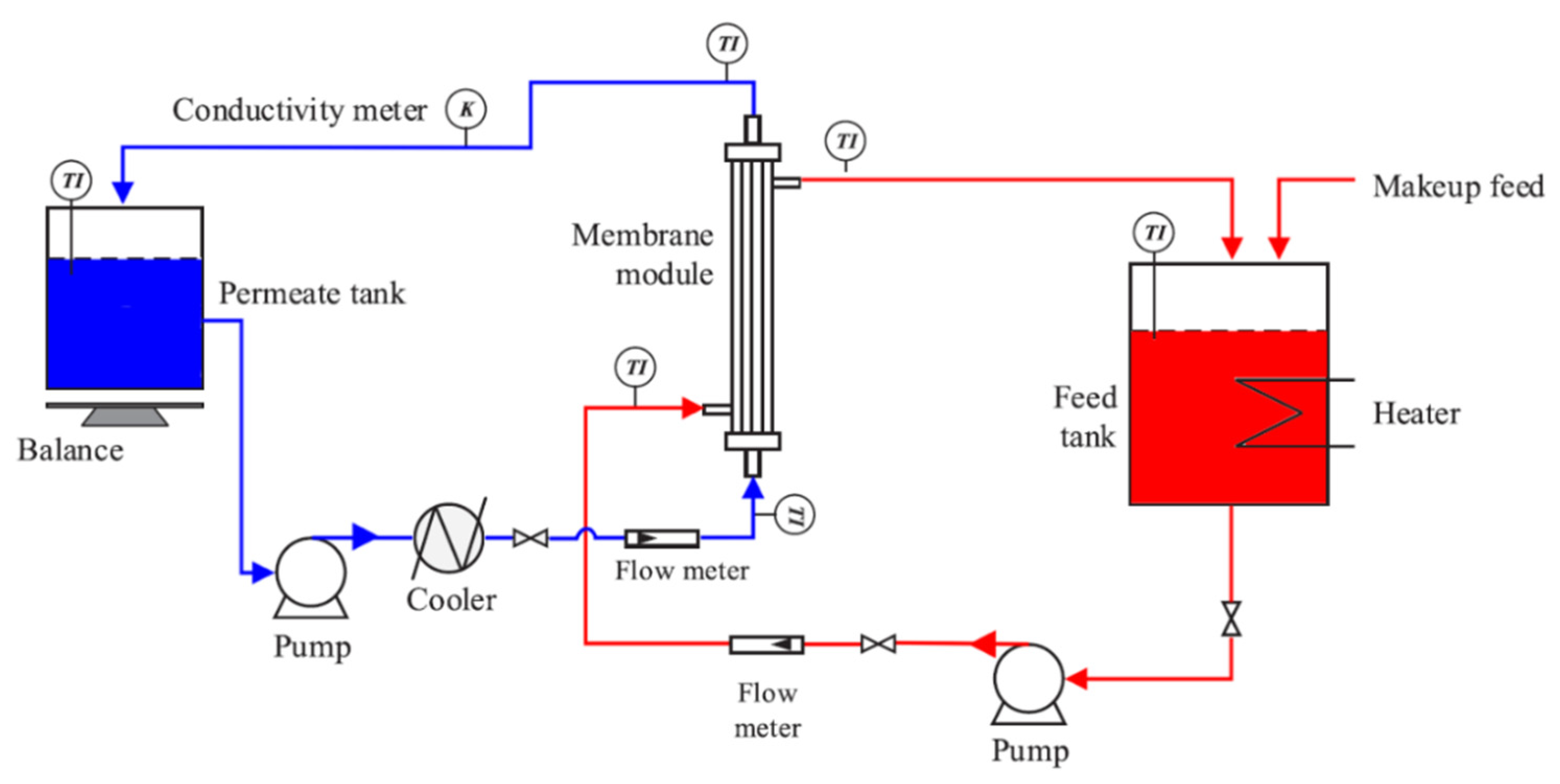
2. Methodology
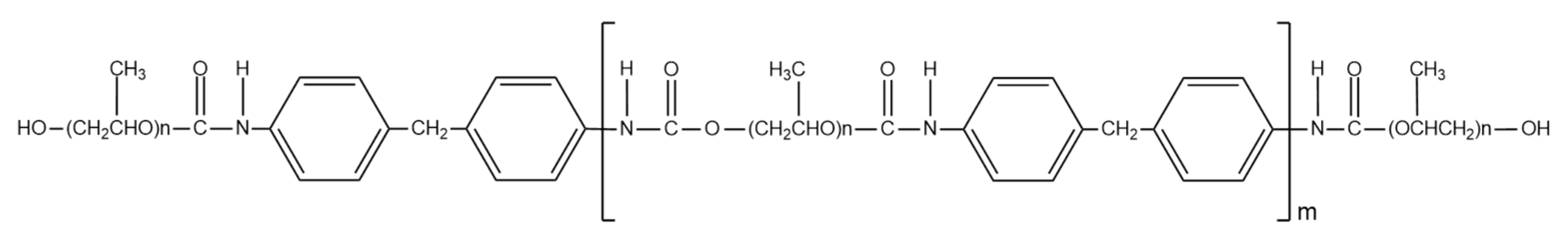
2.1. Materials
2.2. Preparation of PVDF/PEG/LSMM Hollow Fiber Membrane
2.3. Membrane Characterization
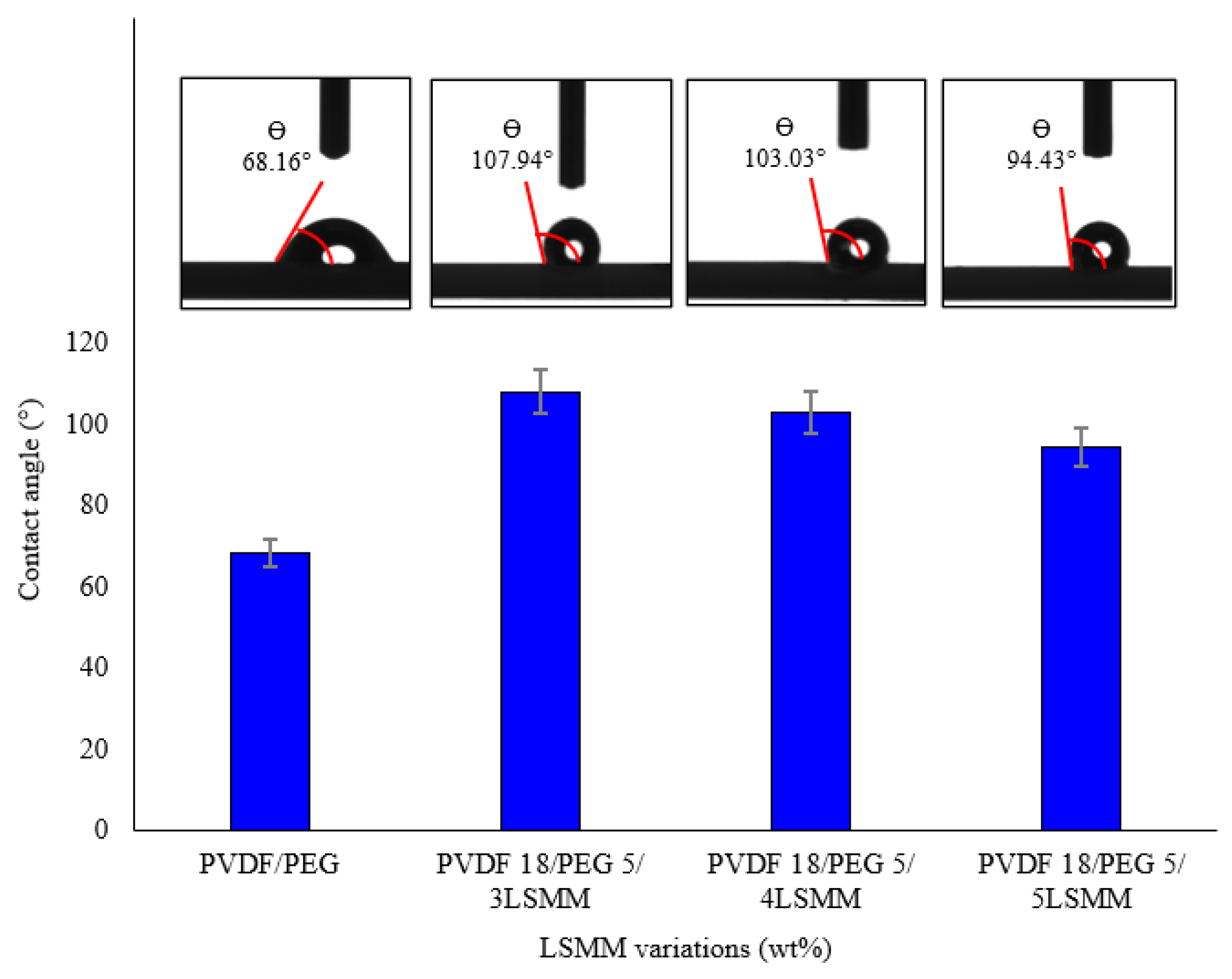
2.3.1. Water Contact Angle (WCA)
2.3.2. Field Emission Scanning Electron Microscope (FESEM)
2.3.3. Differential Scanning Calorimetry (DSC)
2.4. Membrane Performance
2.4.1. Permeate Flux
2.4.2. Salt Rejection
3. Results and Discussion
3.1. Water Contact Angle of PVDF/PEG/LSMM/BSMM Membranes
3.2. Characteristics Study of Modified PVDF Membrane
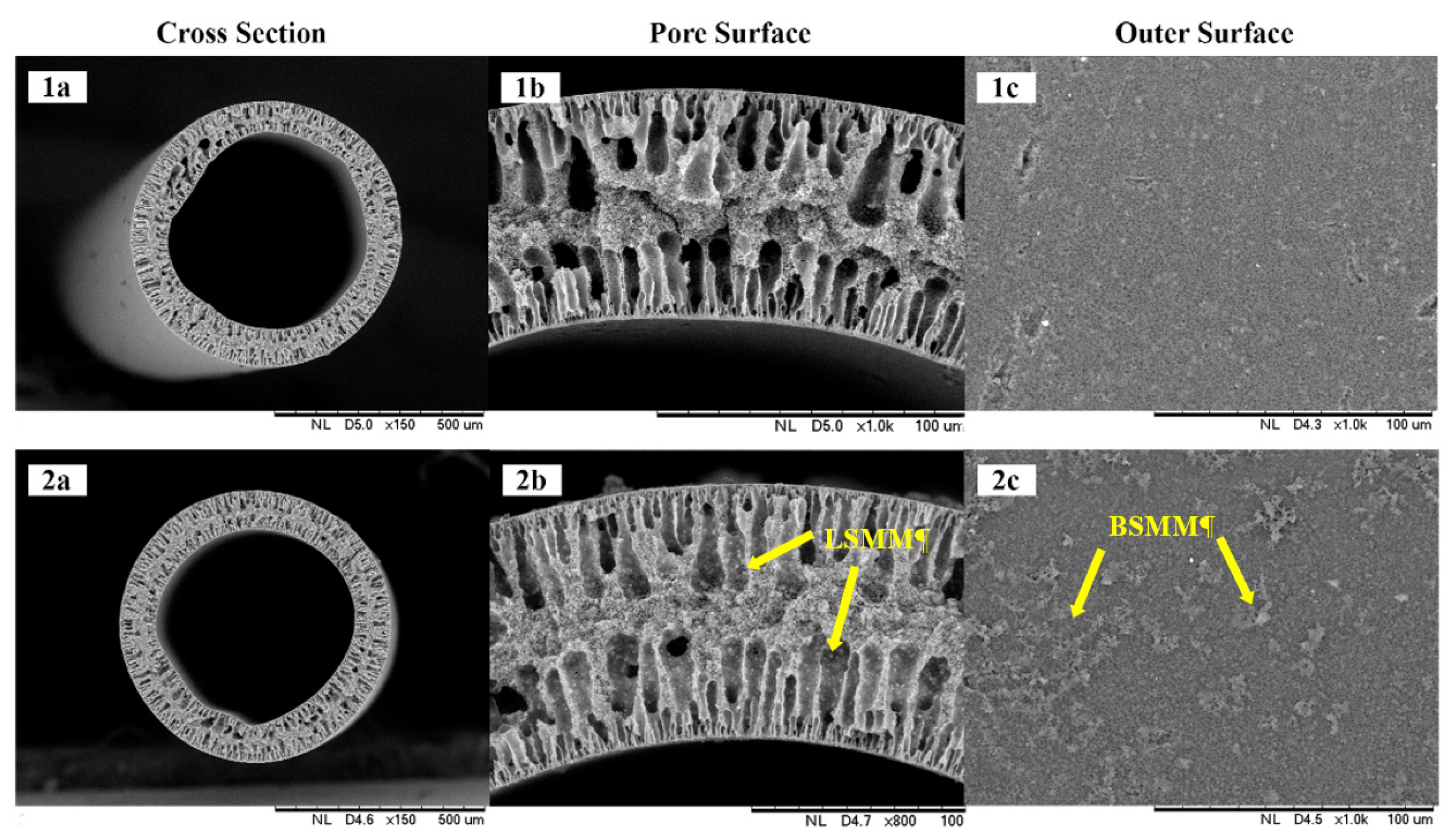
3.2.1. Morphological Structure
3.2.2. Thermal Stability
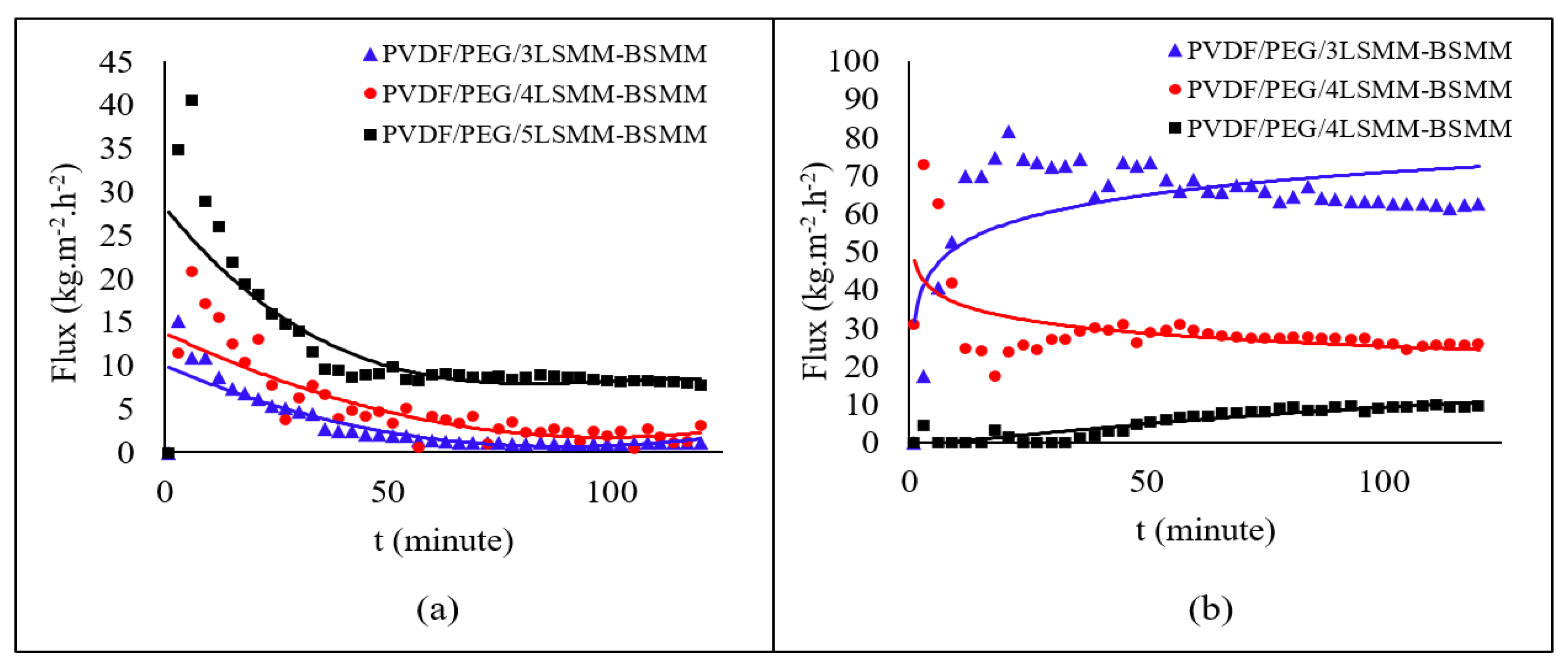
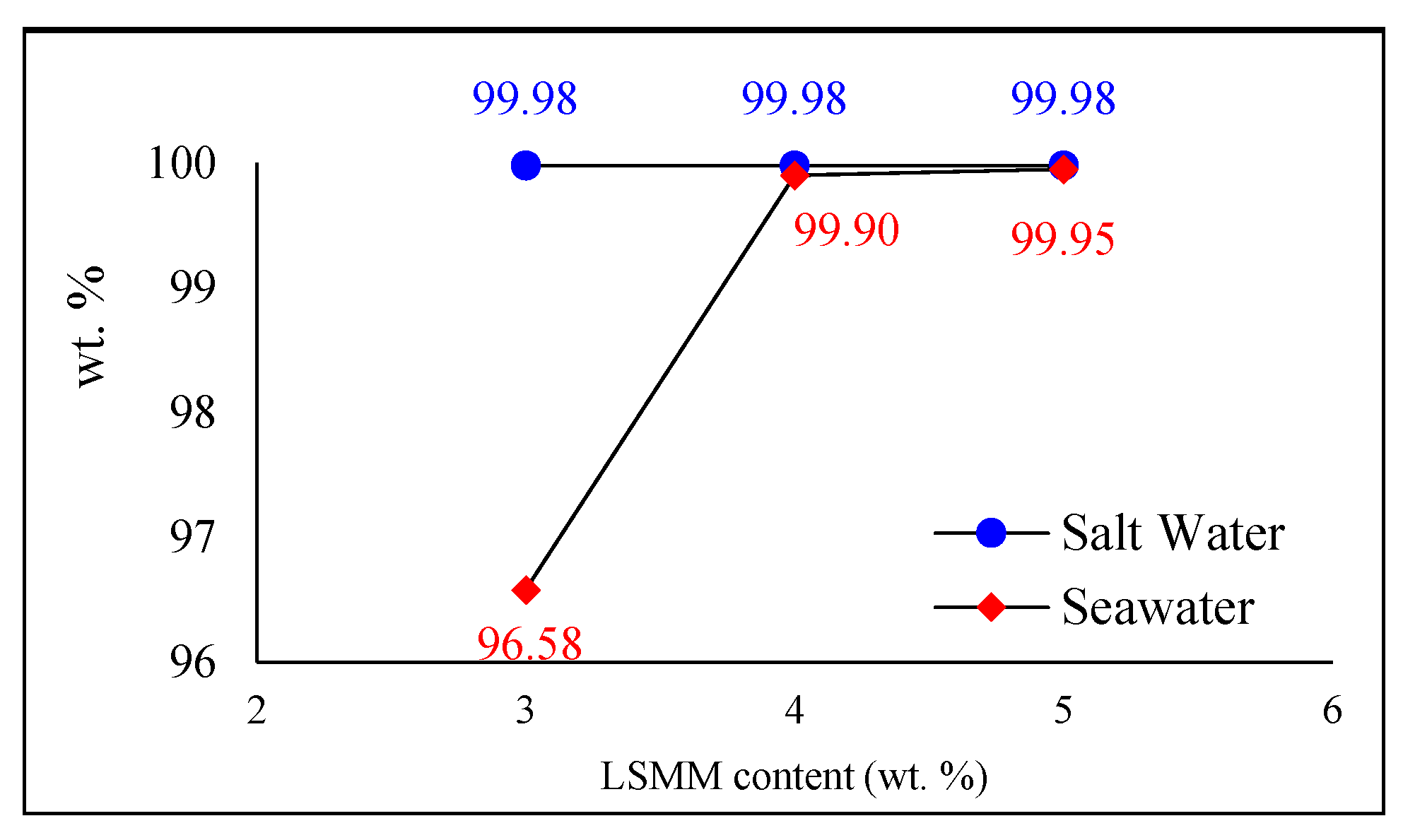
3.3. DCMD Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Askari, M.; Liang, C.Z.; Choong, L.T.; Chung, T.-S. Optimization of TFC-PES hollow fiber membranes for reverse osmosis (RO) and osmotically assisted reverse osmosis (OARO) applications. J. Membr. Sci. 2021, 625, 119156. [Google Scholar] [CrossRef]
- Ezugbe, E.O.; Rathilal, S. Membrane Technologies in Wastewater Treatment. Membranes 2020, 10, 89. [Google Scholar] [CrossRef]
- Camacho, L.M.; Dumée, L.; Zhang, J.; Li, J.; Duke, M.; Gomez, J.; Gray, S. Advances in Membrane Distillation for Water Desalination and Purification Applications. Water 2013, 5, 94–196. [Google Scholar] [CrossRef] [Green Version]
- Liang, C.Z.; Askari, M.; Choong, L.T.; Chung, T.S. Ultra-strong polymeric hollow fiber membranes for saline dewatering and desalination. Nat. Commun. 2021, 12, 2338. [Google Scholar] [CrossRef]
- Hou, D.; Wang, J.; Qu, D.; Luan, Z.; Ren, X. Fabrication and characterization of hydrophobic PVDF hollow fiber membranes for desalination through direct contact membrane distillation. Sep. Purif. Technol. 2009, 69, 78–86. [Google Scholar] [CrossRef]
- Alkhudhiri, A.; Darwish, N.; Hilal, N. Membrane distillation: A comprehensive review. Desalination 2012, 287, 2–18. [Google Scholar] [CrossRef]
- Lou, A.; Lior, N. Critical review of membrane distillation performance criteria. Desalination Water Treat. 2016, 57, 20093–20140. [Google Scholar]
- Tai, Z.S.; Aziz, M.H.A.; Othman, M.H.D.; Ismail, A.F.; Rahman, M.A.; Jaafar, J. An Overview of Membrane Distillation. In Membrane Separation Principles and Applications, Johor, Malaysia; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 251–281. [Google Scholar]
- Mansourizadeh, A.; Aslmahdavi, Z.; Ismail, A.; Matsuura, T. Blend polyvinylidene fluoride/surface modifying macromolecule hollow fiber membrane contactors for CO2 absorption. Int. J. Greenh. Gas. Control. 2014, 26, 83–92. [Google Scholar] [CrossRef]
- Dang, H.T.; Amelot, C.; Rana, D.; Narbaitz, R.M.; Matsuura, T. Performance of a newly developed hydrophilic additive blended with different ultrafiltration base polymers. J. Appl. Polym. Sci. 2010, 116, 2205–2215. [Google Scholar] [CrossRef]
- Patil, D.K.; Shirsat, D.S.P. Membrane Distillation Review and Flux prediction in Direct contact Membrane distillation Process. Int. Res. J. Eng. Technol. 2017, 4, 829–845. [Google Scholar]
- Ali, I.; Bamaga, O.A.; Gzara, L.; Bassyouni, M.; Abdel-Aziz, M.H.; Soliman, M.F.; Drioli, E.; Albeirutty, M. Assessment of Blend PVDF Membranes, and the Effect of Polymer Concentration and Blend Composition. Membranes 2018, 8, 13. [Google Scholar] [CrossRef] [Green Version]
- Omidvar, M.; Soltanieh, M.; Mousavi, S.M.; Saljoughi, E.; Moarefian, A.; Saffaran, H. Preparation of hydrophilic nanofiltration membranes for removal of pharmaceuticals from water. J. Environ. Health Schi. Eng. 2015, 13, 42. [Google Scholar] [CrossRef] [Green Version]
- Kusuma, N.C.; Purwanto, M.; Sudrajat, M.A.; Jaafar, J.; Othman, M.H.D.; Aziz, M.H.A.; Raharjo, Y.; Qtaishat, M.R. Fabrication and characterization of modified PVDF hollow fiber membrane coated with hydrophobic surface modifying macromolecules for desalination application. J. Environ. Chem. Eng. 2021, 9, 105582. [Google Scholar] [CrossRef]
- Khayet, M.; Essalhi, M. Effects of surface modifying macromolecules on the structural characteristics of different structured and nanofibrous membranes. In Proceedings of the Structural Membranes, International Conference on Textile Composites and Inflatable Structures, International Center for Numerical Methods in Engineering, Barcelona, Spain, 19–21 October 2015. [Google Scholar]
- Khosravi, M.; Ghadimi, A.; Mansourpour, Z.; Ghaee, A.; Sadatnia, B. Effect of different additives on separation performance of flat sheet PVDF membrane contactor. J. Chem. Pet. Eng. 2018, 52, 159–169. [Google Scholar]
- Kim, D.S.; Kim, D.H.; Lee, B.S.; Moon, G.Y.; Lee, H.K.; Yong, N.S.; Rhim, J.W. Effect of surface modifying macro-molecules on the properties of poly(vinylidene fluoride) membranes. J. Ind. Eng. Chem. 2009, 15, 393–397. [Google Scholar] [CrossRef]
- Aziz, M.H.A.; Othman, M.H.D.; Hashim, N.A.; Rahman, M.A.; Jaafar, J.; Hubadillah, S.K.; Tai, Z.S. Pretreated aluminium dross waste as a source of inexpensive alumina-spinel composite ceramic hollow fibre membrane for pretreatment of oily saline produced water. Ceram. Int. 2018, 45, 2069–2078. [Google Scholar] [CrossRef]
- Ahmad, N.A.; Leo, C.P.; Ahmad, A.L.; Ramli, W.K.W. Membranes with great hydrophobicity: A review on preparation and characterization. Sep. Purif. Rev. 2015, 44, 109–134. [Google Scholar] [CrossRef]
- Mokhtar, N.M.; Lau, W.J.; Ng, B.C.; Ismail, A.F.; Veerasamy, D. Preparation and characterization of PVDF membranes incorporated with different additives for dyeing solution treatment using membrane distillation. J. Desalin. Water Treat. 2015, 56, 1999–2012. [Google Scholar] [CrossRef]
- Hubadillah, S.K.; Tai, Z.S.; Othman, M.H.D.; Harun, Z.; Jamalludin, M.R.; Rahman, M.A.; Jaafar, J.; Ismail, A.F. Hydrophobic ceramic membrane for membrane distillation: A mini review on preparation, characterization, and applications. Sep. Purf. Technol. 2019, 217, 71–84. [Google Scholar] [CrossRef]
- Zhang, Q.; Lu, X.; Zhao, L. Preparation of Polyvinylidene Fluoride (PVDF) Hollow Fiber Hemodialysis Membranes. Membranes 2014, 4, 81–95. [Google Scholar] [CrossRef]
- Salim, N.E.; Jaafar, J.; Ismail, A.F.; Othman, M.; Rahman, M.A.; Yusof, N.; Qtaishat, M.R.; Matsuura, T.; Aziz, F.; Salleh, W.N.W. Preparation and characterization of hydrophilic surface modifier macromolecule modified poly (ether sulfone) photocatalytic membrane for phenol removal. Chem. Eng. J. 2018, 335, 236–247. [Google Scholar] [CrossRef]
- Liu, X.; Yang, G.; Lipik, V.T. Effect of fluorine atoms amount and fluorinated acrylic chain length chemically attached to hydroxyl groups on the hydrophobic properties of cotton fabrics. Mod. Chem. Appl. 2017, 5, 1–4. [Google Scholar]
- Laganà, F.; Barbieri, G.; Drioli, E. Direct contact membrane distillation: Modelling and concentration experiments. J. Membr. Sci. 2000, 166, 1–11. [Google Scholar] [CrossRef]
- Wang, K.; Abdala, A.; Hilal, N.; Khraisheh, M. Mechanical Characterization of Membranes. In Membrane Characterization; Elsevier: Amsterdam, The Netherlands, 2017; pp. 259–306. [Google Scholar] [CrossRef]
- Kandeel, H.S.; Badawya, N.A.; Hamadaa, A.A.; El-sayed, M.; Fathy, M.; Al-Gamal, A.A.G.; Moghny, T.A. Desalination aspects of PSSA-g-PEG copolymer and its graphene composite membranes. Int. J. Chem. Sci. 2018, 16, 277. [Google Scholar] [CrossRef]
- Etemadi, H.; Yegani, R.; Seyfollahi, M.; Rabiee, M. Synthesis, characteriation, and anti-fouling properties of cellulose acetate/polyethylene glycol-grafted nanodiamond nanocomposite membranes for humic acid removal from contaminated water. Iran. Polym. J. 2018, 27, 381–393. [Google Scholar] [CrossRef] [Green Version]
- Gryta, M. Effectiveness of Water Desalination by Membrane Distillation Process. Membranes 2012, 2, 415–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abd-Elghany, M.; Klapotke, T.M. A review on differential scanning calorimetry technique and its importanec in the field of energetic materials. Phys. Sci. Rev. 2018, 3, 1–14. [Google Scholar]
- Prayitno, J. Aspek Mikrobiologi Dalam Pengolahan Air Siap Minum Menggunakan Membran Reverse Osmosis. J. Rekayasa Lingkung. 2020, 12, 175–184. [Google Scholar] [CrossRef]
- Francis, L.; Ghaffour, N.; Al-Saadi, A.S.; Amy, G. Performance of different hollow fiber membranes for seawater desalination using membrane distillation. Desalination Water Treat. 2014, 55, 2786–2791. [Google Scholar] [CrossRef] [Green Version]
- Qtaishat, M.; Rana, D.; Matsuura, T. Effect of surface modifying macromolecules stoichiometric ration on composite hydrophobic/hydrophilic membranes characteristics and performance in direct contact membrane distillation. Am. Inst. Chem. Eng. J. 2009, 55, 3145–3152. [Google Scholar] [CrossRef]
- Singha, N.R.; Karmakar, M.; Chattopadhyay, R.K.; Roy, S.; Dev, M.; Mondal, H.; Mahapatra, M.; Dutta, A.; Mitra, M.; Roy, J.S.D. Structures, properties, and performances-relationships of polymeric membranes for pervaporative desalination. Membranes 2019, 9, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nalley, E.M.; Tuttle, L.J.; Barkman, A.L.; Conklin, E.E.; Wulstein, D.M.; Richmond, R.H.; Donahue, M.J. Water quality thresholds for coastal contaminant impacts on corals: A systematic review and meta-analysis. Sci. Total Environ. 2021, 794, 148632. [Google Scholar] [CrossRef] [PubMed]
- Qazi, S. Standalone Photovoltaic (PV) Systems for Disaster Relief and Remote Areas; Elsevier Inc.: Amsterdam, The Netherlands, 2017; Chapter 4; pp. 113–138. [Google Scholar]


| Membrane Designation | PVDF (wt. %) | PEG (wt. %) | DMAc (wt. %) | LSMM (wt. %) | BSMM (wt. %) |
|---|---|---|---|---|---|
| PVDF/PEG | 18 | 5 | 77 | 0 | 0 |
| PVDF/PEG/3LSMM-BSMM | 18 | 5 | 74 | 3 | 1 |
| PVDF/PEG/4LSMM-BSMM | 18 | 5 | 73 | 4 | 1 |
| PVDF/PEG/5LSMM-BSMM | 18 | 5 | 72 | 5 | 1 |
| Spinning Conditions | Value |
|---|---|
| Bore Fluid | Distilled Water |
| OD/ID spinneret size (mm) | 1.30/0.55 |
| Air gap (cm) | 10 |
| Bore fluid flow rate (mL·min−1) | 0.6 |
| Gear pump rotation (rpm) | 5 |
| Take-up drum rate (rpm) | 4 |
| Membrane | Thickness (µm) | Mean Pore Diameter (µm) | Surface Porosity (%) |
|---|---|---|---|
| PVDF/PEG | 165 | 0.35 | 83.50 |
| PVDF/PEG/3LSMM-BSMM | 183 | 0.23 | 77.70 |
| PVDF/PEG/4LSMM-BSMM | 181 | 0.23 | 77.27 |
| PVDF/PEG/5LSMM-BSMM | 257 | 0.13 | 73.93 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Purwanto, M.; Kusuma, N.C.; Sudrajat, M.A.; Jaafar, J.; Nasir, A.M.; Aziz, M.H.A.; Othman, M.H.D.; Rahman, M.A.; Raharjo, Y.; Widiastuti, N. Seawater Desalination by Modified Membrane Distillation: Effect of Hydrophilic Surface Modifying Macromolecules Addition into PVDF Hollow Fiber Membrane. Membranes 2021, 11, 924. https://doi.org/10.3390/membranes11120924
Purwanto M, Kusuma NC, Sudrajat MA, Jaafar J, Nasir AM, Aziz MHA, Othman MHD, Rahman MA, Raharjo Y, Widiastuti N. Seawater Desalination by Modified Membrane Distillation: Effect of Hydrophilic Surface Modifying Macromolecules Addition into PVDF Hollow Fiber Membrane. Membranes. 2021; 11(12):924. https://doi.org/10.3390/membranes11120924
Chicago/Turabian StylePurwanto, Mochammad, Nindita Cahya Kusuma, Ma’rup Ali Sudrajat, Juhana Jaafar, Atikah Mohd Nasir, Mohd Haiqal Abd Aziz, Mohd Hafiz Dzarfan Othman, Mukhlis A Rahman, Yanuardi Raharjo, and Nurul Widiastuti. 2021. "Seawater Desalination by Modified Membrane Distillation: Effect of Hydrophilic Surface Modifying Macromolecules Addition into PVDF Hollow Fiber Membrane" Membranes 11, no. 12: 924. https://doi.org/10.3390/membranes11120924
APA StylePurwanto, M., Kusuma, N. C., Sudrajat, M. A., Jaafar, J., Nasir, A. M., Aziz, M. H. A., Othman, M. H. D., Rahman, M. A., Raharjo, Y., & Widiastuti, N. (2021). Seawater Desalination by Modified Membrane Distillation: Effect of Hydrophilic Surface Modifying Macromolecules Addition into PVDF Hollow Fiber Membrane. Membranes, 11(12), 924. https://doi.org/10.3390/membranes11120924










