Membranes for the Capture and Screening of Waterborne Plutonium Based on a Novel Pu-Extractive Copolymer Additive
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of PVDF-g-EGMP Copolymer
2.3. Screening of PVDF-g-EGMP Copolymer for 242Pu Uptake
2.4. Casting PVDF Membranes with PVDF-g-EGMP
2.5. Permeability Studies
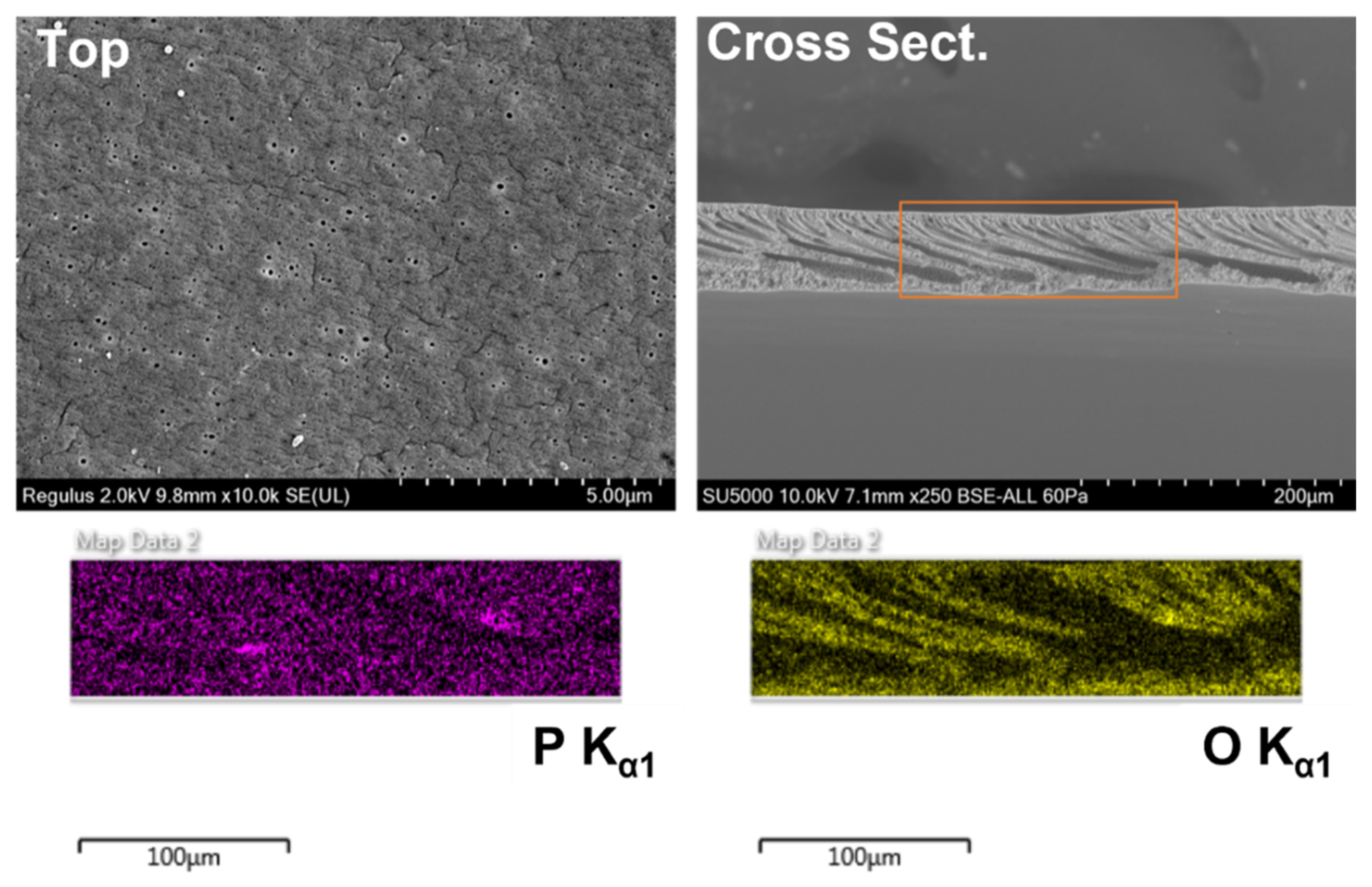
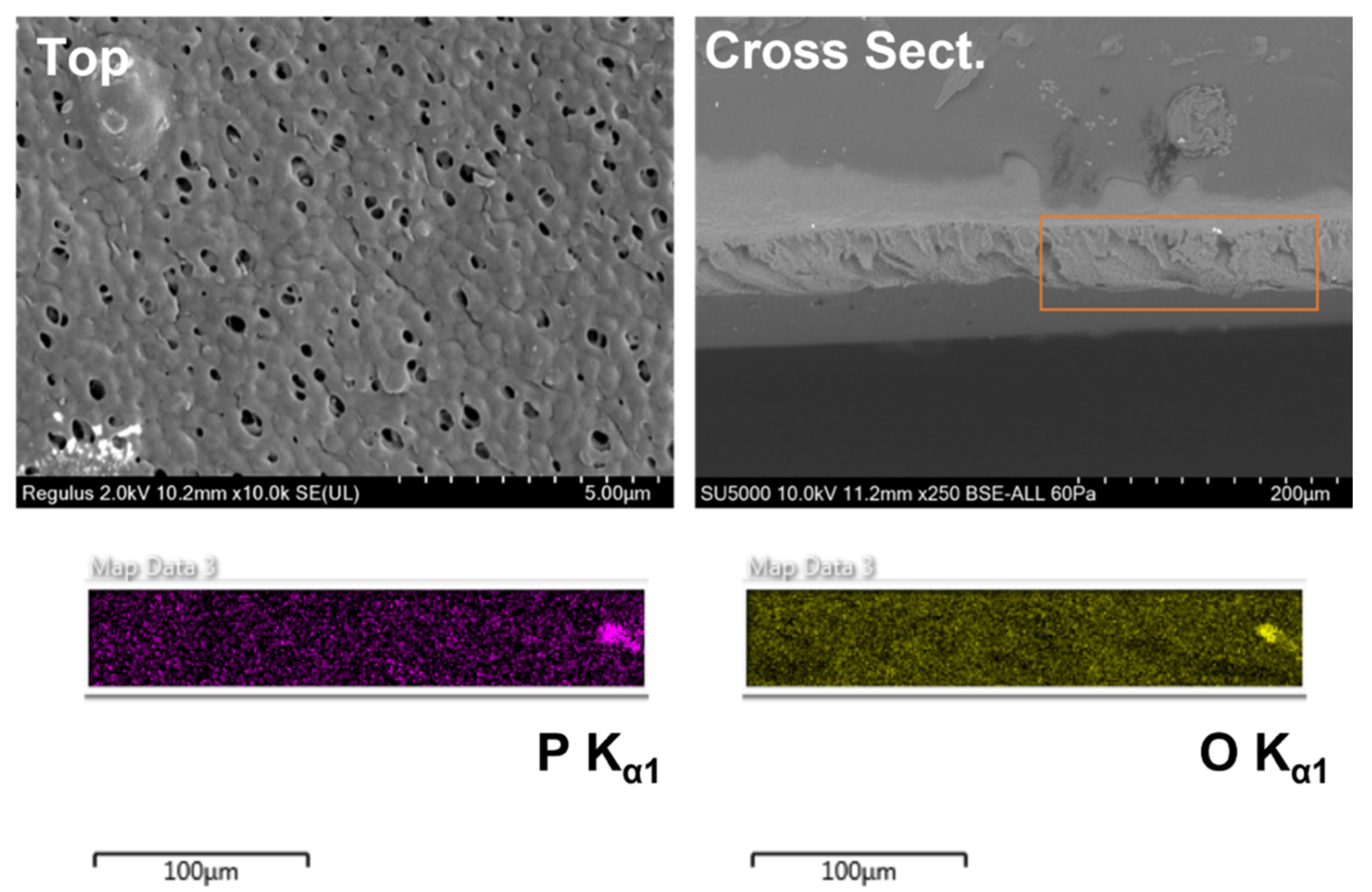
2.6. SEM Analysis of Membrane Morphologies
2.7. Alpha Spectrometry Measurements
2.8. Direct Flow Filtration of 238Pu Solutions through PVDF-g-EGMP Membranes
3. Results
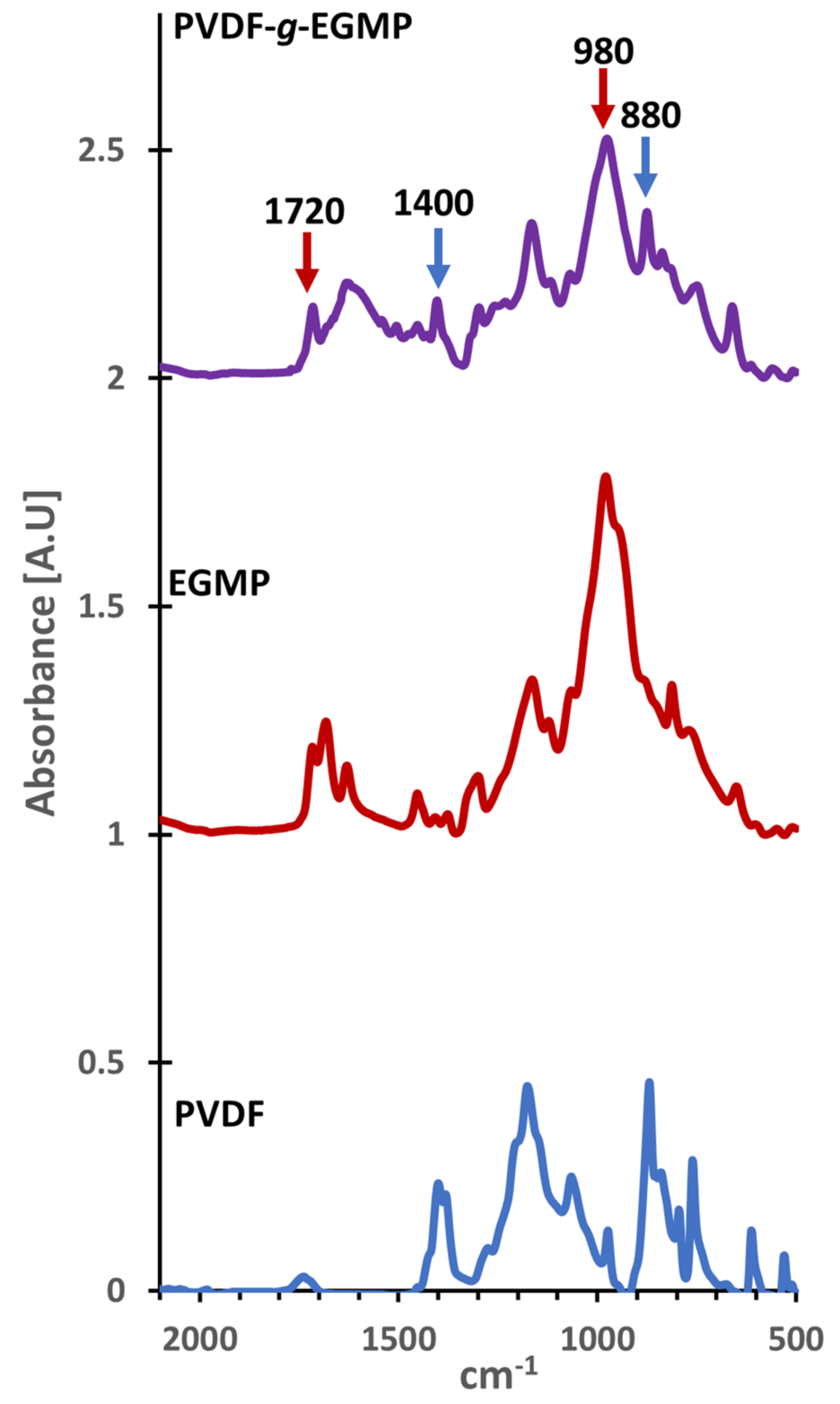
3.1. PVDF-g-EGMP Synthesis and Characterization
3.2. Permeability Measurements of PVDF-g-EGMP Membranes
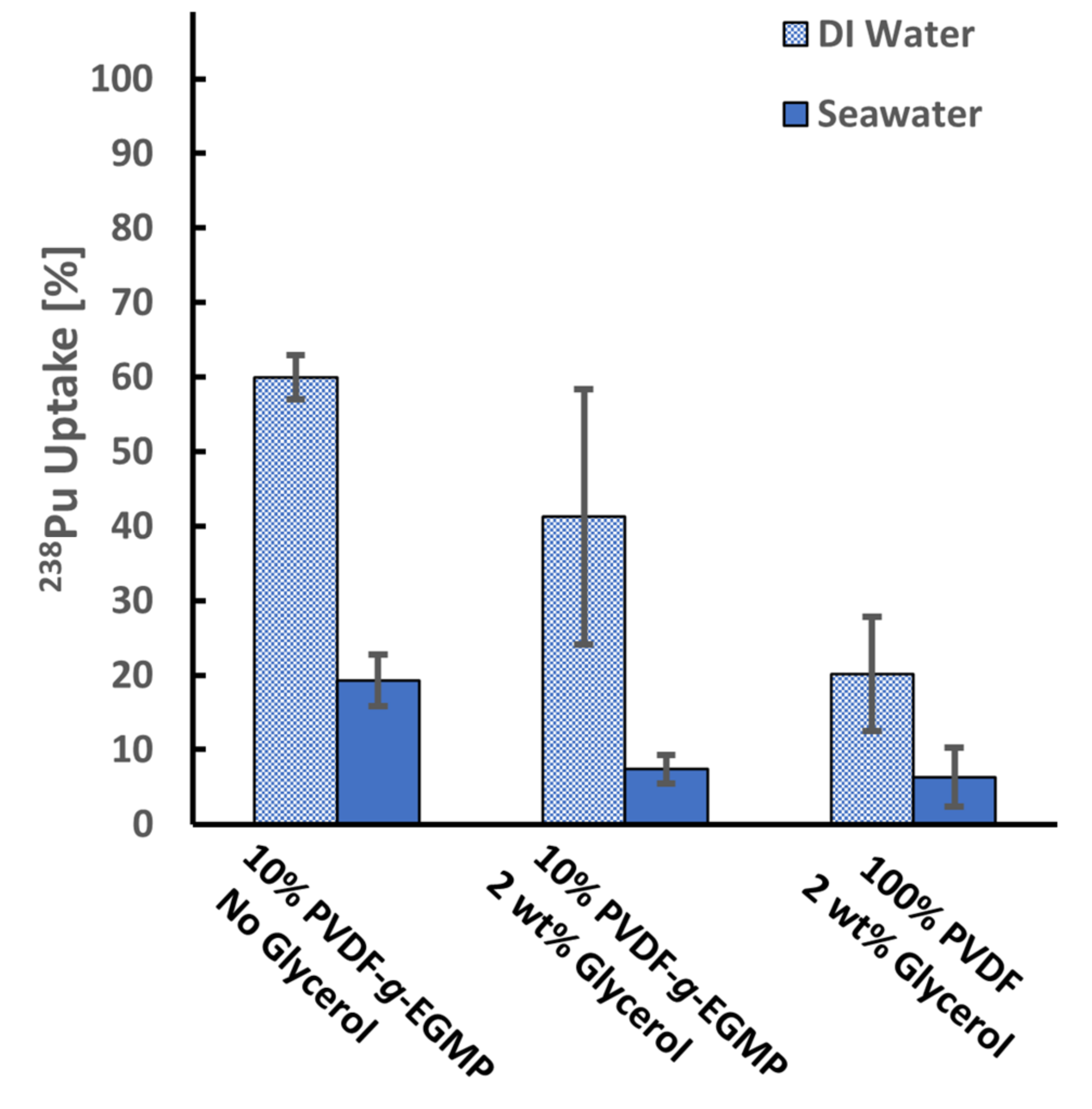
3.3. 242Pu Uptake by PVDF-g-EGMP Copolymer
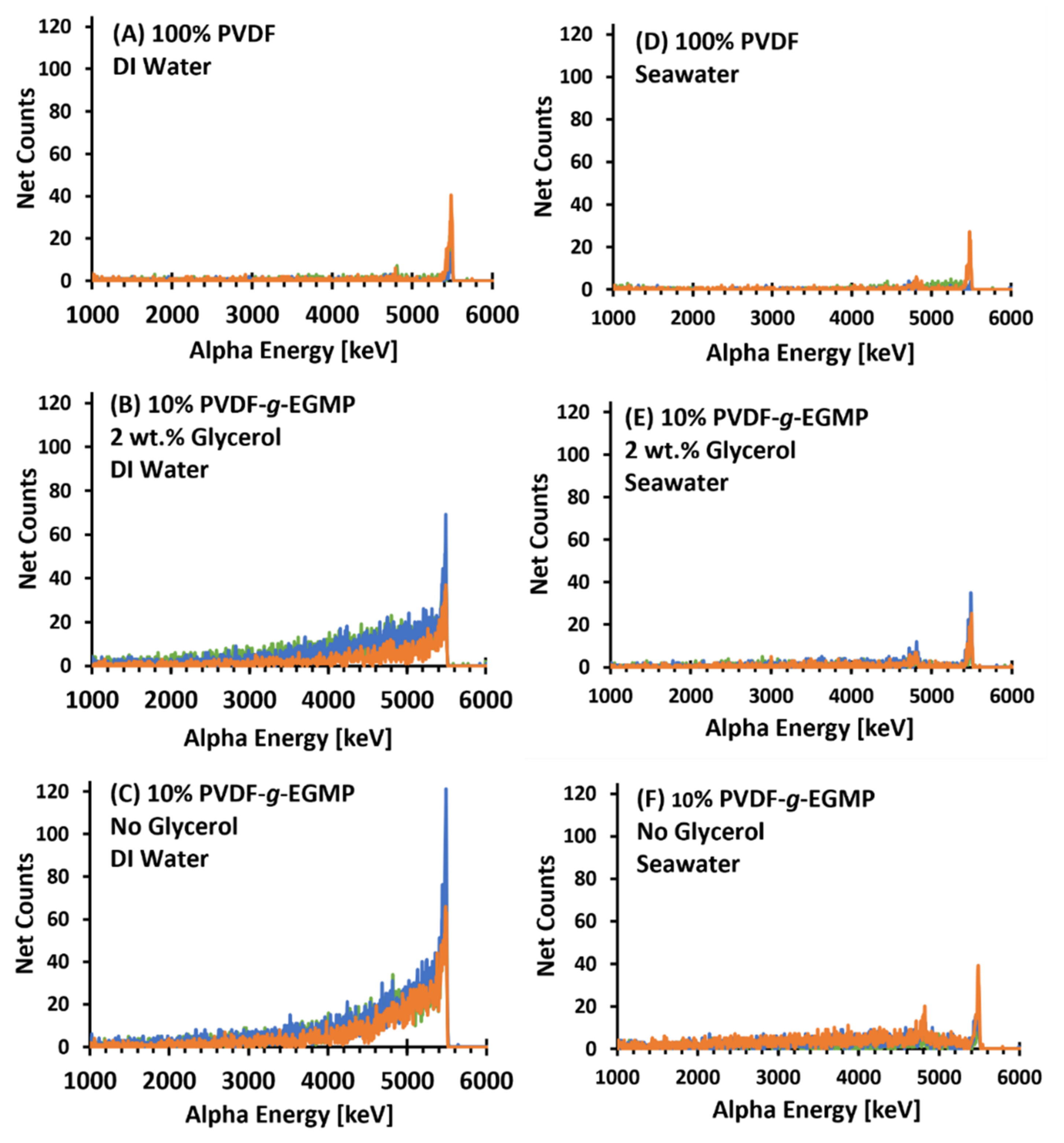
3.4. Direct Flow Filtration and Alpha Spectrometry Measurements with 10% PVDF-g-EGMP Membranes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- UNSCEAR. 2000 Report Vol. II: Sources and Effects of Ionizing Radiation; UNSCEAR: New York, NY, USA, 2000; pp. 518–520. [Google Scholar]
- Moody, K.J.; Hutcheon, I.D.; Grant, P.M. Nuclear Forensic Analysis, 2nd ed.; CRC Press: New York, NY, USA, 2015; pp. 140–150. [Google Scholar]
- Qiao, J.; Hou, X.; Miro, M.; Roos, P. Determination of Plutonium Isotopes in Waters and Environmental Solids: A Review. Anal. Chim. Acta 2009, 652, 66–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reis, A.S.; Temba, E.S.C.; Kastner, G.F.; Monteiró, R.P.G. Combined Procedure Using Radiochemical Separation of Plutonium, Americium, and Uranium Radionuclides for Alpha-Spectrometry. J. Radioanal. Nucl. Chem. 2011, 287, 567–572. [Google Scholar] [CrossRef]
- Rim, J.H.; Gonzales, E.R.; Armenta, C.E.; Ünlü, K.; Peterson, D.S. Developing and Evaluating Di(2-ethylhexyl) Orthophosphoric Acid (HDEHP) Based Polymer Ligand Film (PLF) for Plutonium Extraction. J. Radioanal. Nucl. Chem. 2013, 296, 1099–1103. [Google Scholar] [CrossRef]
- Gonzáles, E.R.; Kingensmith, A.L.; Peterson, D.S. Rapid Separation and Extraction of Radioactive Analytes onto Filters and Surfaces. Proc. Radiochem. 2011, 1, 195–200. [Google Scholar] [CrossRef]
- Hanson, S.K.; Mueller, A.H.; Oldham, W.J. Kläui Ligand Thin Films for Rapid Plutonium Analysis by Alpha Spectrometry. Anal Chem. 2014, 86, 1153–1159. [Google Scholar] [CrossRef] [PubMed]
- Mhatre, A.M.; Chappa, S.; Paul, S.; Pandey, A.K. Phosphate-Bearing Polymer Grafted Glass for Plutonium(IV) Ion-Selective Alpha Spectrometry. J. Anal. At. Spectrom. 2017, 32, 1566–1570. [Google Scholar] [CrossRef]
- Locklair, W.D.; Mannion, J.M.; Husson, S.M.; Powell, B.A. Uptake of Plutonium on a Novel Thin Film for Use in Alpha Spectrometry. J. Radioanal. Nucl. Chem. 2016, 307, 2333–2338. [Google Scholar] [CrossRef] [Green Version]
- Darge, A.W.; Gera, Y.; DeVol, T.A.; Husson, S.M. Uranium Concentration Using Reactive Polymer Thin Films for Spectroscopic Analysis. React. Funct. Polym. 2020, 157, 104761. [Google Scholar] [CrossRef]
- Vasudevan, T.; Das, S.; Sodaye, S.; Pandey, A.K.; Reddy, A.V.R. Pore-Functionalized Polymer Membranes for Preconcentration of Heavy Metal Ions. Talanta 2009, 78, 171–177. [Google Scholar] [CrossRef]
- Duval, C.E.; Darge, A.W.; Ruff, C.; DeVol, T.A.; Husson, S.M. Rapid Sample Preparation for Alpha Spectroscopy with Ultrafiltration Membranes. Anal. Chem. 2018, 90, 4144–4149. [Google Scholar] [CrossRef]
- Foster, J.C.; DeVol, T.A.; Husson, S.M. Extractive Thin-Film Composite Membranes for the Isotopic Screening of Plutonium in Water. React. Funct. Polym. 2021, 167, M105020. [Google Scholar] [CrossRef]
- Hester, J.F.; Banerjee, P.; Won, Y.Y.; Akthakul, A.; Acar, M.H.; Mayes, A.M. ATRP of Amphiphilic Graft Copolymers Based on PVDF and Their Use as Membrane Additives. Macromolecules 2002, 35, 7652–7661. [Google Scholar] [CrossRef]
- Conroy, N.A.; Wylie, E.M.; Powell, B.A. A Novel Method for Tracer Concentration Plutonium(V) Solution Preparation. Anal. Chem. 2016, 88, 4196–4199. [Google Scholar] [CrossRef]
- Das, S.; Pandey, A.K.; Vasudevan, T.; Athawale, A.A.; Manchanda, V.K. Adsorptive Preconcentration of Uranium in Hydrogels from Seawater and Aqueous Solutions. Ind. Eng. Chem. Res. 2009, 48, 6789–6796. [Google Scholar] [CrossRef]
- Cai, X.; Lei, T.; Sun, D.; Lin, L. A Critical Analysis of the α, β, and γ Phases in Poly(vinylidene fluoride) Using FTIR. RSC Adv. 2017, 7, 15382–15389. [Google Scholar] [CrossRef] [Green Version]
- Gestring, I.; Mewes, D. Degassing of Molten Polymers. Chem. Eng. Sci. 2002, 57, 3415–3426. [Google Scholar] [CrossRef]
- Feng, C.; Shi, B.; Li, G.; Wu, Y. Preparation and Properties of Microporous Membrane from Poly(vinylidene fluoride-co-tetrafluoroethylene) (F2.4) for Membrane Distillation. J. Membr. Sci. 2004, 237, 15–24. [Google Scholar] [CrossRef]
- Parvole, J.; Jannasch, P. Poly(arylene ether sulfone)s With Phosphonic Acid And Bis(phosphonic acid) on Short Alkyl Side Chains for Proton-Exchange Membranes. J. Mater. Chem. A 2008, 18, 5547–5556. [Google Scholar] [CrossRef]
- Tamura, Y.; Sheng, L.; Nakazawa, S.; Higashihara, T.; Ueda, M. Polymer Electrolyte Membranes Based on Polystyrenes with Phosphonic Acid via Long Alkyl Side Chains. J. Polym. Sci. A Polym. Chem. 2012, 50, 4334–4340. [Google Scholar] [CrossRef]
- Chappa, S.; Singha Deb, A.K.; Ali, S.M.; Debnath, A.K.; Aswal, D.K.; Pandey, A.K. Change in the Affinity of Ethylene Glycol Methacrylate Phosphate Monomer and its Polymer Anchored on a Graphene Oxide Platform towards Uranium(VI) and Plutonium(IV) ions. J. Phys. Chem. B 2016, 120, 2942–2950. [Google Scholar] [CrossRef]
- Zhu, X.; Alexandratos, S.D. Development of a New Ion-Exchange/Coordinating Phosphate Ligand for the Sorption of U(VI) and Trivalent Ions from Phosphoric Acid Solutions. Chem. Eng. Sci. 2015, 127, 126–132. [Google Scholar] [CrossRef] [Green Version]
- Suresh, P.; Duval, C.E. Poly(acid)-Functionalized Membranes to Sequester Uranium from Seawater. Ind. Eng. Chem. Res. 2020, 59, 12212–12222. [Google Scholar] [CrossRef]
- Breite, D.; Went, M.; Prager, A.; Schulze, A. Tailoring Membrane Surface Charges: A Novel Study on Electrostatic Interactions during Membrane Fouling. Polymers 2015, 7, 2017–2030. [Google Scholar] [CrossRef] [Green Version]
- Breite, D.; Went, M.; Prager, A.; Schulze, A. The Critical Zeta Potential of Polymer Membranes: How Electrolytes Impact Fouling. RSC Adv. 2016, 6, 98180–98189. [Google Scholar] [CrossRef]
- Foster, J.C.; Starstrom, S.A.; DeVol, T.A.; Powell, B.A.; Husson, S.M. Functionalized Polymer Thin Films for Plutonium Capture and Isotopic Screening from Aqueous Sources. Anal. Chem. 2020, 92, 5214–5221. [Google Scholar] [CrossRef] [PubMed]
- Stein, G.E.; Laws, T.S.; Verduzco, R. Tailoring the Attraction of Polymers Towards Surfaces. Macromolecules 2019, 52, 4748–4802. [Google Scholar] [CrossRef] [Green Version]
- Hester, J.F.; Banerjee, P.; Mayes, A.M. Preparation of Protein-Resistant Surfaces on Poly(vinylidene fluoride) Membranes via Surface Segregation. Macromolecules 1999, 32, 1643–1650. [Google Scholar] [CrossRef]
- Hester, J.F.; Mayes, A.M. Design and Performance of Foul-Resistant Poly(vinylidene fluoride) Membranes Prepared in a Single-Step by Surface Segregation. J. Membr. Sci. 2002, 202, 119–135. [Google Scholar] [CrossRef]
- Horwitz, E.P.; Chiarizia, R.; Dietz, M.L.; Diamond, H. Separation and preconcentration of actinides from acidic media by extraction chromatography. Anal. Chim. Acta 1993, 281, 361–372. [Google Scholar] [CrossRef]


| Membrane Dope Solutions | ||||
|---|---|---|---|---|
| DMF | PVDF534K | PVDF-g-EGMP | Glycerol | |
| Membrane | [g] | [g] | [g] | [g] |
| 10% PVDF-g-EGMP No glycerol | 47.2 | 7.5 | 0.83 | 0 |
| 10% PVDF-g-EGMP 2 wt% glycerol | 46.1 | 7.5 | 0.83 | 1.11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Foster, J.C.; DeVol, T.A.; Husson, S.M. Membranes for the Capture and Screening of Waterborne Plutonium Based on a Novel Pu-Extractive Copolymer Additive. Membranes 2022, 12, 3. https://doi.org/10.3390/membranes12010003
Foster JC, DeVol TA, Husson SM. Membranes for the Capture and Screening of Waterborne Plutonium Based on a Novel Pu-Extractive Copolymer Additive. Membranes. 2022; 12(1):3. https://doi.org/10.3390/membranes12010003
Chicago/Turabian StyleFoster, James C., Timothy A. DeVol, and Scott M. Husson. 2022. "Membranes for the Capture and Screening of Waterborne Plutonium Based on a Novel Pu-Extractive Copolymer Additive" Membranes 12, no. 1: 3. https://doi.org/10.3390/membranes12010003
APA StyleFoster, J. C., DeVol, T. A., & Husson, S. M. (2022). Membranes for the Capture and Screening of Waterborne Plutonium Based on a Novel Pu-Extractive Copolymer Additive. Membranes, 12(1), 3. https://doi.org/10.3390/membranes12010003







